To investigate eating behavior and serum concentration of triglycerides in children and adolescents with autistic spectrum disorder.
MethodsCross-sectional study conducted in the neurodevelopment nucleus, from October 2018 to April 2019 in a neurodevelopment center in the city of Pelotas/RS. Blood samples were collected, and serum was essayed for triglycerides by colorimetric enzymatic reaction. The Children's Eating Behavior Questionnaire was applied to the parents. The comparison between two or three categories of variables was performed with nonparametric tests. Linear regression was used to access the association between the log triglyceride serum concentration and the score above or below the median score of the food response and emotional overeating subscales.
ResultsSixty patients were evaluated. The average age was 8.6±3.2 years and most were white (75 %), male (80 %), and overweight (66%). Half of the sample had elevated triglycerides. Triglyceride concentrations were higher among overweight children and adolescents with higher median scores on the “food response” and “emotional overeating” subscales. In the adjusted analysis, the association between triglycerides and higher scores on subscales reflecting interest in food remained significant.
ConclusionChildren and adolescents with autistic spectrum disorder present high triglyceride concentrations associated with a greater interest in food. Knowledge of this eating behavior may provide more effective nutritional intervention in this population.
Autistic spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in two central domains: social communication; restrictive and repetitive behaviors and interests.1 It is estimated that Brazil has approximately two million individuals with ASD,2 who have a long road ahead of them in order to receive adequate treatment.2
In 2014, Evans et al.3 reported that the food intake of individuals with ASD is denser in calories, but insufficient in micronutrients when compared to individuals with typical development3,4; additionally, eating difficulties are present and related to inadequate eating habits.3 However, these comprise a set of genetic and environmental influences5 and, with them, the development of eating behaviors is acquired from the earliest years of life.5,6
Serum triglyceride (TG) concentrations are a metabolic indicator of atherogenic potential,7 and their elevated concentrations in serum are directly proportional to the increased formation of striae in the aorta, the appearance of atherosclerotic lesions, and cardiovascular disease (CVD).7 Studies indicate a higher prevalence of hypertriglyceridemia in individuals with ASD when compared to the general population.8–10
The increase in TG serum concentrations usually occurs in the presence of overweight, a reduction in the levels of high-density lipoprotein (HDL), and an increase in small and dense low-density lipoprotein (LDL). Children and adolescents with ASD are exposed to multiple risk factors for CVD, with hypertriglyceridemia and obesity representing the most frequent factors.11
Although the risk for CVD is present from childhood, few studies have aimed at evaluating them in the pediatric age group, particularly in ASD. To date, there are no studies in the literature that assessed the association between serum TG concentrations and the eating behavior of children and adolescents with ASD. Based on these principles, this study aimed to investigate the eating behavior and serum TG concentrations in children and adolescents with ASD.
Materials and methodsThis was a cross-sectional study carried out at the Neurodevelopment Center of Faculdade de Medicina of Universidade Federal de Pelotas, in the city of Pelotas, state of Rio Grande do Sul, Brazil. Patients were screened from October 2018 to April 2019. Patients who met the following inclusion criteria were invited to participate in the present study: i) age between 3 and 18 years old; ii) absence of a medical diagnosis of neuropathies and congenital heart disease. Of the 82 children eligible for outpatient care, 74 parents or guardians showed interest in participating in the study. Of these, 14 patients did not undergo the blood collection, as they had some difficulty in performing it, representing a loss of 18%. Sixty patients were included in the present study.
All enrolled patients were scheduled for outpatient care specific for the research protocol. The application of questionnaires, scales, and anthropometric measurements was carried out by postgraduate students, previously trained for the present study. The interviews were performed in the presence of the patient’s main caregiver, stratified by time spent with the child or adolescent. In most cases, the child’s caregiver was their mother.
