to describe the frequency and the factors associated with cholelithiasis in obese adolescents.
Methodsthis was a cross-sectional descriptive study performed with the adolescents between 10 and 19 years of age treated at the Child and Adolescent Obesity Outpatient Clinic from May to December of 2011. Obesity was defined as body mass index (BMI)>P97, and overweight as BMI>P85, for age and gender, according to the 2007 World Health Organization reference. A questionnaire concerning the presence of signs and symptoms, such as abdominal pain, nausea, vomiting, and intolerance to fat, was administered. Patients were asked about how many kilograms they had lost and in how much time. Laboratory parameters were: triglycerides, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels. Cholelithiasis and hepatic steatosis were diagnosed by ultrasonography.
Resultscholelithiasis was diagnosed in 6.1% (4/66) of the obese adolescents, most of whom were female (3/4); hepatic steatosis was identified in 21.2% (14/66). Intolerance to dietary fat was reported by all patients with cholelithiasis (4/4) and by 17.7% (11/62) of the group without cholelithiasis (p=0.001). The average weight loss was 6.0±2.9kg in the patients with cholelithiasis and 3.2±4.8kg in the group without cholelithiasis (p=0.04). However, there was no difference between the two groups regarding the time of weight loss (p=0.11).
Conclusionscholelithiasis and hepatic steatosis are frequent among obese adolescents and should be investigated systematically in the presence or absence of symptoms.
descrever a frequência e os fatores associados à litíase biliar em adolescentes obesos.
Métodosestudo descritivo tipo corte transversal com adolescentes entre 10 e 19 anos atendidos em ambulatório de obesidade infanto-juvenil, no período de maio a dezembro de 2011. A obesidade foi definida como índice de massa corporal>P97 e o sobrepeso>P85, para idade e sexo, segundo o referencial OMS 2007. Foi aplicado um questionário com dados relacionados à presença de sinais e sintomas, como: dor abdominal, náusea, vômito e intolerância à gordura. Os pacientes foram questionados sobre quantos quilos perderam e em quanto tempo. As variáveis laboratoriais foram: triglicerídeos, colesterol total, lipoproteína de alta densidade (HDL) e lipoproteína de baixa densidade (LDL), aspartato aminotransferase (AST) e alanina aminotransferase (ALT). A litíase biliar e a esteatose hepática foram diagnosticadas por ultrassonografia.
Resultadosa litíase biliar foi diagnosticada em 6,1% (4/66) dos adolescentes obesos, a maioria do sexo feminino (3/4); a esteatose hepática foi identificada em 21,2% (14/66). Intolerância à gordura da dieta foi referida por todos os portadores de litíase biliar (4/4) e por 17,7% (11/62) do grupo sem litíase biliar (0,001). A média de perda de peso foi de 6,0±2,9kg nos pacientes com litíase biliar e 3,2±4,8kg no grupo sem litíase biliar (p=0,04). Porém, em relação ao tempo de perda não houve diferença entre os dois grupos (p=0,11).
Conclusõesa litíase biliar e a esteatose hepática são frequentes entre adolescentes obesos e devem ser investigadas sistematicamente na presença ou ausência de sintomas.
The prevalence of obesity in children and adolescents continues to rise in many countries. In the United States, obesity has more than doubled in children and tripled in adolescents over the last 30 years.1,2 In Brazil, a study of 4,914 children aged 4 to 6 years conducted in the public schools of Rio Grande do Sul and Santa Catarina found a prevalence of obesity of 14.4% and 7.5%, respectively.3 In Bahia, a study that included 1,056 children aged 0 to 5 years found a 15.2% prevalence of overweight/obesity.4
Several studies have described the deleterious effects of obesity, such as metabolic syndrome, cardiovascular disease, joint disease, polycystic ovary syndrome, fatty liver, gallstones, as well as social and psychological problems.5–7 Cholelithiasis is a recognized comorbidity in obese adults,8 although little studied in pediatric patients.9,10 Another epidemiological finding is the higher frequency of cholecystectomy in this age group that has been observed in recent years.11
Infants, children and adolescents with cholelithiasis appear to comprise three different populations regarding pathogenesis and predisposing factors.10 In the prepubertal cases, black pigment calculi predominate, which are associated with hemolysis, parenteral nutrition, cirrhosis, and heart valve replacement.12 Cholesterol calculi are the most frequent during and after adolescence,12 when changes in estrogen metabolism may result in increased bile litogenicity and formation of this type of gallstones.13
Particular attention must be given to obese, dyslipidemic adolescents, pregnant women, and oral contraceptive users, as these individuals are more likely to develop gallstones, in addition to the high percentage of idiopathic cases.10,14 Overweight adolescents are twice as likely to have gallstones when compared to adolescents with normal body mass index (BMI).14 For the obese, the chance increases by four-fold, and for those with severe obesity, the likelihood of having this condition is six-fold higher.14
Clinically silent cholelithiasis is increasingly diagnosed as an incidental finding during imaging examinations, particularly abdominal ultrasound. In adults, 50% to 70% of cases are asymptomatic,15 and progression to symptomatic disease is relatively low, ranging from 10% to 25%.15 Conversely, most children and adolescents present symptoms, from unspecific abdominal pain symptoms to biliary symptoms, such as biliary colic and jaundice.9,10,13
The upper transabdominal ultrasound examination is the diagnostic method of choice, with a sensitivity and specificity greater than 95%, and the capacity to show calculus size and location. The image is characterized by hyperrefringence and presence of acoustic shadow. The exam begin with the patient in the supine position and the patient can be moved to the left posterior oblique or upright position to demonstrate stone mobility.16 Schweizer et al.13 recommend observing patients with obesity or other risk factors for gallstone formation through repeated control ultrasounds for at least ten years.
