The goal of this article is to provide an account of language development in the brain using the new information about brain function gleaned from cognitive neuroscience. This account goes beyond describing the association between language and specific brain areas to advocate the possibility of predicting language outcomes using brain-imaging data. The goal is to address the current evidence about language development in the brain and prediction of language outcomes.
SourcesRecent studies will be discussed in the light of the evidence generated for predicting language outcomes and using new methods of analysis of brain data.
Summary of the dataThe present account of brain behavior will address: (1) the development of a hardwired brain circuit for spoken language; (2) the neural adaptation that follows reading instruction and fosters the “grafting” of visual processing areas of the brain onto the hardwired circuit of spoken language; and (3) the prediction of language development and the possibility of translational neuroscience.
ConclusionsBrain imaging has allowed for the identification of neural indices (neuromarkers) that reflect typical and atypical language development; the possibility of predicting risk for language disorders has emerged. A mandate to develop a bridge between neuroscience and health and cognition-related outcomes may pave the way for translational neuroscience.
O objetivo deste trabalho é apresentar um relato sobre o desenvolvimento da linguagem no cérebro utilizando as novas informações sobre função cerebral obtidas na neurociência cognitiva. o relato vai além da descrição da associação entre linguagem e áreas específicas do cérebro e defende a possibilidade de predizer os resultados de linguagem por meio de dados de imagens cerebrais. O objetivo é tratar das evidências atuais sobre desenvolvimento da linguagem no cérebro e abordar a possibilidade de predição de resultados de linguagem.
FontesEstudos recentes serão discutidos em face das evidências geradas pela predição de resultados de linguagem e pelo uso de novos métodos de análise de dados cerebrais.
Resumo dos dadosEste relato de comportamento cerebral abordará: (1) o desenvolvimento de um circuito cerebral de linguagem falada; (2) a adaptação neural que segue a instrução da leitura e incentiva a “inserção” de áreas de processamento visual do cérebro no circuito de linguagem falada; e (3) a predição do desenvolvimento da linguagem e a possibilidade de uma neurociência translacional.
ConclusõesAs imagens cerebrais permitiram a identificação de índices neurais (neuromarcadores) que refletem o desenvolvimento da linguagem típico e atípico; surge a possibilidade de prever o risco de disfunções de linguagem. A responsabilidade de desenvolver uma ligação entre neurociência e resultados relacionados a saúde e cognição pode abrir o caminho para a neurociência translacional.
One of the key challenges for the neurosciences is to become faster and more effective at producing evidence that transforms health and education-related practice. Neuroscientific studies of neuronal markers hold promise for more successful health and education policies. The obstacles to making predictions from brain data involve producing more generalizable, robust results; establishing communication channels with education and healthcare decision-makers and professionals; and addressing ethical issues that arise from making such predictions. The present article focuses on a specific type of evidence: functional brain data obtained from noninvasive brain imaging to predict developmental and clinical outcomes. Recent studies will be discussed in the light of the evidence generated for predicting language outcomes and of using new methods of analysis of brain data.
With the advent of magnetic resonance imaging (MRI) and its varied functional and structural procedures, studies of the brain mechanisms that underpin cognition and psychiatric disorders have flourished.1 Studies using functional MRI (fMRI) have unveiled specific neural circuitry and mechanisms for language acquisition, language disorders, and language-related processes.2–4 Language development has been shown to be amenable to investigation by MRI procedures.
Research on the brain's structural and functional indices that predict language outcome5–11 may be an effective means to translate new evidence into applications.4–12 However, the influence of the research outside the scientific community appears to fall short of its promise.1 The production of generalizable models of clinical and educational outcomes based on these indices (neuromarkers) is an exciting possibility for changing the way health and education-related decisions benefit from brain imaging. A recent review13 advocated the application of brain measures in the prediction of outcomes in several fields, including education and learning; health-related behaviors and responses to treatments; and relapse for alcohol, smoking, and drug addiction. Prediction was defined as producing generalizable models that effectively identify outcomes in out-of-sample individuals; in the interest of the present article, this would mean establishing neuromarkers that can be applied to more diverse populations outside the smaller-sample brain imaging studies.13 Prediction, in this broad sense, is the ultimate test of a neuromarker after passing the test of reliable within-subject and in-sample predictions.
