To compare LISA with INSURE technique for surfactant administration in preterm with gestational age (GA) < 36 weeks with RDS in respect to the incidence of pneumothorax, bronchopulmonary dysplasia (BPD), need for mechanical ventilation (MV), regional cerebral oxygen saturation (rSO2), peri‑intraventricular hemorrhage (PIVH) and mortality.
MethodsA systematic search in PubMed, Embase, Lilacs, CINAHL, SciELO databases, Brazilian Registry of Randomized Clinical Trials (ReBEC), Clinicaltrials.gov, and Cochrane Central Register of Controlled Trials (CENTRAL) was performed. RCTs evaluating the effects of the LISA technique versus INSURE in preterm infants with gestational age < 36 weeks and that had as outcomes evaluation of the rates of pneumothorax, BPD, need for MV, rSO2, PIVH, and mortality were included in the meta-analysis. Random effects and hazard ratio models were used to combine all study results. Inter-study heterogeneity was assessed using Cochrane Q statistics and Higgin's I2 statistics.
ResultsSixteen RCTs published between 2012 and 2020 met the inclusion criteria, a total of 1,944 preterms. Eleven studies showed a shorter duration of MV and CPAP in the LISA group than in INSURE group. Two studies evaluated rSO2 and suggested that LISA and INSURE transiently affect brain autoregulation during surfactant administration. INSURE group had a higher risk for MV in the first 72 h of life, pneumothorax, PIVH and mortality in comparison to the LISA group.
ConclusionThis systematic review and meta-analyses provided evidence for the benefits of the LISA technique in the treatment of RDS, decreasing CPAP time, need for MV, BPD, pneumothorax, PIVH, and mortality when compared to INSURE.
Respiratory distress syndrome (RDS) is a condition that has a high incidence in premature newborns (NB), and it is one of the main causes of morbidity. Despite this, management has gradually evolved over the years and has resulted in greater survival, especially in the 24 to 26 weeks of gestational age (GA).1,2 Its main cause is surfactant deficiency, a fundamental substance in lung mechanics, responsible for reducing surface tension and preventing alveolar collapse during expiration.3
Thus, in the absence of surfactant, the NB has difficulty in performing inspiration, causing a large work of breathing and causing respiratory failure in the first hours of life. Major complications include pneumothorax, need for mechanical ventilation, bronchopulmonary dysplasia (BPD), peri‑intraventricular hemorrhage (PIVH), and mortality.4 Guidelines for the management of RDS determine that surfactant replacement therapy plays an essential role in treatment, due to its effectiveness in reducing morbidity. Recent protocols recommend that early rescue should be standard as soon as clinical signs of RDS occur.5
Among the surfactant administration techniques, one of the most frequently used is called Intubation-Surfactant-Extubation (INSURE), in which surfactant is administered after intubation, followed by rapid extubation. However, its use should be cautious, since intubation and mechanical ventilation (MV) with positive pressure, even for a short period, may be related to lung and tracheal injuries.6
Recently, less invasive surfactant administration (LISA) has been developed in which a thin intratracheal catheter is introduced into the airway during spontaneous breathing using continuous positive airway pressure (CPAP).7 Application of LISA while using CPAP is associated with less alveolar damage compared to MV,8 being a strategy of choice for the management of RDS in many hospital centers.9-11
Several randomized clinical trials (RCT) compared the LISA versus INSURE method and showed that LISA presented a decrease in the need and time of MV,8 and consequently, a reduction in the rate of BPD12 and death.13 A meta-analysis using pooled data from RCT that analyzed LISA versus control, covering various therapies such as INSURE, MV only, or CPAP, showed that the LISA technique reduces the risk of BPD and death among NB with a 36-week GA.14
A systematic review with meta-analysis carried out comparing the use of tracheal intubation and LISA included studies that did not clearly determine the use of the INSURE protocol in the control group.15 On the other side, an excellent Cochrane Database of Systematic Review and meta-analysis including 10 randomized clinical trials showed that administration of surfactant via thin catheter is associated with reduced risk of death or BPD, less intubation in the first 72 h, and reduced mortality than INSURE, suggesting more studies to confirm and refine these findings, clarify whether surfactant therapy via thin tracheal catheter provides benefits.16
Thus, exclusively comparing outcomes involving safety and efficacy between the two methods of surfactant administration is mandatory, and understanding the best strategy for pulmonary surfactant administration may improve the future quality of life of preterm infants.
Materials and methodsType of studySystematic Review and meta-analysis, submitted to the International Prospective Register of Ongoing Systematic Reviews (PROSPERO), an international database of prospective registry of systematic reviews in the health area, under registration number: CRD42021241287. In addition, the study followed the PRISMA Statement and the Cochrane Collaboration Recommendations; and used Review Manager Software 5.4.
Eligibility criteriaRCT that evaluated the effects of the LISA technique versus INSURE in preterm NB < 36 weeks GA and whose endpoints were pneumothorax, BPD, need for mechanical ventilation, mortality, regional cerebral oxygen saturation and peri‑intraventricular hemorrhage were included.
Research questionP (Population) - Premature infants with RDS and GA of less than 36 weeks.
I (Intervention) - Administration of LISA.
C (Comparator) - Compared to INSURE administration.
O (Outcome) - Mortality, bronchopulmonary dysplasia, pneumothorax, need for mechanical ventilation, regional cerebral oxygen saturation, and peri‑intraventricular hemorrhage.
T (Type of Studies) - Randomized clinical trials.
Search sourcesThe bibliographic searches were carried out in the following electronic databases: PubMed, Embase, Lilacs, CINAHL, SciELO, and search at Registro Brasileiro de ensaios clínicos randomizados (ReBEC), Clinicaltrials.gov and Cochrane Central Register of Controlled Trials (CENTRAL). The search terms were built specifically for each of the databases used, considering their specificities and in order not to neglect any article that would fulfill the inclusion criteria of this work. Also, a search was carried out in the references of the articles found in the databases. Articles published and indexed in these databases in the last ten years and available in Portuguese or English were included.
Search termsThe search terms were built specifically for each of the databases used − PubMed, Embase, Cinahl Lilacs and SciELO −, considering their specificities and in order not to neglect any article that could meet the inclusion criteria of this work (Table 1).
Search terms used in the database.
After carrying out the research using these search strategies, the generated list of articles was downloaded, which was inserted into the Zotero Reference Manager, in which each article found was subject to the inclusion and exclusion criteria determined to, finally, select the articles that are part of this Systematic Review. Study selection was performed by two independent researchers (N. M and A. F), initially by reading the titles and abstracts and, later, by reading the complete version of the articles.
Disagreements regarding the inclusion of studies were resolved by consensus and with a third evaluator (RCS). The selection of articles for this Systematic Review did not limit the results by date, therefore, all articles that emerged because of the search terms were submitted to the decision of inclusion or not by the researchers.
In the meta-analysis, the included trials for administering surfactant were randomized or quasi-randomized studies selected in the systematic review during the last ten years.