This was the first Brazilian study on cholelithiasis in obese adolescents. The aim of this study was to describe the frequency and factors associated with cholelithiasis in a group of obese adolescents.
MethodsThis was a descriptive, cross-sectional study conducted at the Child and Adolescent Obesity Outpatient Clinic of Instituto de Saúde Elpídio de Almeida (ISEA), Campina Grande, Paraíba, Brazil. All adolescents aged 10 to 19 years treated between May and December of 2011 who were obese (BMI>97th percentile) or overweight (BMI>85th percentile) for age and gender, according to the 2007 World Health Organization (WHO) reference charts, were included.17 BMI was calculated by the Quetelet index (BMI=weight/height[2]). Clinical and laboratory characteristics of 66 patients of both genders were analyzed. Patients with anemia, genetic syndromes, pregnant patients, users of corticosteroid therapy or oral contraceptives, or those who habitually used or abused alcohol were excluded.
Clinical variables included: waist circumference, presence of signs and symptoms, such as abdominal pain, nausea, vomiting, and intolerance to fat. Patients were asked about how many kilograms they had lost and in how much time. Laboratory variables were: triglycerides, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels, as well as the ultrasonographic diagnosis of cholelithiasis and hepatic steatosis.
Anthropometric measurements were obtained at a single weight measurement on a calibrated platform scale, in addition to the height measurement with a stadiometer. The techniques used were standardized by the WHO.18 The adolescent was positioned in the orthostatic position in the center of the scale, barefoot with heels placed together, straight back, arms hanging at the sides, and wearing light clothes.
Abdominal circumference (AC) was measured using a non-extensible measuring tape with the patient in the standing position, using as reference the midpoint between the last rib and the iliac crest, at the time of expiration. AC was measured in centimeters in accordance with Taylor et al.19
Cholelithiasis was identified on ultrasound, using SA 8000 EX Medison equipment (Samsung Medison), with a multi-frequency convex transducer of 3-7MHz, by a single specialist trained in diagnostic imaging.
After the informed consent was signed by the adolescent and the parent/guardian, data collection was initiated through interviews and physical examination, followed by laboratory tests and ultrasound assessment. Patients with cholelithiasis were referred to the surgical outpatient clinic for evaluation and management.
Data analysis was performed using the Statistical Package for Social Sciences (SPSS) software 21.0. Initially, all variables were analyzed descriptively. Student's t-test was used to compare the means of two groups (presence or absence of cholelithiasis). When the assumption of data normality was rejected, the nonparametric Mann-Whitney test was used. To test the homogeneity between proportions, the chi-squared test or Fisher's exact test were used, when the expected frequencies were < 5. The multivariate logistic regression model was used to study the several factors influencing the occurrence of cholelithiasis. The significance level used for the tests was set at 5%.
The project was approved by the Research Ethics Committee on Human Subjects of the Hospital Universitário Alcides Carneiro (HUAC), under protocol number 20091412-053.
ResultsThe present study included 66 patients, 40 of whom (60.6%) were females, with a mean age of 14.3±2.2 years, who resided in Campina Grande and surrounding cities.