The assessed outcome was serum TG concentrations. Blood samples were obtained without prior fasting in a clinical laboratory through venipuncture. Serum TG concentrations were determined by the enzymatic colorimetric method and the results were expressed as mg/dL. This study considered the following as cutoff points for high TG concentrations without fasting: ≥ 85 mg/dL for children between 0 and 9 years, and ≥100 mg/dL for children and adolescents from 10 to 19 years of age.12 According to the Brazilian Consensus for Standardization of Laboratory Measurements of the Lipid Profile, prior fasting is not necessary to evaluate the lipid profile, since the fed state predominates during most of the day and thus, the patient is more exposed to lipids present in this condition when compared to the fasting state, better representing cardiovascular risk.12
The main exposure was the eating behavior, which had its subjective perception assessed by the Children's Eating Behavior Questionnaire (CEBQ), translated and validated to the Portuguese language.13 This questionnaire has 35 questions, divided into eight subscales in two domains: i) behaviors that reflect “interest in food,” consisting of the subscales: a) response to food, b) enjoyment of food, c) desire to drink, and d) emotional overeating; ii) “lack of interest in food” behaviors, consisting of the subscales: a) emotional undereating, b) response to satiety, c) slow eating, and d) selectivity. The questionnaires were answered with responses on a five-point Likert scale, according to how often they experienced each behavior. The instrument score ranges from 1 to 5: never (1), rarely (2), sometimes (3), often (4), and always (5). Subsequently, the score of the questions belonging to the same subscale was added, obtaining a median value and interquartile range (IQR) for each. This result was used dichotomously in the analyses (lower than the median; equal to or greater than the median).
The age variable was collected in years and subsequently categorized into the following ranges: ≤5, 6 to 10, and ≥11 years. Maternal schooling was obtained in full years of study. Medication use was collected openly and further categorized into antipsychotic medication (yes, no). The child’s gender was categorized as male or female.
Anthropometric measurements of height and weight were measured by previously trained and standardized nutritionists. The patients were weighed while wearing light clothing, on a digital scale (TRENTIN®, RS, Brazil) with a capacity of 150kg and 100g accuracy. Height was obtained with a 213-cm vertical stadiometer coupled to the scale, with 0.1cm accuracy. The nutritional status of the participants was assessed according to the body mass index (BMI) for age as a Z-score. For this purpose, the 2006 and 2007 World Health Organization (WHO) proposals14,15 were used as a reference. To calculate the Z-score, the software Anthro® and Anthro Plus® (WHO; Software for assessing growth of the world's children and adolescents. Geneva: WHO, 2009) were used. Individuals were considered overweight when their BMI was >+1 Z-score.14,15
The collected data were double entered using the EpiData® program (EpiData® program, version 3.1; The EpiData Association, Odense, Denmark, 2003–2005) for further typing consistency analysis and the analyses were carried out using the Stata® statistical package (Stata Statistical Software: Version 11. College Station, TX, USA). Descriptive analyses are presented as mean±standard deviation, median and interquartile range (IQR) for continuous variables, and as proportions for categorical variables. Since the outcome showed an asymmetric distribution, the comparison between categories was performed using the nonparametric Mann-Whitney and Kruskal–Wallis tests.
The linear regression was performed to assess the association between the log of concentration of TG. For this analysis, log transformation of the serum TG concentration was performed and the β coefficients of the crude and adjusted analyses were presented in their exponential form. This result should be interpreted as the percentage of change in serum TG concentrations in relation to the reference group, for instance: the β coefficient was 1.10, which means 10% higher than the reference group. Potential adjustment confounding factors in the regressions were age of patients, use of antipsychotic drugs, and maternal schooling. The level of statistical significance was set at p<5%.
The project was approved by the Research Ethics Committee of Faculdade de Medicina of Universidade Federal de Pelotas, under protocol No. 2.835.793. The informed consent was obtained in two copies, signed by the parents or guardians of the children and adolescents.
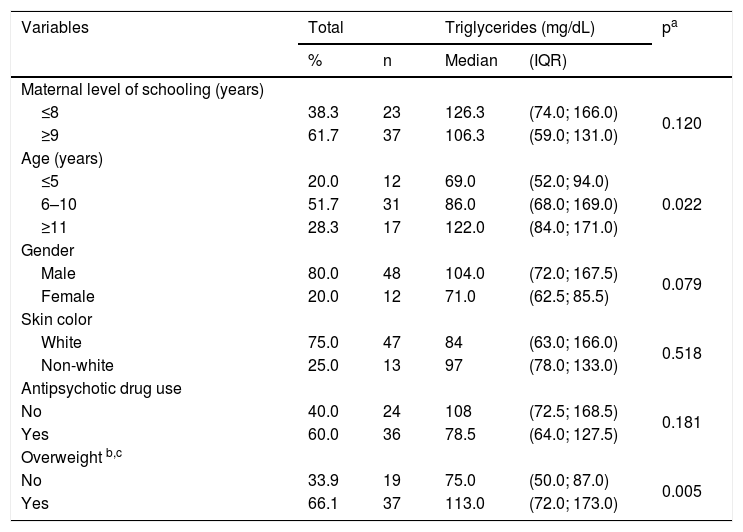
ResultsThe study participants were on average 8.6±3.2 years old. Most were males (80%), with white skin color (75%), and attended an educational institution (92 %). Most (62 %) of the mothers had nine or more years of schooling. Regarding the nutritional status, most children and adolescents (66%) were overweight. The median serum TG level was 86.5 (IQR 67.5; 136.5) mg/dL, and was significantly higher in the age group ≥11 years and in those who were overweight (Table 1).