Noninvasive brain imaging measures: fMRIThe present article focuses on evidence from fMRI investigations of language development. This technique identifies changes in metabolism and oxygen levels in the brain.14,15 Though the increase in metabolism is not a direct measure of neuronal activity, the blood-oxygen level dependent (BOLD) signal acquired during fMRI procedures reflects neural responses: there is a correlation between electrical discharges from neurons and the BOLD signal.16,17 Other MRI procedures, such as diffusion-tensor imaging, used for investigations of white matter pathways, are increasingly being used in multimodal studies of language processes, disorders, and development.13,18–21 Other methods, such as event-related potentials, have also effectively predicted language outcomes: neonatal event-related potentials (as early as 36h after birth) were predictive of language outcomes at 8 years of age.22 Multimodal investigations that combine procedures eliminate the shortcomings of the different procedures.23 A more in-depth discussion of multimodal investigation and methods is beyond the scope of the present article.
Spoken language development: a resilient process, its milestones, and hardwired brain circuitryEarly language development and emerging communication abilities are fundamental for the development of cognitive and social skills.24 Language development is one of the key factors associated with the development of mental capital; this means ensuring that each person is given the chance to achieve their full cognitive potential, and is allowed to prosper and flourish within modern society.25
The United States Committee on Integrating the Science of Early Childhood Development produced an evidence-based report on early brain development, language, socialization, and self-regulation. In the words of the report: spoken language is resilient, literacy is fragile.24 These two key aspects of oral and written language development, resilience and fragility, have been investigated in the light of brain imaging. There are language milestones (behavioral markers) associated with neural signatures of language. The milestones provide evidence that helps establish predictive brain–behavior relationships for language.
Spoken language develops without instruction. The process of development occurs in almost all children despite economic and social hardships, as well as lack of instruction (but such hardships influence qualitative and quantitative aspects of language and cognitive development).24,26–28 The brain circuits that develop for spoken language are hardwired in the brain. The components of the language circuitry are associated with levels of auditory comprehension processes; the key centers and processes include: (1) the primary auditory cortex, which processes raw auditory information; (2) the posterior temporal and inferior parietal cortices, which process the systematic organization of word sounds; (3) the middle temporal cortex, which is associated with accessing word meaning; (4) and the inferior frontal cortex, which processes the structure of language. Over the past 25 years, noninvasive brain imaging has provided robust and replicable evidence about the brain circuits that develop for spoken language. It is worth mentioning that the brain areas involved depend on the granularity of the language process being investigated.2,3,9,29,30
At times there are delays in oral language development. The age at which a child begins to speak is a behavioral marker of such delay. Children usually produce their first words between 10 and 15 months of age. A delay in speaking indicates atypical language development, and is one of the indicators of risk for reading disorder.31–33 Language milestones such as age at speaking can be evaluated in terms of the typical neural circuits that develop in the child.
A recent study of early and late talkers identified cortical and subcortical markers of children who spoke their first words at 1.2 years (early) versus those who spoke at 2.5 years (late).34 In addition to better performance in language tests at 8 years of age, early talkers (also at 8 years of age) showed more activation in subcortical structures associated with the learning of rule-based systems (putamen and thalamus); these subcortical structures are at the basis of learning new linguistic skills.35,36 Early milestones as simple as uttering two to three word sentences are strong indicators of language outcome, and the effects of age at speaking are associated with markers of brain development. Identifying early behaviors and understanding the consequences brain development provide a path for cognitive neuroscience to inform early intervention. Brain–behavior relationships help understand the typical interaction between psychological and biological processes, and the missing biological pieces in atypical development.