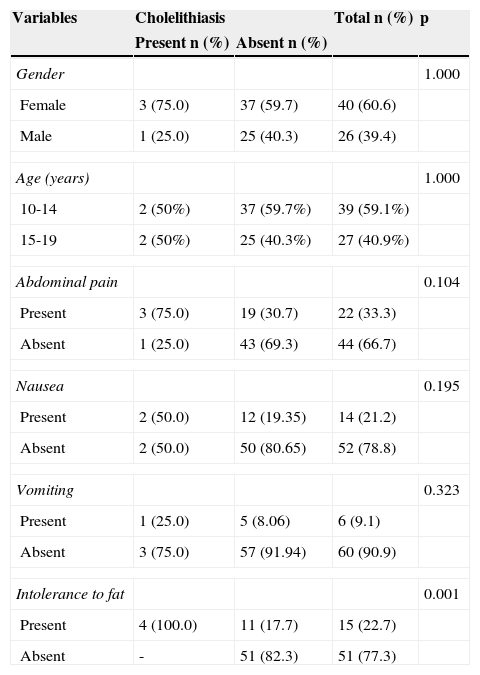
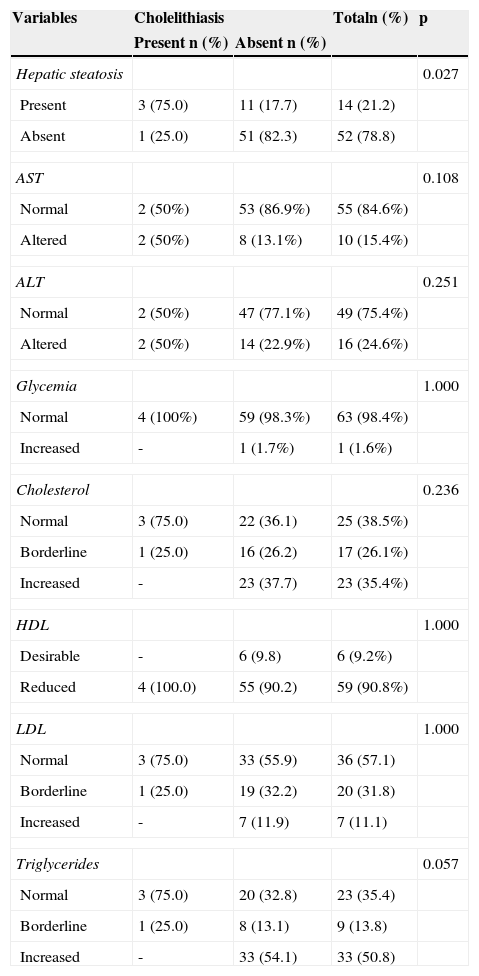
Cholelithiasis was diagnosed in three of the 66 (4.5%) obese adolescents. By including an adolescent who had undergone cholecystectomy, cholelithiasis affected four of the 66 adolescents (6.1%), with a predominance of the female gender (3/4). Hepatic steatosis was identified in 21.2% patients (14/66), of whom 57.1% (8/14) had the mild form and 42.9% (6/14) a moderate form of the disease. Hepatic steatosis was observed in 75% (3/4) of patients with cholelithiasis and in 17.7% (11/62) of those without cholelithiasis; this difference was significant (p=0.02).
All patients with cholelithiasis reported intolerance to fatty foods (4/4), which was also mentioned by 11 of the 62 (17.7%) patients without cholelithiasis, showing a significant difference (p=0.001). Other symptoms presented no significant difference between the groups with and without cholelithiasis (Table 1). The mean weight loss was 6.0±2.9kg in cholelithiasis patients and 3.2±4.8kg in the group without cholelithiasis (p=0.04). However, in relation to time of weight loss, there was no difference between the two groups (p=0.11).
Distribution of medical history variables according to the presence of cholelithiasis.
| Variables | Cholelithiasis | Total n (%) | p | |
|---|---|---|---|---|
| Present n (%) | Absent n (%) | |||
| Gender | 1.000 | |||
| Female | 3 (75.0) | 37 (59.7) | 40 (60.6) | |
| Male | 1 (25.0) | 25 (40.3) | 26 (39.4) | |
| Age (years) | 1.000 | |||
| 10-14 | 2 (50%) | 37 (59.7%) | 39 (59.1%) | |
| 15-19 | 2 (50%) | 25 (40.3%) | 27 (40.9%) | |
| Abdominal pain | 0.104 | |||
| Present | 3 (75.0) | 19 (30.7) | 22 (33.3) | |
| Absent | 1 (25.0) | 43 (69.3) | 44 (66.7) | |
| Nausea | 0.195 | |||
| Present | 2 (50.0) | 12 (19.35) | 14 (21.2) | |
| Absent | 2 (50.0) | 50 (80.65) | 52 (78.8) | |
| Vomiting | 0.323 | |||
| Present | 1 (25.0) | 5 (8.06) | 6 (9.1) | |
| Absent | 3 (75.0) | 57 (91.94) | 60 (90.9) | |
| Intolerance to fat | 0.001 | |||
| Present | 4 (100.0) | 11 (17.7) | 15 (22.7) | |
| Absent | - | 51 (82.3) | 51 (77.3) | |
Family history of cholelithiasis was positive in three of four (75%) patients with cholelithiasis and in 22 of 61 (36.1%) patients without it, but this difference was not significant (p=0.28). One adolescent had been adopted.