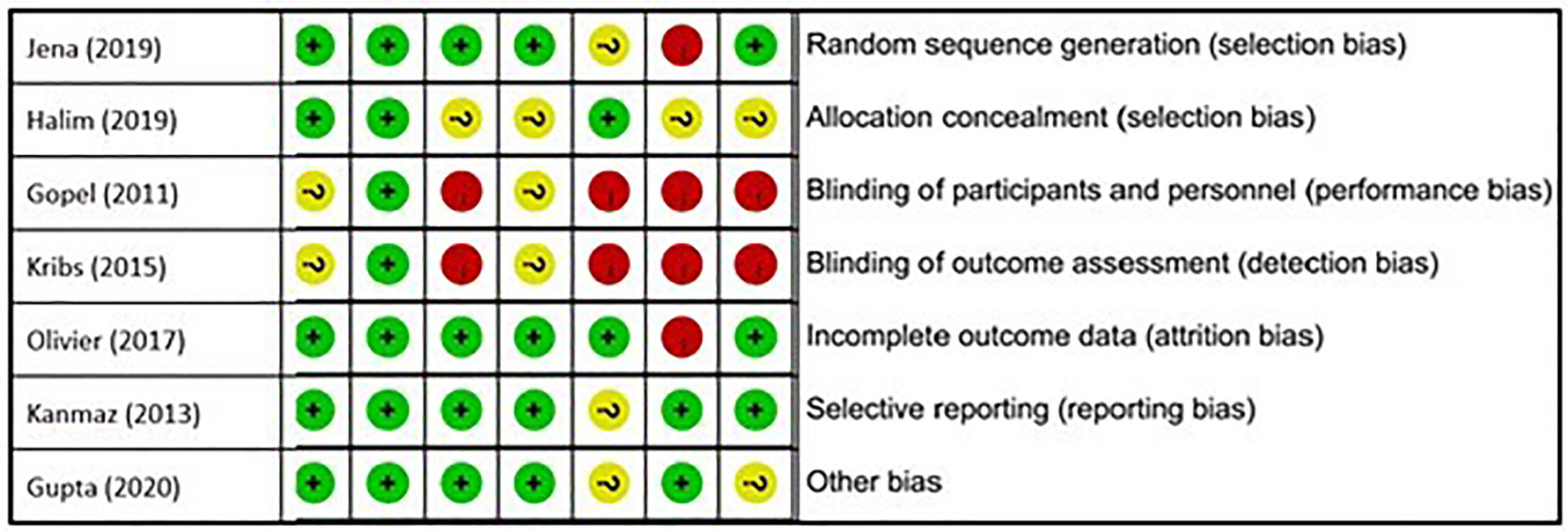
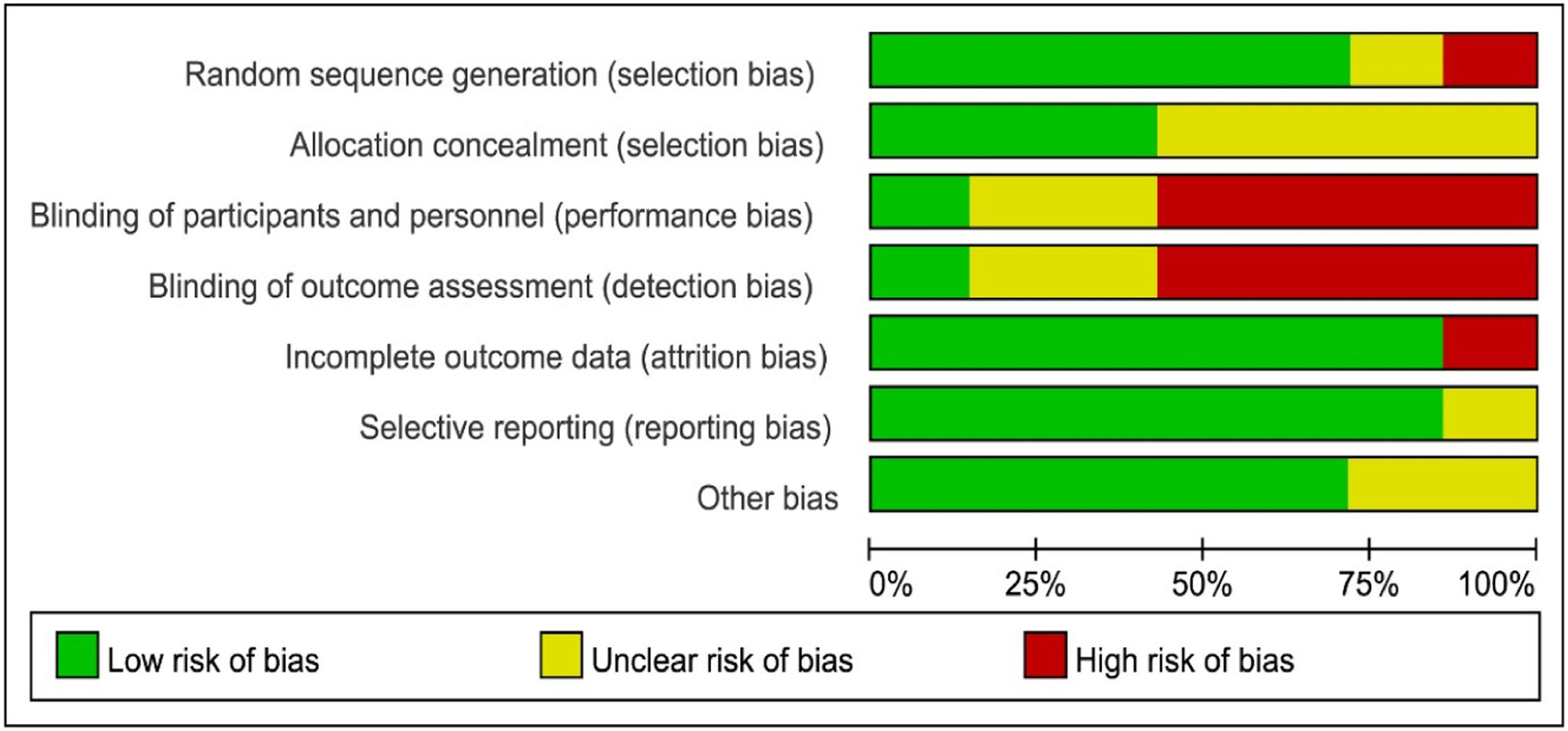
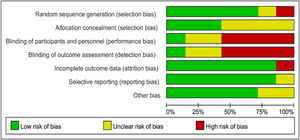
Quality of evidence and risk of bias assessmentThe methodological qualities of the studies were assessed by two researchers. The quality of evidence from the selected studies was assessed using the GRADE checklist (Grading of Recommendations, Assessment, Development, and Evaluation), while the risk of bias was assessed using the Cochrane collaboration tool (ROB 1.0 tool). The review authors' judgments about each risk of bias item are presented as percentages across all included studies (Figures 1 and 2).
The meta-analysis was performed using Comprehensive Meta-Analysis version 3.3 (Biostat, Englewood, NJ, USA). Odds ratio (OR) with a 95% confidence interval (CI) and P-value were calculated from the data provided in each study. A random-effects model was used to combine all study results. Data extracted from each study were used to calculate the frequency of patients in each variable studied (need for MV in the first 72 h of life, BPD, pneumothorax, mortality, and PIVH) and then a meta-analysis was performed to compare the LISA and INSURE groups through from Review Manager Software 5.4. Random effects and hazard ratio models were used to combine all study results. Inter-study heterogeneity was assessed using Cochrane Q statistic and Higgin's I2 statistics were derived from Q Statistic; with low, moderate, and high I2 values of 25%, 50%, and 75%, respectively.17
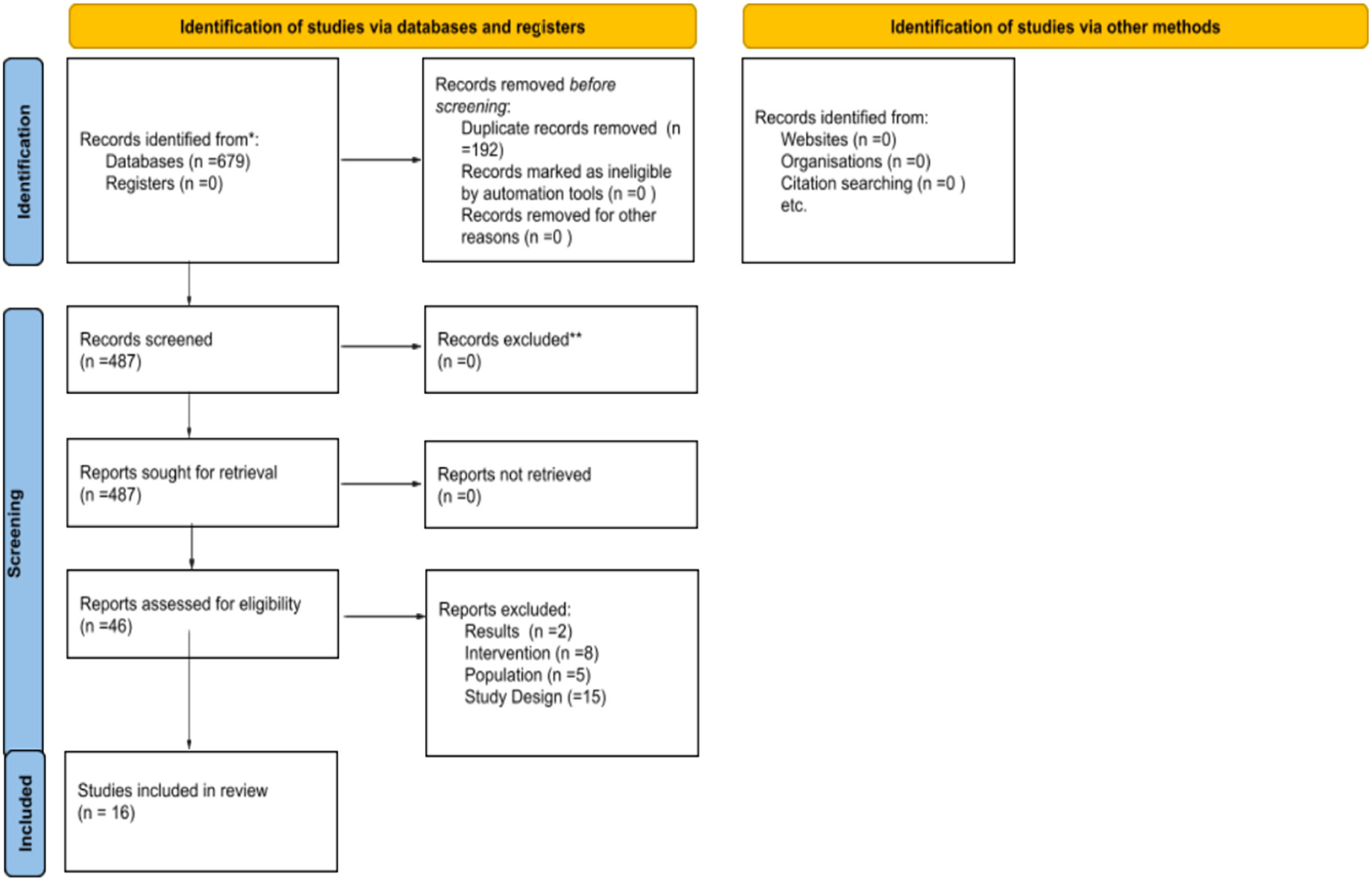
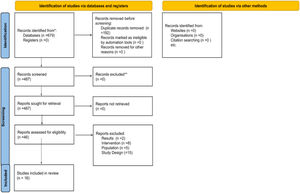
ResultsA total of 679 articles were identified, after initial screening and removal of duplicates, 487 articles remained, of which 46 were selected for detailed analysis. After analysis, 16 articles met the eligibility criteria and were included in this systematic review, with a total of 1944 patients (Figure 3).