Serum triglyceride concentrations according to sociodemographic characteristics, antipsychotic drug use, and presence of overweight in children and adolescents with autistic spectrum disorder. Neurodevelopment Center, Pelotas, 2018–2019 (n=60). IQR, interquartile range.
| Variables | Total | Triglycerides (mg/dL) | pa | ||
|---|---|---|---|---|---|
| % | n | Median | (IQR) | ||
| Maternal level of schooling (years) | |||||
| ≤8 | 38.3 | 23 | 126.3 | (74.0; 166.0) | 0.120 |
| ≥9 | 61.7 | 37 | 106.3 | (59.0; 131.0) | |
| Age (years) | |||||
| ≤5 | 20.0 | 12 | 69.0 | (52.0; 94.0) | 0.022 |
| 6–10 | 51.7 | 31 | 86.0 | (68.0; 169.0) | |
| ≥11 | 28.3 | 17 | 122.0 | (84.0; 171.0) | |
| Gender | |||||
| Male | 80.0 | 48 | 104.0 | (72.0; 167.5) | 0.079 |
| Female | 20.0 | 12 | 71.0 | (62.5; 85.5) | |
| Skin color | |||||
| White | 75.0 | 47 | 84 | (63.0; 166.0) | 0.518 |
| Non-white | 25.0 | 13 | 97 | (78.0; 133.0) | |
| Antipsychotic drug use | |||||
| No | 40.0 | 24 | 108 | (72.5; 168.5) | 0.181 |
| Yes | 60.0 | 36 | 78.5 | (64.0; 127.5) | |
| Overweight b,c | |||||
| No | 33.9 | 19 | 75.0 | (50.0; 87.0) | 0.005 |
| Yes | 66.1 | 37 | 113.0 | (72.0; 173.0) | |
Fifty percent of the children and adolescents had high serum TG concentrations for age (Table 1), and 30 % had hypertriglyceridemia, characterized by TG concentrations above the 95th percentile of the reference curve for age (data not shown).
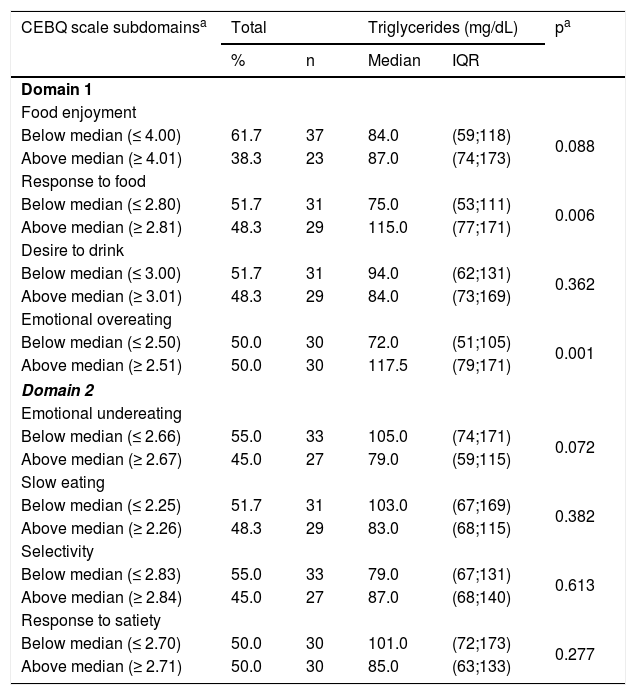
The CEBQ subscales for food enjoyment, food response, desire to drink, emotional overeating, emotional undereating, satiety response, slow eating, and selectivity showed medians of 4.0, 2.8, 3.0, 2.5, 2.6, 2.7, 2.2, and 2.8, respectively.
