An interesting review of acute respiratory distress (ARDS) definitions has been recently published in the Jornal de Pediatria, focusing on actual needs in terms of research and clinical care of pediatric ARDS.1
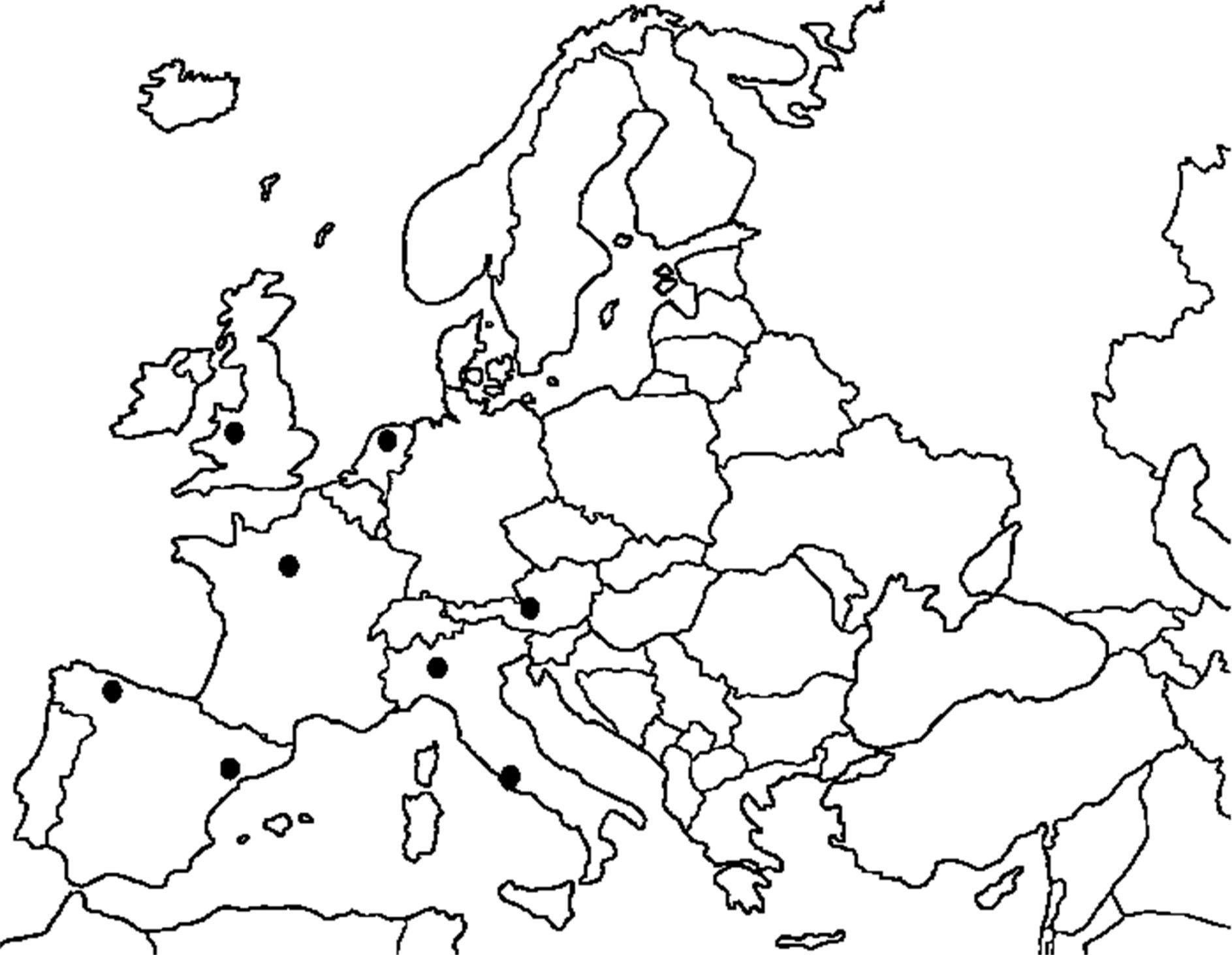
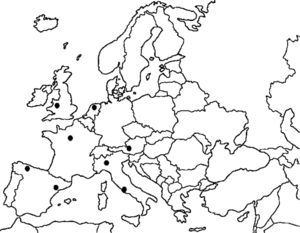
Unfortunately, timing prevented the consideration of an important step forward in this field. The European Society for Pediatric and Neonatal Intensive Care (ESPNIC), together with some members of the original ARDS Task Force, have set up an international collaborative project to validate the new Berlin definition for infants and toddlers.2 This project is the first initiative linking different pediatric intensive care units (PICU) in order to reach enough statistical power to address a specific research need. Figure 1 shows the ESPNIC net for this project. Indeed, as Fioretto et al. summarized,1 no specific pediatric validation had ever been conducted, even though some children were included in the original ARDS definition proposed by Ashbaugh et al. in 1967.3
Fioretto et al. described several possible limitations of the Berlin definition: however, some of the points raised by these authors should not be considered as a limitation, since the new Berlin definition is not supposed to be a predictive tool, but rather a framework to define a syndrome for epidemiology, clinical care, and research.