Flowchart of studies included in the systematic review. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/.
The size of the populations of NB included in the studies ranged from n = 20 to n = 350 neonates. All studies involved premature infants, with gestational age (GA) ranging from 25 to 36 weeks (Table 2).18-29 All studies excluded previously intubated NB and those with major congenital anomalies.
Characteristics of the studies included in the systematic review.
| Author (year) | Participants (N-I/C) | Group Intervention | Group Control | Primary endpoint | Results |
|---|---|---|---|---|---|
| Gupta et al. (2020)18 | 58 (29/29) | Insertion of a probe by direct laryngoscopy using forceps. After placement of the probe, the laryngoscope was removed. | The neonates were intubated for surfactant replacement therapy, on positive pressure ventilation and a self-inflating resuscitation bag. Neonates were extubated immediately after surfactant administration and placed back on NIPPV. | Need for IMV in the first 72 h of life. | Need for IMV in 72h:There was no statistically significant difference between the LISA group (10.34%) and the INSURE group (20.69%).Persistence of the ductus arteriosus,PIVH > grade 2, BPD and BPD outcome/pre-discharge mortality: No differences were observed between the two groups.Hospital stay:Neonates in the INSURE group stayed in the hospital longer than in the LISA group (mean 41.6 days vs 29.76 days).Need for a second dose of surfactant: There was no difference. |
| Mirnia et al. (2013)13 | 80 (38/42) | Tracheal instillation via catheter during spontaneous breathing under nCPAP. | The neonates were intubated, received positive pressure ventilation for 30 s while surfactant was administered. After surfactant instillation, they were placed on nCPAP immediately. | – | Duration of ventilation:There was no difference between the duration of mechanical ventilation between the two groups (p < 0.2), but the mean total duration of CPAP was shorter in the LISA group, in contrast to the INSURE group (p < 0.01).Morbidities:There was no difference in the prevalence of BPD, patent ductus arteriosus, PIVH, pneumothorax, sepsis and retinopathy of prematurity between the two groups.O2 supplement need and length of stay: Lower rates in the LISA group compared to the INSURE group, but not statistically significant. |
| Jena et al. (2019)19 | 350 (175/175) | Direct laryngoscopy was performed and the catheter or feeding tube was inserted through the vocal cords to the desired depth. Meanwhile, the CPAP prong was fitted to the face. After placement of the catheter, the laryngoscope was removed. Surfactant was administered as a single bolus over 60 to 90 s and the tracheal catheter was immediately withdrawn. | The infants were intubated and administered as in the intervention group, while they received PPV with a T piece resuscitator. After extubation, nCPAP was started as in the intervention group. | Effect of the LISA technique on the need for MV in the first 72 h of life. | Need for MV:There was a significant reduction in the need for MV in the LISA group (19% vs 40%, p < 0.01).Second dose of surfactant and morbidities:There was no difference between the two groups in the need for the second dose of surfactant, EOS, PDA, PIVH and mortality before hospital discharge.In addition, duration of oxygen therapy, necrotizing enterocolitis and duration of NICU stay were significantly shorter in the LISA group. |
| Baskabadi et al. (2019)20 | 40 (20/20) | Direct laryngoscopy was performed to place a feeding tube into the infant's trachea. Then, during spontaneous breathing using nCPAP, surfactant was used for 1–3 min through the feeding tube, then the feeding tube was removed and NCPAP continued. | The infants on NCPAP were intubated for surfactant administration and received the same amount of surfactant through the tracheal tube, then for 30–60 s they were submitted to bag-valve mask ventilation, then the tracheal tube was removed and NCPAP was restarted. | – | Duration of ventilation and duration of hospitalization:The use of LISA reduced the need for MV in infants (p = 0.027), did not increase the side effects of RDS, and did not change the duration of the need for NCPAP and the duration of hospitalization (p > 0.05). |
| Olivier et al. (2017)21 | 45 (24/21) | Laryngoscopy was performed while patients received nCPAP support in which a sterile, flexible probe with forceps was inserted. | Surfactant was administered only after intubation based on the judgment of the attending physician. | Need for MV or the development of a pneumothorax requiring chest drainage. | Mechanical ventilation or pneumothorax:The incidence of the primary outcome was significantly lower in the intervention group (p < 0.001). |
| Kribs et al. (2015)22 | 211 (107/104) | Laryngoscopy was performed while the child was breathing with the aid of nasal CPAP, then a catheter was introduced with forceps at an angle of approximately 120°; the catheter was fixed in this position and the laryngoscope removed. The infant's mouth was closed, and the surfactant was manually instilled for 30 to 120 s per minibolus. | The infants were intubated, mechanical ventilation was started, and surfactant was administered through the endotracheal tube. Sedation and analgesia for intubation were not routinely used.After administration, the infants were extubated and placed on non-invasive ventilation. | Survival without bronchopulmonary dysplasia at 36 weeks GA, as determined by a standardized test. | Bronchopulmonary dysplasia:In the intervention group, 67.3% of all infants survived without BPD compared to 58.7% in the control group, i.e., no significant difference. The absolute risk reduction for the primary outcome was 8.6% (p = 0.20).Mechanical ventilation:Duration was shorter in the intervention group. No significant differences were observed in duration of respiratory support, use of supplemental oxygen, or incidence of pulmonary hemorrhage.Pneumothorax:The occurrence was significantly lower in the intervention vs control group (4.8% vs 12.6%; p = 0.04).Intraventricular hemorrhage:The intervention group had significantly less severe PIVH (10.3% vs 22.1%; p = 0.02).Duration of hospitalization:It was not significantly lower in the intervention group (103 vs 105 days; p = 0.11). |
| Han et al. (2020)23 | 298 (151/147) | A laryngoscope was introduced through which the catheter tip was positioned with the aid of forceps up to 1.0 cm below the vocal cords. The laryngoscope and forceps were removed, and the child's mouth closed. The surfactant was instilled for 60 to 300 s per minibolus.The catheter was removed immediately after administration. Sedation and analgesia were not used. During surfactant administration, nCPAP therapy was continued. | They were intubated and received positive pressure ventilatory support. The surfactant was administered through an endotracheal tube. Positive pressure ventilatory support was performed following predefined patterns. Extubation criteria were established as FiO 2 < 0.3 and mean airway pressure (MAP) < 8 cm H2O. | Difference in BPD morbidity between two groups of infants with LISA and INSURE at 36 weeks corrected gestational age. | Bronchopulmonary dysplasia:There were no clear benefits of LISA therapy on the incidence of BPD, but there was a trend towards a reduction in the incidence of BPD in the intervention group (29/151 vs. 138/147, 19.2 vs. 25.9%, p = 0.170).BPD and PDA morbidity:There was a significant reduction in morbidity in the BPD intervention group (9/31, 29.0 vs. 14/20, 70.0%, p = 0.004) and PDA (9/31, 29.0, vs. 13/21, 65.0%, p = 0.011).Duration of ventilatory support:There were no differences in the duration of nCPAP respiratory support and supplemental oxygen between the two groups.Duration of hospitalization:The infants in both groups remained in the NICU for almost 40 days.Persistence of the ductus arteriosus:Children in the INSURE group had higher rates compared to children in the LISA group (60.5 vs. 41.1%, p = 0.001). |
| Bao et al. (2015)24 | 90 (47/43) | A 16-gage, 130 mm vascular catheter was marked to indicate the desired insertion depth (28–29 weeks: 1.5 cm, 30–32 weeks: 2 cm). While the neonates were on nCPAP, direct laryngoscopy was performed, and the catheter was inserted beyond the vocal cords to the required depth. Surfactant was administered in a standard dose with 5 bolus or more over 3–5 min. The tracheal catheter was immediately removed, and the infants were left on nCPAP. | Surfactant instillation via endotracheal tube was performed with a few brief mechanical ventilations, a standard dose of surfactant was always divided into 2 or 3 bolus. The endotracheal tube was removed as soon as clinically possible after PS instillation, and the infant was switched to nCPAP. The entire procedure lasted about 3 min and took place without continuous distending pressure. | – | Mechanical ventilation:The duration of MV and nCPAP was significantly shorter in the intervention group.There were no significant differences in both rates of MV in the first 72 h and mean duration of oxygen need.Mortality and morbidities:There were no differences in mortality or in the incidence of bronchopulmonary dysplasia, intraventricular hemorrhage, retinopathy of prematurity and necrotizing enterocolitis. |
| Mosayebi et al. (2017)6 | 53 (24/26) | The surfactant was instilled through a thin tracheal catheter, which was then removed. | The infants were first intubated, administered surfactant by passing a feeding tube through the endotracheal tube, and then extubated after 30 s of positive pressure ventilation. | – | Ventilatory support:The amount of oxygen needed by the LISA group was consistently lower than the other group in the first 48 h of life. The overall mean FiO2 was 42.5 ± 19.6 in the LISA group and 48.4 ± 21.6 in the INSURE group (p = 0.009).Duration of hospitalization:The mean length of stay in the neonatal intensive care unit was 7.3 ± 7.2 days in the MIST group and 9 ± 10.4 days in the INSURE group (p = 0.81).Complications:In terms of early and late complications, no difference was observed between the two groups. |