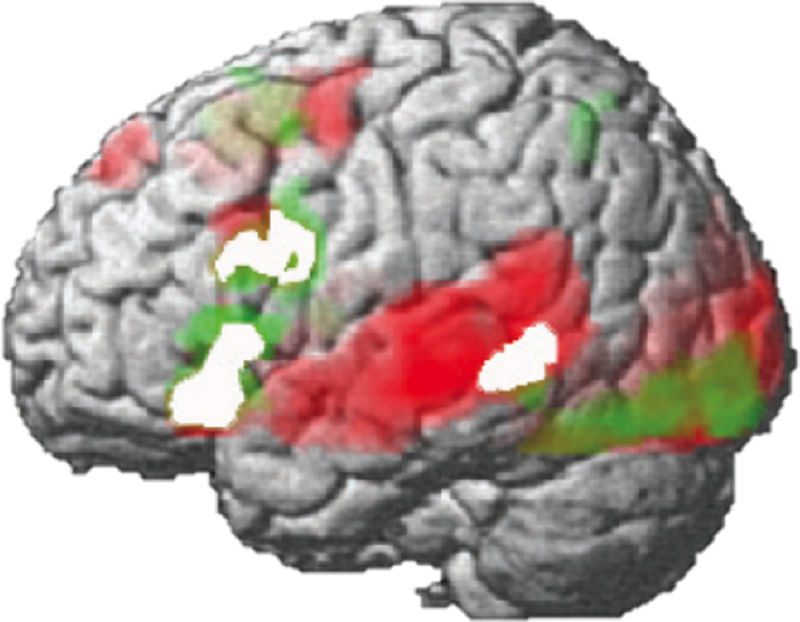
The development of spoken language is resilient and also remarkably similar across languages. Recently, a brain imaging study of four different languages (Spanish, English, Hebrew, and Chinese) showed the universality of the language network in the brain.9 In all languages, the traditional left frontal–temporal network of the brain was activated for listening comprehension, and it was remarkably overlapping. Such findings provide the basis for future models of prediction of language outcome across cultures. There is invariance in structures that develop for speech comprehension despite the different characteristics of sound and structure of spoken languages. The study also showed a common brain signature for reading in the four languages. Despite the significant differences in writing systems (alphabetic and ideographic, for example), the brain signature for reading is largely similar in the four languages; it is also largely constrained by the speech processing system.9 Earlier studies showed how the centers that adapt for processing print are constrained by the centers hardwired for spoken language (of course, with the exception of visual centers). Once children learn to read, the centers for processing print are grafted onto a left-lateralized network of language areas hardwired for spoken language. This has been shown for English and for Portuguese, for example.29,30Fig. 1 demonstrates the overlap in centers involved for speech and print processing for Portuguese (adapted from Buchweitz et al.).29 Similar centers were identified in the study of four different languages.
Based on the notion that there is a hardwired language circuitry, and that instruction-dependent reading development is constrained by this circuitry, it is possible to reframe this information in the light of prediction of language outcome. By knowing what underlying biological centers have to develop and the adaptations that follow from learning, it is possible to establish a neural account of spoken language and reading development within the framework of prediction. Some of the neural adaptations to reading are discussed below.
Reading development: a fragile process and its associated adaptations in the brainLearning to read is instruction-dependent, and it systematically changes the human visual circuitry and the way language sounds are processed. Studies show lasting effects on the processing of auditory word-form. Illiterate adults are incapable of deleting or adding sounds from pseudowords (words that do not exist, but are made up of regular consonant and vowel structures).37 The systematic changes in brain function that take place are likely candidates for neuromarkers of typical and poor reading development. One such change has been identified in illiterate adults: they do not process made-up words using the same brain centers literate adults do38 and they have difficulty repeating back made-up words and making word associations based on sound.39 These difficulties follow from not having learned the visual boundaries of words and the corresponding boundaries in sound.
One of the consequences of learning to read is that the human ability to recognize mirror images slows down (contrary to natural human mirror-imaging abilities, learning to read means “unlearning” to mirror “b” and “d,” for example).40 When one learns to read, a specific center in the occipitotemporal region reprograms itself; it adapts from its original function of processing faces and objects to specializing in the identification of the visual form of words.41–43 The activation of the occipitotemporal region in association with the presentation of letters and words is a marker of reading development. The more a child learns to read, the more the occipitotemporal region activates in response to visual processing of words. The specific region that adapts to the recognition of letters and words has been coined the visual word form area (VWFA).43–45 In illiterate adults, the VWFA does not light up when words are presented visually. It does not activate for visual linguistic stimuli differently than it activates for nonlinguistic visual stimuli. Also, the activation of the region is modulated by how late one learns to read. The VWFA activates significantly more in people who have learned to read as children than in those who learned to read only as adults. The different levels of activation of the VWFA correlate with reading fluency.44 The activation of the VWFA in early years is predictive of reading outcomes and is a marker of fluent reading.6,42,45
Pathways from the human visual circuitry to other centers in the brain develop with reading. Two pathways, or routes, develop in parallel: the dorsal, or phonological route and the ventral, or lexical route. The left temporoparietal region is part of the dorsal route of the brain that develops with reading. The dorsal pathway includes left temporoparietal and left inferior frontal regions. The development of the dorsal pathway is associated with learning print and sound associations.43 Brain imaging has also revealed that activation of the dorsal pathway develops with age46; and that the temporoparietal component of the pathway is hypoactive in dyslexic children.33,47 It is a brain circuitry that develops with learning to read and fails to develop when there are obstacles to reading fluently. The ventral pathway, in turn, develops with reader fluency and with the learning of irregularities of language (such as one vowel having more than one possible sound).