In addition, concerns were expressed regarding the application of the new Berlin criteria to the pediatric population, as there were no children in their original development population.4,5 This is the reason why the Respiratory Failure Section of ESPNIC started the above-mentioned project to evaluate the reliability of the new Berlin definition in a homogeneous and adequately large pediatric population. The project focused on the early pediatric age (range: 30 days to 18 months), since especially at this age, the syndrome is distinctly different from ARDS in adults.2,6 In fact, infants and toddlers present peculiarities regarding lung development, respiratory system mechanics, and co-morbidities, which are responsible for the peculiar epidemiology and prognosis of ARDS in these patients.6
The main results demonstrated that the new Berlin definition has the same reliability both for the pediatric and adult patients in terms of mortality and need for extracorporeal life support.2 To aid the clinical application of the definition, a set of chest X-rays with an interpretation guide and a list of ARDS risk factors, as estimated by researchers participating in this collaborative effort, were established. Both are practical tools that have proven to be helpful in clinical practice and research.2,4,7,8
However, the ESPNIC collaborative work validating the new Berlin criteria for pediatric ARDS patients has some limitations that have already been pointed out.2,9 Besides the retrospective character of this pediatric validation study, only one of the several secondary variables that have been tested in adults (i.e. standardized minute ventilation [(Vecorr) = minute ventilation x worst PaCO2/40]) could be tested.2 However, other variables (such as lung volume estimation, surfactant amount and activity, biomarkers) could have been tested, and the new Berlin definition could have been more tailored to pediatric patients with an adequate prospective study population. In fact, the Murray lung injury score reviewed by Fioretto el al. has already been modified for pediatric ARDS,10 but it was never subjected to further validation studies. Finally, other pediatric ages had not been considered: while ARDS in adolescents could be considered as very similar to the syndrome in adults, neonates deserve a specific project to define the syndrome and distinguish it from other forms of neonatal lung injury.
Thus, the ESPNIC collaborative work was an initial and substantial step forward, and disseminated a validated ARDS definition for a particular pediatric population, answering a specific need of pediatric intensivists. Clearly, many other questions remain open, and they can be addressed only with similar international collaborative projects. Such studies are needed, given the complex reality of a syndrome with multiple causes and co-morbidities such as ARDS. Furthermore, it is necessary to study larger pediatric populations in order to reach an adequate statistical power, since ARDS is significantly less frequent in children and neonates than in adult patients.
We are looking forward to proceed with other similar projects in order to answer some of the open questions described above. To do this, and to achieve more representative results, a worldwide collaborative work between the Respiratory Failure Section of ESPNIC and other non-European researchers and clinical centers will be needed.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: De Luca D, Kneyber M, Rimensberger PC. International collaborative research for pediatric and neonatal lung injury: the example of an ESPNIC initiative to validate definitions and formulate future research questions. J Pediatr (Rio J). 2014;90:209–11.