| Göpel et al. (2011)25 | 220 (108/112) | The infants received surfactant treatment during spontaneous breathing through a thin catheter inserted into the trachea by laryngoscopy if they required an inspired fraction of oxygen greater than 0.30. | The infants in the standard care group were assigned to receive CPAP, rescue intubation, and surfactant treatment if needed. | Any mechanical ventilation, or not being ventilated, but having a PCO2 greater than 65 mm Hg (8.6 kPa) or an FiO2 greater than 0.060 or both, for more than 2 h between 25 and 72 h of age. | Need for mechanical ventilation:The number of infants who received MV during the hospitalization was lower in the intervention group than in the control group (28% vs 46%; p = 0·008).Duration of ventilatory support:The total number of ventilation days was 599 in the control group vs 242 days in theintervention group. |
| Bertini et al. (2017)26 | 20 (10/10) | A flexible nasogastric tube was placed in the trachea after direct visualization of the vocal cords with a laryngoscope and forceps. After placement of the catheter, the laryngoscope was removed, and surfactant was administered intratracheally within 30 to 60 s. After instillation, the catheter was immediately removed. The nCPAP support was maintained during the procedure. | Performed with endotracheal tube and surfactant was instilled into the trachea within 30 s. After surfactant instillation, mechanical ventilation was performed for 1 min using a T piece device set at 18/5 cm H 2 O. Then, patients were immediately extubated and nCPAP resumed. | Changes in measurement of cerebral regional oxygenation induced by LISA and INSURE proceduresand the possible differences between them. | Regional cerebral oxygen saturation:The LISA and INSURE procedures transiently decreased rSO2C in both groups, but the decrease was greater in the LISA group (p ≤ 0.001). |
| Mohammadizadeh et al. (2015)27 | 38 (19/19) | Using the laryngoscope, a flexible catheter was inserted into the trachea and fixed with the aid of forceps at an angle of 120° This was fixed with two fingers and the laryngoscope removed. Then, surfactant was injected into the trachea for 1–3 min. At the end of the procedure, to ensure that the drug was not accidentally injected into the stomach, the orogastric tube was aspirated. During the procedure, nCPAP was applied continuously. After surfactant administration, FIO2 was gradually decreased as in the control group. | The surfactant was administered through a 2.5F or 3F endotracheal tube inserted into the trachea. The child was temporarily separated from the CPAP. After bolus injection of drug into the trachea, positive pressure ventilation was applied using neopuff and continued for at least 1 min or until SpO2 reached 87% or greater. The endotracheal tube was then removed, and the child was switched back to nCPAP at the previous pressure.FIO 2 was decreased at a rate of 5% every 1–2 min while SpO2 was maintained at the above-desired level. | Need for mechanical ventilation up to 72 h after birth. | Need for mechanical ventilation:There was no significant difference between the groups regarding the need formechanical ventilation during the first 72 h of birth (3 [15.8%] in the control group vs. 2 [10.5%] in theintervention group; p = 0.99).Duration of ventilatory support:The duration of oxygen therapy in the intervention group (fine catheter inserted into the trachea) was significantly shorterthan the control group (endotracheal tube) (243.7 ± 74.3 h vs. 476.8 ± 106.8 h; p = 0.018).Adverse events:The number of adverse events during surfactant administration was significantly lower in the intervention group than in the control group (6 [31.6%] vs.12 [63.2%]; p = 0.049).Morbidities and mortality:There was no significant difference between the two groups in terms of intraventricular hemorrhage rate, mortality and chronic lung disease. |
| Yang et al. (2020)28 | 97 (47/50) | A gastric tube with an outer diameter of 2 mm was marked to indicate the desired insertion depth (32–34 weeks: 2 cm, 34–36 weeks: 2.5 cm). While the child was breathing via CPAP, a direct laryngoscope was introduced while the probe was grasped with Magill forceps to the desired position. The laryngoscope and clamp were removed. The infant's mouth was closed, and the surfactant was slowly injected over 1 to 3 min. After this step, 1 ml of air was introduced, and the gastric tube was removed. | The infants in the INSURE group were treated with tracheal intubation and positive pressure artificial ventilation. Positive pressure ventilation continued for 3 min after surfactant injection and NCPAP was used after extubation. | – | There were no significant differences in reflux, asphyxia, Bradycardia (< 100 beats/min), apnea, FiO2, changes in PaO2 and PaCO2 1 hour after treatment between the groups.During administration, blood pressure and SpO2 in the LISA group were more stable, and significant differences between the 2 groups were observed.However, there were no significant differences in complications and outcomes between the 2 groups. |
| Li et al. (2016)29 | 44 (22/22) | Surfactant via LISA was administered within 6 h of birth. The detailed protocol for LISA was derived from the literature. | Surfactant via INSURE was administered within 6 h of birth. The detailed protocol for INSURE was derived from the literature. | To compare the effect of the two methods of surfactant administration on brain autoregulation. | Regional cerebral oxygen saturation: INSURE and LISA caused a transient impairment of brain autoregulation in infants with RDS, LISA was better than the INSURE technique in terms of duration of effect (< 5 min for LISA vs. 5–10 min for INSURE). |
| Kanmaz et al. (2013)12 | 200 (100/100) | A laryngoscope was used to introduce a flexible and sterile nasogastric tube. The desired insertion depths beyond the vocal cords for preterm infants at 25 to 26, 27 to 28, and 29 to 32 weeks GA were 1.0, 1.5, and 2.0 cm, respectively. After placement of the catheter, the laryngoscope was removed. Surfactant was prepared and administered as a 1 bolus over 30 to 60 s and the tracheal catheter was immediately withdrawn. During the procedure, CPAP support was not interrupted. | Patients were first intubated orally with a double-lumen endotracheal tube and surfactant was instilled into the trachea within 30 s. During surfactant instillation, manual lung inflation was performed using a T piece device with a pressure of 20/5 cm H2O, and then the patient was promptly extubated. Soon after extubation, nCPAP support was restarted, depending on the intervention group. No premedication, such as sedation or atropine, was used during either procedure. | Need for mechanical ventilation in the first 72 h of life. | Need for mechanical ventilation:The need for mechanical ventilation in the first 72 h of life was significantly lower in the LISA group when compared to the INSURE group (30% vs 45%, p = 0.02).Duration of ventilatory support:The average duration of nCPAP and MV were significantly shorter in the LISA group (P values 0.006 and 0.002, respectively).Bronchopulmonary dysplasia:The rate was significantly lower among children treated with LISA and the incidence of moderate to severe BPD among patients who survived the disease was significantly higher in the INSURE Group (20.2% vs 10.3%, p = 0.009).Mortality:Overall mortality rates were similar in both groups (16% and 13%, p = 0.68). |
| Halim et al. (2019)8 | 100 (50/50) | Surfactant was administered with the aid of a nasogastric tube. The upper respiratory tract was visualized with a laryngoscope and the catheter was passed 1–2 cm beyond the vocal cords. Surfactant was delivered within 1–3 min in small doses, while the child continued to breathe with nCPAP, during and after the procedure.If catheterization was not possible within 20–30 s, the procedure was interrupted and tried again when the infant was stable. The tracheal catheter was removed immediately after the procedure. | Infants were intubated and surfactant was successfully administered in 2–3 doses with an endotracheal tube at the same dose as the intervention group, while they received positive pressure ventilation via a T-piece resuscitator. After a brief period of positive pressure ventilation for 15–20 min, the endotracheal tube was removed, and the infants were placed on nCPAP. | Need for mechanical ventilation. | Need for mechanical ventilation:The need for invasive mechanical ventilation was significantly higher in the INSURE group (60% (n = 30) vs. 30% (n = 15), p < 0.05} compared to the LISA group.Duration of mechanical ventilation:The duration of mechanical ventilation was also significantly longer in the INSURE group with a median of 71 (IQR 62) vs. 40 (IQR 75) hours, p < 0.05 when compared to the LISA group.Morbidities:No significant differences were observed in either group based on complication rate (pneumothorax, PDA, pulmonary hemorrhage). In the INSURE group 5 (10%) developed pneumothorax compared with 2 (4%) in the LISA group (p = 0.625).Mortality:There were better survival rates in the LISA group (62% discharged, 38% died) compared with the INSURE group (44% discharged, 56% died), with no significant value. |
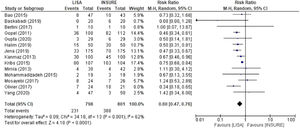
The study interventions had as a primary objective to assess the need for MV in the first 72 h of life. Six studies showed a statistically significant difference in the primary endpoint between the groups, showing a lower need for MV in the first 72 in the LISA group participants. The other studies also suggested a lower need for MV in the first 72 h in patients in the LISA group, although this was not significant. In addition, three studies showed satisfactory results regarding the duration of MV and CPAP in the LISA group, when compared to the INSURE group.