In the group of patients with cholelithiasis, mean BMI (37.9±9.1kg/m2) was higher than in the group without cholelithiasis (30±4kg/m2), but the difference was not significant (p=0.18). The mean AC was also greater among adolescents with (109.4±24.7cm) when compared to patients without cholelithiasis (91.4±10.2cm), although the difference was not significant (p=0.14).
One patient who had moderate hepatic steatosis also presented elevated blood glucose, but no cholelithiasis. The results of lipid and aminotransferase profile are shown in Table 2.
Distribution of complementary examination variables according to the presence of cholelithiasis.
| Variables | Cholelithiasis | Totaln (%) | p | |
|---|---|---|---|---|
| Present n (%) | Absent n (%) | |||
| Hepatic steatosis | 0.027 | |||
| Present | 3 (75.0) | 11 (17.7) | 14 (21.2) | |
| Absent | 1 (25.0) | 51 (82.3) | 52 (78.8) | |
| AST | 0.108 | |||
| Normal | 2 (50%) | 53 (86.9%) | 55 (84.6%) | |
| Altered | 2 (50%) | 8 (13.1%) | 10 (15.4%) | |
| ALT | 0.251 | |||
| Normal | 2 (50%) | 47 (77.1%) | 49 (75.4%) | |
| Altered | 2 (50%) | 14 (22.9%) | 16 (24.6%) | |
| Glycemia | 1.000 | |||
| Normal | 4 (100%) | 59 (98.3%) | 63 (98.4%) | |
| Increased | - | 1 (1.7%) | 1 (1.6%) | |
| Cholesterol | 0.236 | |||
| Normal | 3 (75.0) | 22 (36.1) | 25 (38.5%) | |
| Borderline | 1 (25.0) | 16 (26.2) | 17 (26.1%) | |
| Increased | - | 23 (37.7) | 23 (35.4%) | |
| HDL | 1.000 | |||
| Desirable | - | 6 (9.8) | 6 (9.2%) | |
| Reduced | 4 (100.0) | 55 (90.2) | 59 (90.8%) | |
| LDL | 1.000 | |||
| Normal | 3 (75.0) | 33 (55.9) | 36 (57.1) | |
| Borderline | 1 (25.0) | 19 (32.2) | 20 (31.8) | |
| Increased | - | 7 (11.9) | 7 (11.1) | |
| Triglycerides | 0.057 | |||
| Normal | 3 (75.0) | 20 (32.8) | 23 (35.4) | |
| Borderline | 1 (25.0) | 8 (13.1) | 9 (13.8) | |
| Increased | - | 33 (54.1) | 33 (50.8) | |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The frequency of cholelithiasis in this study (6.1%) was high. To date, the only study of cholelithiasis in patients with childhood obesity was performed in Germany by Kaechele et al.,20 who found a frequency of 2% among 493 hospitalized obese children and adolescents.
Later, a population-based study conducted in that same country by Kratzer et al.21 found a frequency of 1% in 307 adolescents aged 12 to 18 years. In that study, two of the three adolescents with gallstones were obese and thus, the authors concluded that obesity appears to be a risk factor in the development of gallstones in childhood and adolescence.
The high frequency observed in the present study is probably related to environmental factors, such as diet. Diets low in fiber with a high intake of refined sugars and fats contribute to gallstone formation and are related to the development of obesity, which is considered a risk factor for cholelithiasis.19,22,23 Moreover, in the present sample all participants were overweight or obese.
In the United States, a cohort study performed by Koebnick et al.14 detected 766 cases of cholelithiasis among 510,816 adolescents who participated in the study, a prevalence of 0.1%. The authors observed that the prevalence of gallstones increased with increasing weight, but the association was stronger in females than in males. The present study confirmed this finding, since three of the four adolescents with lithiasis were female. Several studies have shown a higher frequency of cholelithiasis in the female gender.8,22
The female gender is associated with cholelithiasis, especially during the childbearing years. Estrogens increase the secretion of cholesterol and decrease the secretion of bile salts, whereas progestogens act by reducing the secretion of bile salts and the emptying of the gallbladder, leading to stasis.24
The frequency of hepatic steatosis (21.2%) was lower than that reported by Lira et al.,25 who observed it in 27.7% of the 172 adolescents, and greater than that reported by Schwimmer et al.,26 who reported it in 9.6% among children and adolescents.