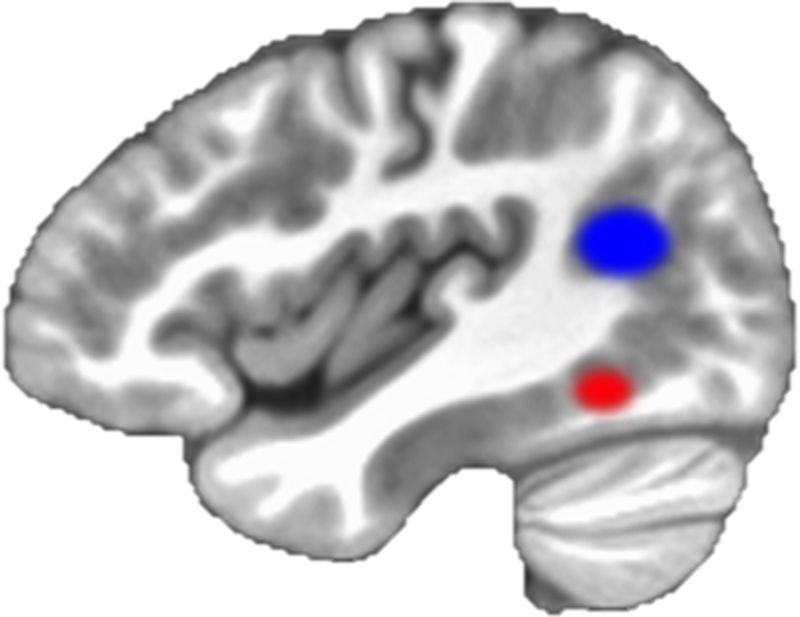
Brain imaging has produced evidence about markers of reading development, and the importance of early instruction for reading. The question is no longer whether brain imaging has the potential to inform practices and generate predictions. The scientific question, it seems, lies in producing more generalizable evidence and replicating studies with larger populations; the political and ethical issues should follow. One promise for prediction lies in the interaction between brain imaging and machine learning algorithms, which is briefly addressed below. Table 1 shows studies that identified neural markers in association with reading development. Fig. 2 shows a rendering of the two centers discussed above, the occipitotemporal and the temporoparietal regions.
Brain regions associated with reading development.
| Region (left hemisphere) | Brain–behavior relationship | Reference |
|---|---|---|
| Occipitotemporala | Recognition of letters; adaptation of the visual circuit to reading. | 6,9,40,43,45 |
| Temporoparietal | Development of the phonological route (letter-sound associations) | 33,44,47,48 |
In sum, cognitive neuroscience and brain imaging have produced evidence of brain and behavior relationships that inform the basis of typical and atypical language development. The challenge is how to make the evidence more reliable to the point of informing educational and health policies. The application of machine learning algorithms to brain imaging data in combination with large-scale brain imaging studies is an exciting possibility to produce more evidence-based educational policies.
A mandate for the neurosciences: prediction of health and education-related outcomesThe application of machine learning algorithms to identify patterns in human activity is changing the way human behavior can be investigated and predicted. These algorithms identify patterns in instant messaging, traffic, and health-related Internet queries (i.e., for the word “influenza”) that may raise red flags for possible epidemics.48 The recent advances in the use of machine learning algorithms have made it possible to reliably identify cognitive states and psychiatric disorders using brain imaging data.49–53
Machine learning algorithms can unveil patterns of brain activity that can be tested on new sets of brain imaging data for their generalizability; patterns associated with human emotions or with clinical populations have been tested on entirely new brain imaging data sets.50,54 Machine learning has been applied to identify autism from the brain data alone,50 to identify cognitive states,51,54,49 to identify brain patterns for emotions,52 and to identify brain patterns that identify when new concepts are learned.55 The issue that emerges is how to support more use of machine learning algorithms and knowledge in the neurosciences. Identification of early markers of health and cognition-related outcomes holds promise for a positive effect in quality of life and in return-on-investment of public funding for science and healthcare. These algorithms can be used to test whether the neural markers of language and reading development extrapolate to larger populations, and predict language outcomes. Brain imaging evidence is accumulating and artificial intelligence algorithms continue to improve. Education and healthcare should benefit from these advances.
Conflicts of interestThe author declares no conflicts of interest.
Please cite this article as: Buchweitz A. Language and reading development in the brain today: neuromarkers and the case for prediction. J Pediatr (Rio J). 2016;92(3 Suppl 1):S8–13.