In Halim et al.8 the need for MV was significantly higher in the INSURE group, 60% versus 30% (p < 0.05) compared to the LISA group. Kanmaz et al.12 observed that the LISA technique significantly reduced the need for MV (30% vs 45%, p = 0.02).
Jena et al.19 found a significant reduction in the need for MV in the LISA group, 19% versus 40% in the INSURE group (p < 0.01). Boskabaldi et al.20 also concluded that the LISA technique reduces the need for MV in NBs (p = 0.02). The same results were found in Kribs et al.22 in which the duration of MV was shorter in the LISA group (p = 0.001).
Göpel et al.25 showed that the administration of surfactant using the LISA technique reduces the need for MV. In this study, only 22% of NBs in the LISA group received MV on the 2–3rd day after birth, compared to 43% in the INSURE group. In addition, the total number of ventilation days was 599 days in the INSURE group versus 242 days in the LISA group (< 0.001).
Bao et al.24 did not find significant differences in MV rates in the first 72 h, but the duration of MV and CPAP was significantly shorter in the LISA group when compared to the INSURE group. In Mirnia et al.13 although there were no differences in the duration of MV between the groups, the mean duration of CPAP was shorter in the LISA group, in contrast to INSURE (p < 0.01).
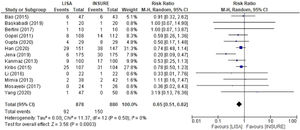
Bronchopulmonary dysplasiaBPD rates were significantly reduced in two studies in the LISA group. Kanmaz et al.12 found that the rate of BPD was significantly lower in the LISA group (13.6%) when compared to the INSURE group (26.2%), and the incidence of moderate to severe BPD among patients who survived the disease was significantly higher in the INSURE group (p = 0.009). Jena et al.19 also concluded that there was a significant decrease in BPD rates in the LISA group, 3% versus 17% (p ≤ 0.01) when compared to INSURE.
In the study by Han et al.23 although the comparison did not show clear benefits with LISA on the incidence of BPD, there was a trend towards a reduction in the incidence of BPD, 19.2% versus 25.9% (p = 0.170).
Pneumothorax, mortality, and peri‑intraventricular hemorrhageAll selected studies investigated at least one of the secondary outcomes, pneumothorax, mortality, and PIVH rates, which were similar between the two groups in most studies, as shown in Table 3.
Pneumothorax, Mortality and PIVH rates.
| Author (year) | Pneumothorax n (%) | Mortality n (%) | PIVH n (%) |
|---|---|---|---|
| Gupta et al. (2020)18 | – | LISA 4 (13.7%)INSURE 9 (31%)p = 0.11 | LISA 0 (0%)INSURE 1 (3.45%)p > 0.99 |
| Mirnia et al. (2013)13 | LISA 2 (6%)INSURE 2 (5%)p = 0.9 | LISA 1 (3%)INSURE 3 (8%)p = 0.3 | LISA 4 (11%)INSURE 1 (2%)p = 0.3 |
| Jena et al. (2019)19 | – | LISA 9 (5%)INSURE 17 (10%)p >0.05 | LISA 5 (3%)INSURE 4 (2%)p > 0.05 |
| Baskabadi et al. (2019)20 | – | – | LISA 1 (5%)INSURE 1 (5%)p = 1 |
| Olivier et al. (2017)21 | LISA 1 (4.1%)INSURE 1 (4.7%)p > 0.05 | – | – |
| Kribs et al. (2015)22 | LISA 5 (4.8%)INSURE 13 (12.6%)p = 0.04 * | LISA 10 (9.3%)INSURE 12 (11.5%)p = 0.59 | LISA 11 (10.3%)INSURE 23 (22.1%)p = 0.02 * |
| Han et al. (2020)23 | – | – | LISA 10 (6.6%)INSURE 10 (6.8%)p = 0.35 |
| Bao et al. (2015)24 | LISA 4 (8.9%)INSURE 3 (7%)p = 0.79 | LISA 1 (2.1%)INSURE 0 (0%)p = 0.34 | LISA 1 (2.1%)INSURE 0 (0%)p = 0.34 |
| Mosayebi et al. (2017)6 | LISA 0 (0%)INSURE 1 (3.8%)p = 0.49 | LISA 1 (3.7%)INSURE 0 (0%)p = 0.98 | LISA 0 (0%)INSURE 1 (3.8%)p = 0.49 |
| Göpel et al. (2011)25 | LISA 4 (4%)INSURE 8 (7%)p = 0.37 | LISA 7 (7%)INSURE 5 (5%)p = 0.56 | LISA 8 (7%)INSURE 6 (5%)p = 0.59 |
| Bertini et al. (2017)26 | – | LISA 1 (10%)INSURE 0 (0%)p = 1 | – |
| Mohammadizadeh et al. (2015)27 | – | LISA 1 (5.2%)INSURE 3 (15.8%)p = 0.60 | LISA 1 (5.2%)INSURE 1 (5.2%)p = 1 |
| Yang et al. (2020)28 | LISA 2 (4.3%)INSURE 3 (6%)p = 0.70 | LISA 0 (0%)INSURE 0 (0%) | LISA 0 (0%)INSURE 0 (0%) |
| Li et al. (2016)29 | – | LISA 0 (0%)INSURE 1 (4.5%)p = 0.31 | LISA 2 (9.1%)INSURE 3 (13.6%)p = 0.60 |
| Kanmaz et al. (2013)12 | LISA 7 (7%)INSURE 10 (10%)p = 0.61 | LISA 16 (16%)INSURE 13 (13%)p = 0.68 | LISA 10 (10%)INSURE 16 (16%)p > 0.05 |
| Halim et al. (2019)8 | LISA 2 (4%)INSURE 5 (10%)p = 0.63 | LISA 19 (38%)INSURE 28 (56%)p > 0.05 | – |
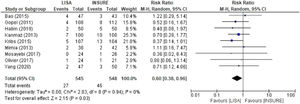
Only in Kribs et al.22 there was a significant effect in favor of the LISA group with lower rates of pneumothorax and PIVH when compared to the INSURE group. Suggesting a higher uncomplicated survival rate in those who received less invasive surfactant.
Regional cerebral oxygen saturationLi et al.29 and Bertini et al.26 evaluated regional brain oxygen saturation (rSO2), monitored using near-infrared spectroscopy (NIRS) technology. The results of Li et al. suggest a transient impairment of cerebral autoregulation during and after the two procedures and concluded that the effect of duration of impairment in the LISA technique was smaller than in the INSURE technique (< 5 min in LISA vs. 5–10 min in INSURE).
Bertini et al.26 showed that both procedures transiently decreased rSO2, and the decrease was greater in the LISA group (p < 0.001). Thus, Li et al.29 and Bertini et al.26 suggest that LISA and INSURE transiently affect brain autoregulation during surfactant administration.