The median serum TG concentration was higher among those above the median in the “food response” subscale (115.0 mg/dL, IQR 77; 171), when compared to children with scores below the median (75.0mg/dL, IQR 53; 111; Table 2). For the “emotional overeating” subscale it was observed that patients above the median had a higher TG concentration, of 117.5mg/dL (IQR 79; 171), when compared to patients below the median, 72.0mg/dL (IQR 51; 105).
Serum triglycerideconcentrations according to the CEBQ scale subdomain scores in children and adolescents with autistic spectrum disorder. Neurodevelopment Center, Pelotas, 2018–2019 (n=60).
| CEBQ scale subdomainsa | Total | Triglycerides (mg/dL) | pa | ||
|---|---|---|---|---|---|
| % | n | Median | IQR | ||
| Domain 1 | |||||
| Food enjoyment | |||||
| Below median (≤ 4.00) | 61.7 | 37 | 84.0 | (59;118) | 0.088 |
| Above median (≥ 4.01) | 38.3 | 23 | 87.0 | (74;173) | |
| Response to food | |||||
| Below median (≤ 2.80) | 51.7 | 31 | 75.0 | (53;111) | 0.006 |
| Above median (≥ 2.81) | 48.3 | 29 | 115.0 | (77;171) | |
| Desire to drink | |||||
| Below median (≤ 3.00) | 51.7 | 31 | 94.0 | (62;131) | 0.362 |
| Above median (≥ 3.01) | 48.3 | 29 | 84.0 | (73;169) | |
| Emotional overeating | |||||
| Below median (≤ 2.50) | 50.0 | 30 | 72.0 | (51;105) | 0.001 |
| Above median (≥ 2.51) | 50.0 | 30 | 117.5 | (79;171) | |
| Domain 2 | |||||
| Emotional undereating | |||||
| Below median (≤ 2.66) | 55.0 | 33 | 105.0 | (74;171) | 0.072 |
| Above median (≥ 2.67) | 45.0 | 27 | 79.0 | (59;115) | |
| Slow eating | |||||
| Below median (≤ 2.25) | 51.7 | 31 | 103.0 | (67;169) | 0.382 |
| Above median (≥ 2.26) | 48.3 | 29 | 83.0 | (68;115) | |
| Selectivity | |||||
| Below median (≤ 2.83) | 55.0 | 33 | 79.0 | (67;131) | 0.613 |
| Above median (≥ 2.84) | 45.0 | 27 | 87.0 | (68;140) | |
| Response to satiety | |||||
| Below median (≤ 2.70) | 50.0 | 30 | 101.0 | (72;173) | 0.277 |
| Above median (≥ 2.71) | 50.0 | 30 | 85.0 | (63;133) | |
IQR, interquartile range; CEBQ, Children’s Eating Behavior Questionnaire.
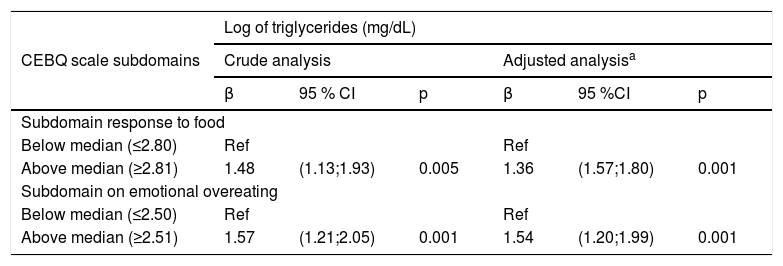
Table 3 shows the regression coefficients. Both the “food response” and “emotional overeating” subdomains were statistically associated with TG concentrations, both in the crude analysis and after adjustment for confounders. Individuals above the median in the “food response” subscale averaged 36 % (95 %CI 0.45; 0.59) greater TG concentration when compared to individuals below the median after adjusting for confounders. Regarding the subscale “emotional overeating,” individuals who were above the median had on average 53 % (95 % CI 0.18; 0.69) greater TG concentration when compared to those below the median.