These authors reported that the prevalence increases with age, ranging from 0.7% from ages 2 to 4 years to 17.3% from ages 15 to 19 years.7 In the present study, the significant association between cholelithiasis and hepatic steatosis is spurious, since obesity is implicated as a risk factor for both.16,22 Regarding the physiopathology, cholelithiasis and hepatic steatosis behave as independent variables.19
The presence of symptoms associated with cholelithiasis in this study was greater than that reported in the literature, as all four patients were symptomatic; however, only intolerance to dietary fat was significant. Ruibal et al.27 studied 123 children and adolescents with cholelithiasis and observed that 66% had symptoms; approximately 35% had abdominal pain associated with vomiting and 27% had isolated abdominal pain.
Wesdorp et al.11 studied the clinical presentation of 82 children and adolescents with cholelithiasis, most with hemolytic disease (32/82) and only three with obesity, and observed that 17% were asymptomatic, 52% had biliary symptoms (biliary colic and jaundice), 24% had unspecific abdominal pain, and 7% had acute abdominal pain with fever. Among patients with biliary symptoms, 10% reported intolerance to fat.
However, certain vague complaints such as symptoms of dyspepsia with certain foods, flatulence, nausea, and bloating that have been in the past attributed to cholelithiasis are not accepted today, mainly because of lack of improvement after cholecystectomy, and are thus attributed to irritable bowel syndrome or other organic or functional diseases.16 The findings in the present study suggest that the symptomatic clinical picture is more often observed in obese adolescents. However, further studies with larger samples are needed in order to identify the most relevant symptoms.
The greater weight loss in cholelithiasis patients shown in the present study is consistent with the literature. Kaechele et al.20 observed that obese children and adolescents with cholelithiasis lost an average of 10.1±7.0kg compared with only 5.8±5.0kg lost in the group without cholelithiasis. Among the ten obese patients with cholelithiasis, nine reported undergoing dieting for weight reduction. As for the time of weight loss, the present study found no difference between the two groups. The best evidence comes from prospective studies, showing that rapid weight loss, as that observed in hypocaloric diets, markedly increases the formation of gallstones.28
The main mechanism of gallstone formation in patients with weight gain or rapid weight loss is reduction of the gallbladder motility and increased excretion of cholesterol in the bile, causing cholesterol supersaturation, with subsequent formation of gallstones.16
In the present study, the reporting of family history of the disease by 75% of adolescents with cholelithiasis is consistent with the literature. In the study by Kaechele et al.,20 three out of 10 (30%) obese patients with cholelithiasis reported positive family history, and the mothers were affected in all three cases. Wesdorp et al.9 observed a lower frequency, with a positive family history in seven out of 82 (8.5%) patients with cholelithiasis.
A cohort study29 with individuals with symptomatic cholelithiasis observed that men with BMI>28.5kg/m2 have a 2.48-fold higher chance of developing cholelithiasis when compared to those with BMI < 22.2kg/m2. Furthermore, individuals with AC>102.6cm have a 2.66-fold higher risk of developing cholelithiasis than individuals with AC < 86.4cm. The authors demonstrated that AC predicts the risk of cholelithiasis regardless of the BMI.29 In the present study, obese adolescents with cholelithiasis had higher BMI and AC measurements when compared with adolescents without cholelithiasis.
Half of obese adolescents with symptomatic cholelithiasis had abnormal levels of aminotransferases, and this finding was consistent with the literature. Wesdorp et al.9 identified elevated liver enzymes in 51% of patients with biliary symptoms, and in 29% of asymptomatic patients, and a higher increase in patients with biliary sludge than in those with cholelithiasis. In the present study, biliary sludge was not diagnosed. Therefore, laboratory tests do not support the diagnosis and treatment plan, that is, the laboratory abnormalities do not contribute to the diagnosis of cholelithiasis and may hinder the decision of whether or not to perform surgery.
Cholelithiasis and hepatic steatosis are common in obese adolescents, and gastrointestinal symptoms should be considered, as they are often underestimated in adolescence, through attribution to a psychosomatic or other type of cause. Therefore, it is recommended to maintain active screening using ultrasound to identify this condition in all symptomatic or asymptomatic obese adolescents.
FundingCoordination of Improvement of Higher Level Personnel (CAPES)
Conflicts of interestThe authors declare no conflicts of interest.