The authors reviewed all articles included in this systematic review to identify those reported subgroup analyses of prematurity. Only Kanmaz et al.12 and Han et al.23 present analyses considering subgroups of prematurity; therefore, performing a meta-analysis of subgroups is not feasible.
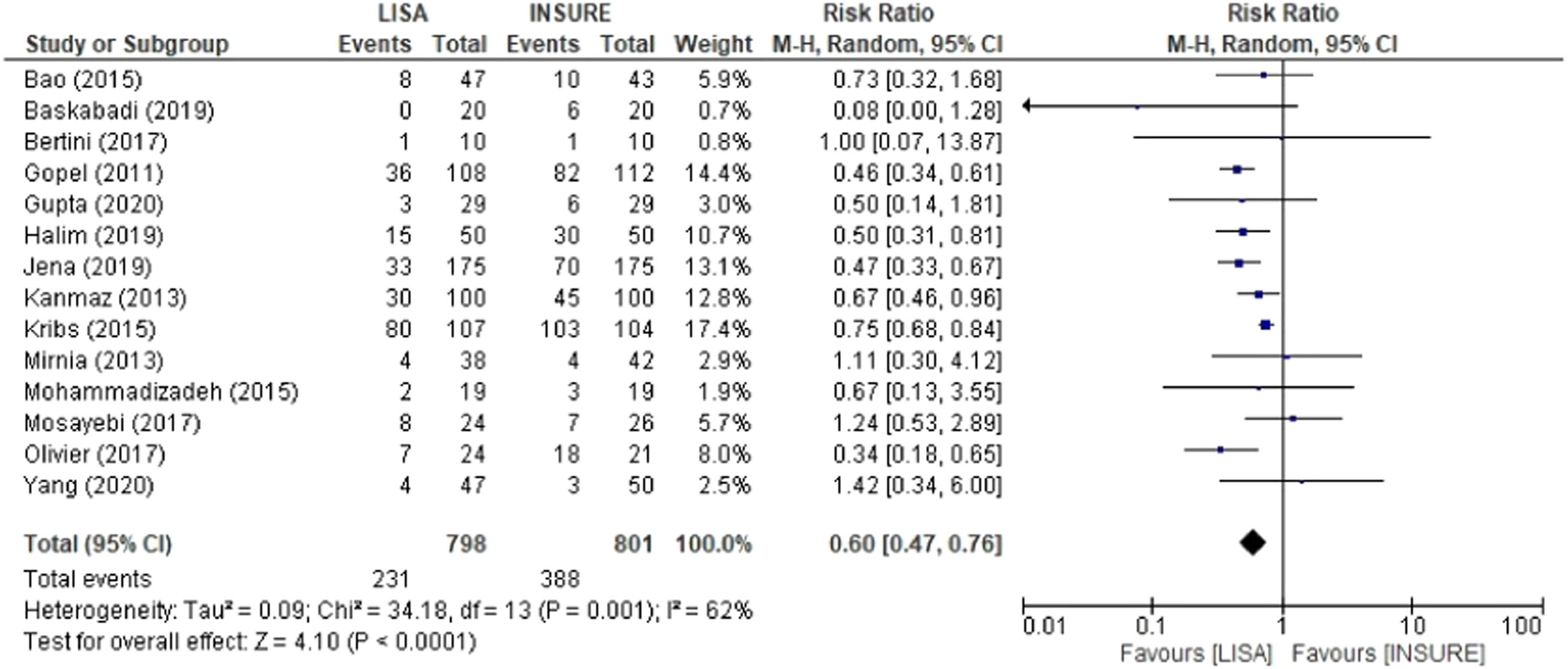
Meta-analysis resultsThe first analysis for comparison included the 14 studies that reported the frequency of patients who required MV in the first 72 h of life. A total of 798 and 801 patients in the LISA and INSURE groups, respectively. The INSURE group had more risk of MV in the first 72 h of life, with an overall risk ratio of 0.60 (95% CI 0.47 – 0.76), compared to the LISA group. Moderate heterogeneity was observed between studies (I2 = 62%) (Figure 4).
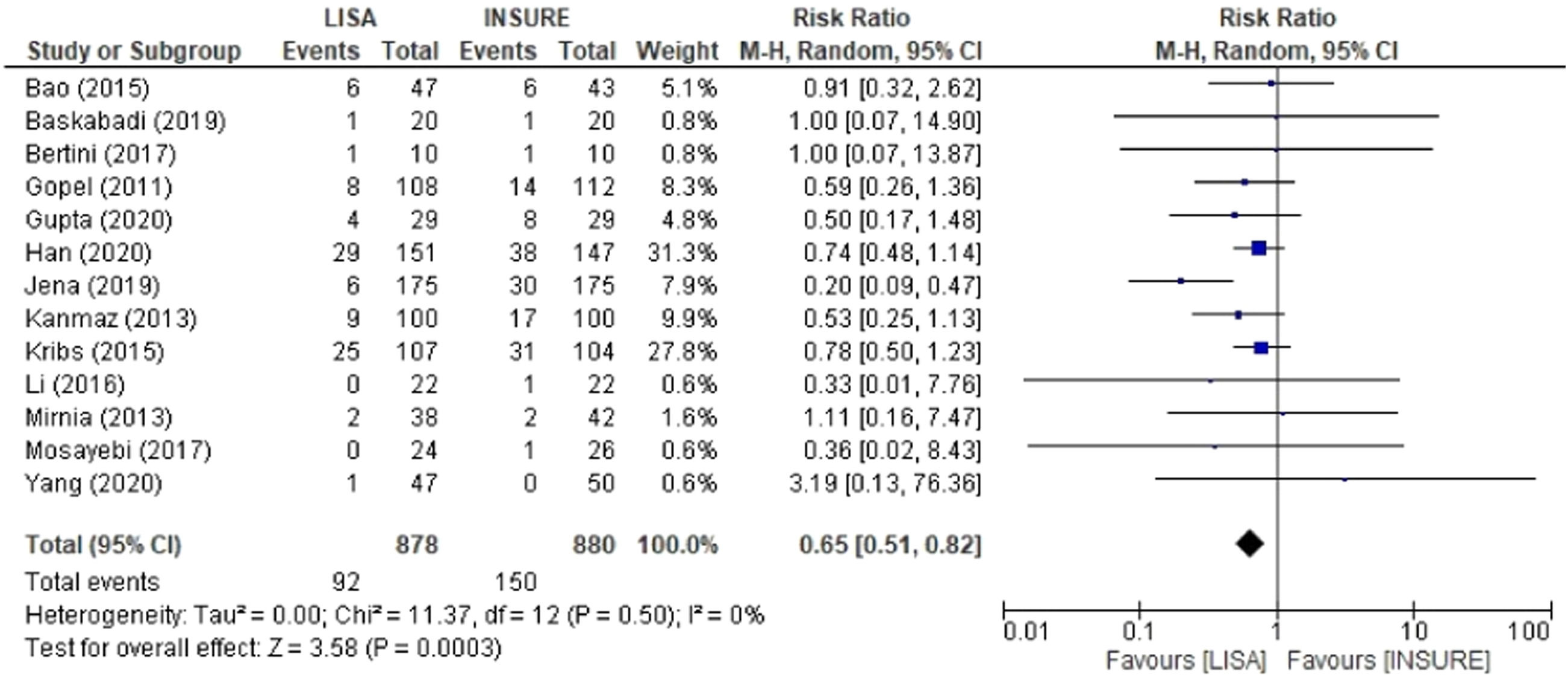
When comparing groups for BPD, 13 studies were included in the analyzes reporting the frequency of this outcome with a total of 878 patients in the LISA group and 880 in the INSURE group. The INSURE group had an increased risk for BPD than the LISA group; 0.65 (95% CI 0.51 – 0.82) (Figure 5).
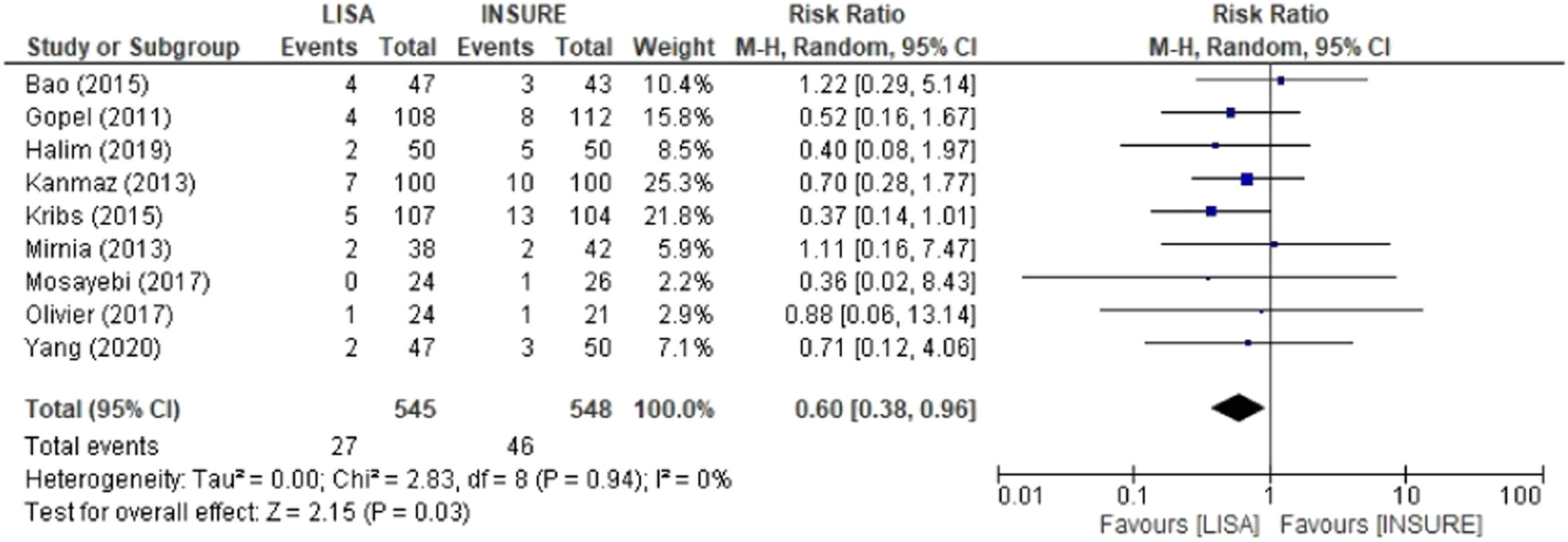
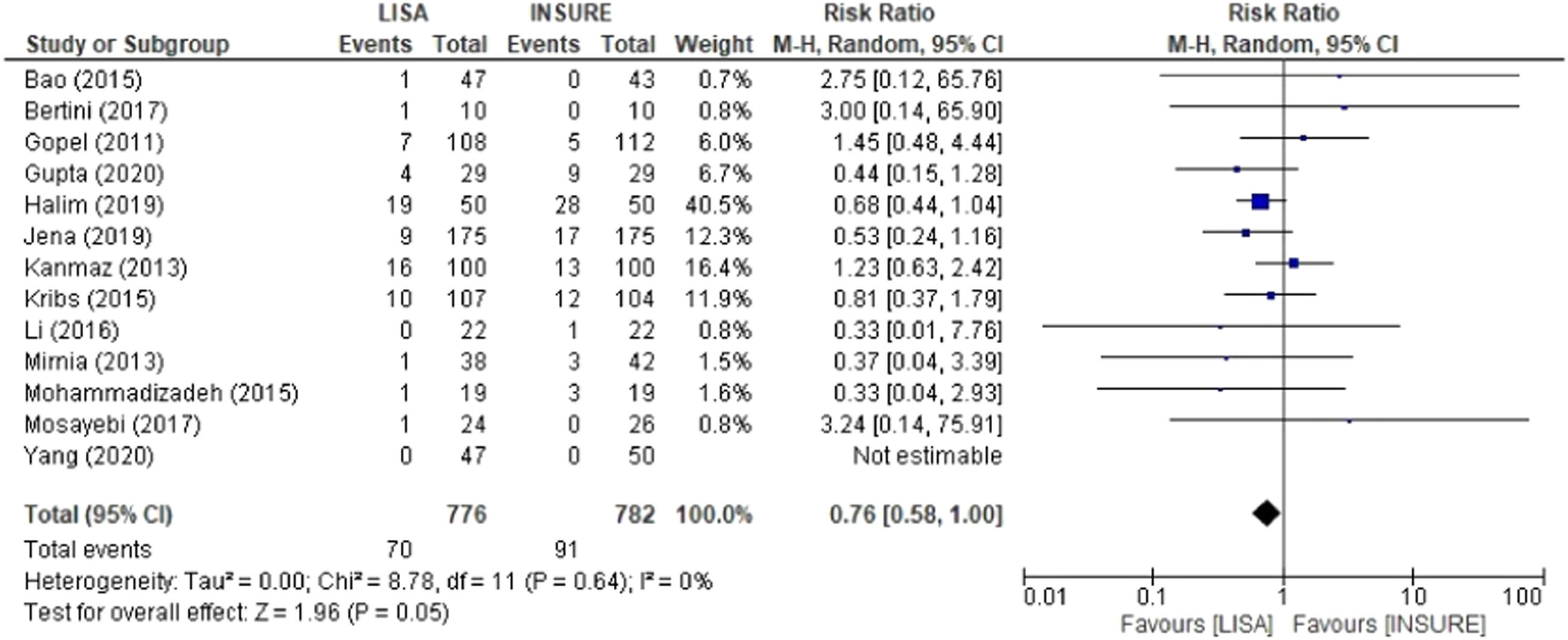
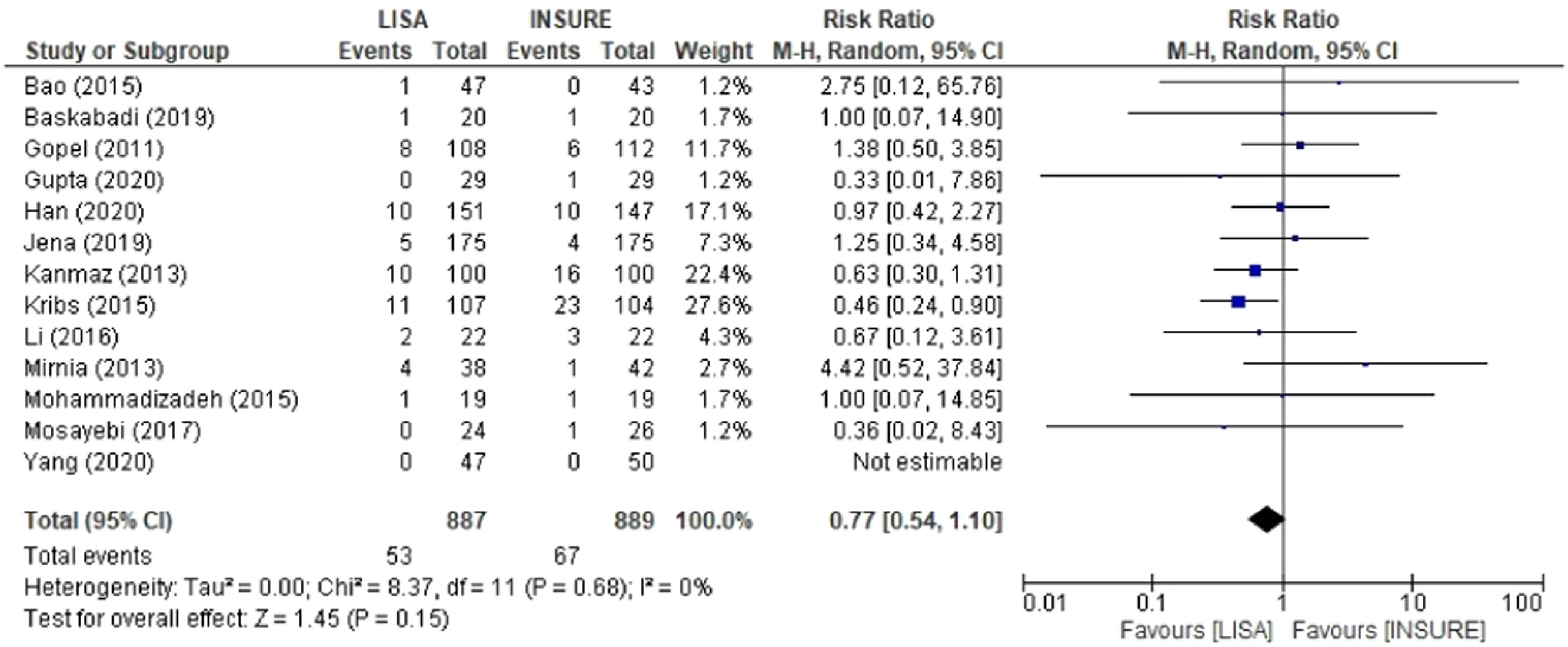
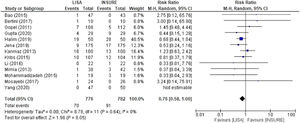
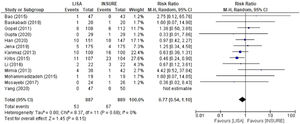
Pneumothorax, mortality and PIVHComparison analyses were also performed for pneumothorax, mortality and PIVH, showing significant risks for the INSURE group. Nine studies reported frequencies of pneumothorax, totaling 545 patients in the LISA group and 548 in the INSURE group, with a hazard ratio of 0.60 (95% CI 0.38–0.96) (Figure 6). Thirteen studies assessed mortality, 776 patients in the LISA group and 782 patients in the INSURE group, and the hazard ratio was 0.76 (95% CI 0.58–1.00) (Figure 7). Thirteen studies reported the frequency of PIVH, a total of 887 patients in the LISA group and 889 in the INSURE group, and the hazard ratio in the meta-analysis was 0.77 (95%CI 0.54–1.10) (Figure 8).
In this study, the primary endpoint was to compare the LISA versus INSURE technique for pulmonary surfactant administration in preterm NB with gestational age (GA) < 36 weeks with RDS with respect to the incidence of pneumothorax, BPD, PIVH need for MV, regional cerebral oxygen saturation, and mortality. The present meta-analyses showed statistically significant differences in favor to LISA administration, with a significantly decreased risk of needing Mechanical ventilation, BPD, pneumothorax, mortality, and PIVH.
Recent studies suggest that the best approach for preterm infants who need surfactant administration during non-invasive respiratory support is the LISA method, as it is less invasive at a time when the neonate is breathing spontaneously.8,12 Cochrane review including 10 randomized clinical trials comparing different methods of surfactant administration found significant advantages in the surfactant administration via a thin catheter, with a decrease in the following: risk of death or BPD, need for assisted breathing in the first 72 h of life, severe PIVH, death during first hospitalization, and BPD among survivors.16 In the systematic review, all 16 randomized clinical trials included showed favorable results to the use of the LISA technique in comparison to INSURE.
Need and time of mechanical ventilationEight studies had as primary objectives to analyze the need for mechanical ventilation in the first 72 h of life after administration of LISA versus INSURE, namely Gupta et al.,18 Jena et al.,19 Boskabaldi et al.,20 Olivier et al.,21 Göpel et al.,25 Mohammadizadeh et al.,27 Kanmaz et al.,12 and Halim et al.,8 Of these studies, 6 suggested that the LISA technique reduced the need for MV in the first 72 h of life. In the other studies that addressed the need for MV, either as a primary or secondary outcome, there was no significant difference between the groups, but administered by the LISA method was not inferior to INSURE.
Kanmaz et al.12 included 200 neonates < 32 weeks of GA in their study, randomized to LISA and INSURE. The LISA group had a significantly lower need for MV in the first 72 h of life, mean duration of nCPAP and MV. Furthermore, CPAP failure in the first 72 h of life was significantly lower in the LISA group when compared to the INSURE group.
Jena et al.19 studied 350 neonates with GA ≤ 34 weeks with RDS randomized between LISA and INSURE. The need for MV in the first 72 h was significantly lower in the LISA than in the INSURE group. In the study by Olivier et al.,21 the need for MV was also significantly lower in the LISA group. However, a limitation of the latter study was that there was no specific criterium for surfactant administration in the control group, and patients in the control group had more severe RDS, as they required oxygen and surfactant administration earlier than those in the LISA group.
In Boskabaldi et al. study,20 the use of the LISA significantly reduced the need for mechanical ventilation in infants but did not change the duration of nCPAP and the duration of hospitalization. Halim et al.8 showed that the need for invasive mechanical ventilation was also significantly lower in the LISA group compared to the INSURE group, but the duration of respiratory support (CPAP) was significantly longer in the LISA group.
In Göpel et al.25 study, including 220 neonates of 26 to 28 weeks GA, the primary outcome analyzed was the need for any type of mechanical ventilation after administration of surfactant by LISA or INSURE methods. The number of neonates who received MV during hospitalization was lower in the LISA group. The total number of ventilation days was 599 in the INSURE group versus 242 days in the LISA group. These results suggested that less invasive surfactant application in premature infants reduces the need for mechanical ventilation.
Gupta et al.18 found no statistically significant difference in the need for MV in the first 72 h of life between LISA and INSURE groups. However, in this study, NIPPV was used as the primary mode of respiratory support whereas, as in most previous studies with LISA, NCPAP was the primary mode of respiratory support. This may have reduced the need for IMV in both study groups, as there is already evidence in the literature supporting nasal intermittent positive pressure ventilation (NIPPV) as the primary mode of respiratory support to decrease the need for IMV.
Bao et al.24 and Mohammadizadeh et al.27 found no significant differences in MV rates in the first 72 h, mortality, nor in the incidence of bronchopulmonary dysplasia, intraventricular hemorrhage, retinopathy of prematurity, and necrotizing enterocolitis, or the duration of respiratory support. In Mohammadizadeh's study,25 the number of infants who experienced adverse events during surfactant administration was significantly lower in LISA than in INSURE group.
Bronchopulmonary dysplasiaRandomized clinical trials by Kribs et al.22 and Han et al.23 had the analysis of BPD as their primary objectives. Kribs et al.22 included 211 neonates < 27 weeks of gestation randomized to LISA and INSURE groups. The primary aim was to analyze BPD-free survival at 36 weeks GA. In LISA group, 67.3% survived without BPD compared to 58.7% in the INSURE group, showing no significant difference between the groups. In another study, 298 neonates with RDS were randomized to LISA and INSURE groups with a trend toward a reduction in the incidence of BPD.21
Previous studies have looked at BPD analysis as secondary outcome, only Kanmaz et al.12 found a significantly lower rate of BPD among children treated with LISA. The incidence of moderate to severe BPD among patients who survived the was significantly higher in the INSURE group, suggesting that LISA shows a tendency to be beneficial.
Pneumothorax, mortality and PIVHNine randomized clinical trials looked at the incidence of pneumothorax, but only Kribs et al.’s study22 showed a significantly lower occurrence of pneumothorax in the LISA group. Although Halim et al.8 found a more than double occurrence of pneumothorax, in the INSURE group, it did not reach a statistical significance.
Thirteen studies evaluated mortality and PIVH rates, none showed a significant difference in mortality. Kribs et al.22 evaluated PIVH grade 3 or 4 and showed that the LISA group also had significantly less severe PIVH. It is noted that LISA is associated with benefits in significant secondary outcomes, which are associated with lifelong disabilities.
Regional saturation of cerebral oxygenTwo studies evaluated changes in regional cerebral oxygen saturation (rSO2) induced by the LISA and INSURE procedures. Bertini et al.26 evaluated NB with GA < 33 weeks and showed that LISA and INSURE transiently decreased rSO2. The decrease was greater in the LISA group. The decrease in rSO2 is up to 55% in the LISA group but the duration of this episode is short (< 1 min). The study also calculated the fractional oxygen extraction rate from brain tissue (cFTOE), and it was higher in the LISA group, suggesting a compensatory mechanism to maintain adequate brain oxygenation during the technique.
Li et al.29 evaluated NB with GA < 32 weeks to detect rSO2 and mean arterial pressure (MAP) simultaneously. The correlation of the ScO2 and MAP coefficient (rScO2−MAP) was evaluated in both groups. It is suggested a transient impairment of brain autoregulation during and after LISA and INSURE procedures, but it was concluded that the duration of impairment in the LISA technique was shorter than in the INSURE.
The authors couldn't perform a meta-analysis for the outcome of regional saturation of cerebral oxygen, despite the systematic review with a small number of articles has shown results in favor of LISA administration. A variety of types of catheters and instruments are used for LISA surfactant administration, the authors did not explore this aspect in the meta-analysis and perhaps there are some relationships with more difficult administration secondary to expertise. Future studies looking specifically for this outcome need to be conducted.
LISA and INSUREThe study by Bertini et al.26 hypothesizes that the most striking effect of the LISA versus INSURE technique is due to patients breathing spontaneously during LISA, while in INSURE they receive positive pressure through invasive support. This appears to facilitate the recruitment of pulmonary alveoli, increasing residual capacity function, improving surfactant distribution, and stabilizing breath control; reasons that lead to better gas exchange and tissue oxygenation. Li et al.29 seem to agree, indicating that the delivery procedure may be the reason for potential damage to brain regulation, particularly in the INSURE group. On the other hand, a recent randomized control trial comparing LISA and INSURE among 26-to-34-week gestation age infants didn't find any difference in the total duration of respiratory support, despite of lesser need for invasive mechanical ventilatory support in the LISA group.30
The systematic review and meta-analysis allowed us to conclude that surfactant administration via the LISA technique decreased the need for MV in the first 72 h of life, BPD, PIVH, pneumothorax, and mortality rates compared to INSURE, proving to be a safe and easily reproducible technique. These findings contribute to a decrease in the economic and social impact of the use of LISA technique in RDS, such as a reduction of hospital length of stay and MV complications, and are closely related to a better survival rate and reduction of associated morbidities.
This work was supported by a grant from Cnpq-Brazil. The funding sources have not been involved in the study.
Institution: Federal University of Rio Grande do Sul and Hospital de Clinicas de Porto Alegre.