Linear regression of serum triglyceride concentrations and subdomain of the CEBQ scale (food enjoyment and emotional overeating) of children and adolescents with autistic spectrum disorder. Neurodevelopment Center, Pelotas, 2018–2019 (n=60).
| Log of triglycerides (mg/dL) | ||||||
|---|---|---|---|---|---|---|
| CEBQ scale subdomains | Crude analysis | Adjusted analysisa | ||||
| β | 95 % CI | p | β | 95 %CI | p | |
| Subdomain response to food | ||||||
| Below median (≤2.80) | Ref | Ref | ||||
| Above median (≥2.81) | 1.48 | (1.13;1.93) | 0.005 | 1.36 | (1.57;1.80) | 0.001 |
| Subdomain on emotional overeating | ||||||
| Below median (≤2.50) | Ref | Ref | ||||
| Above median (≥2.51) | 1.57 | (1.21;2.05) | 0.001 | 1.54 | (1.20;1.99) | 0.001 |
CEBQ, Children’s Eating Behavior Questionnaire; β, coefficient of regression shown in its exponential form.
This study showed that children and adolescents with ASD were characterized by elevated serum TG concentrations and greater interest in food. The behaviors that reflected “interest in food” significantly explained the change in serum TG concentrations in children and adolescents with ASD. The study of eating behaviors is essential in the prevention and treatment of diseases and health problems that may be associated with an inadequate diet.
The serum TG concentration reflects the content of remnant lipoproteins (RL), of chylomicron particles, and of very low density lipoproteins (VLDL).16 RL have high atherogenic potential, since they are rich in cholesterol, which is predominantly captured by scavenger receptors in macrophages.11,17 Thus, the elevated TG concentration contributes to the formation of foam cells and the onset of the atherosclerotic lesion.5
In this sense, research indicates that hypertriglyceridemia in children and adolescents is able to predict the increase in the thickness of the carotid intima media layer and the increase of the carotid artery stiffness.11,16,17 Thus, the early development of hypertriglyceridemia is a predictor of cardiovascular events in young adults.11
In particular, children and adolescents on the autistic spectrum using antipsychotic drugs need to have their cardiovascular risk monitored, since these drugs, independently and synergistically, have an orexigenic and hyperlipemic effect.18,19 In these cases, hypertriglyceridemia is related to excessive weight gain for age, increased adiposity, and insulin resistance.18–20 Particularly, the use of risperidone in the short term causes a significant increase in weight and serum TG concentration.20
Children with ASD tend to prefer unhealthy meals, as observed in studies that showed a high frequency of foods such as processed meats, snacks, and cookies.21 Additionally, individuals with ASD have the habit of eating to deal with different emotional states of happiness, anxiety, and stress, often ingesting sugary drinks throughout the day and eating faster.21 Together, this behavior and increased adiposity are major issues that compromise the health of children and adolescents with ASD, particularly regarding increased TG concentration and the risk of short- and long-term health problems.21
Studies have shown differences in several dimensions of eating behaviors in children with and without excess weight. Neurotypical children with excess weight are more responsive to stimuli such as the taste and color of food, show greater food enjoyment, and are less capable of responding to satiety, when compared with children without excess weight. These aspects cause them to eat more in the absence of hunger and show a greater interest in food. In the case of individuals with ASD, these behaviors may be exacerbated, considering that compulsion is among the most frequent comorbidities.22,23
The CEBQ scale has been broadly used to assess the eating behavior of individuals with typical neurological development.23,24 However, studies that specifically investigated the eating behavior of individuals with ASD indicates that it can be better investigated through instruments that address their behavioral diversity.23–25 However, four studies were found that showed specific instruments to assess the eating behavior of children, young adults, and adults with ASD: two were performed in the United States, one in Sweden, and one in Canada.26 Until the planning of the current research, no studies had been found on the validation of these instruments for other populations, such as the Brazilian population. Despite these considerations, the present results pointed to behaviors and associated risk factors that were similar to those found in samples of Brazilian, Portuguese, Canadian, Dutch, and Chinese children and adolescents using the CEBQ scale.23,26–30 Notably, in 2015, Passos et al.24 applied this scale to children in the city of Pelotas-RS, observing that behaviors that indicated ‘interest in food’ were positively associated with excess weight.24
This study has some limitations; the first refers to the lack of information on the severity of ASD symptoms; the second, to the loss of patients due to the difficulty in collecting blood from the children; the third, which cannot be ignored, refers to the possible difficulty regarding parental interpretation of patient behavior, although these are the best source of information about the child for data collection. Also, the absence of dietary data that could explain the serum concentrations of TG may be considered a limitation. However, diet is considered a mediator for the association between behavior and serum concentrations, rather than a confounding factor for this association. The external validation of results is limited to population groups similar to that of the present study. This study’s strengths include the application of standardized instruments, trained staff, and the use of a biochemical marker.
Children and adolescents with ASD had high TG concentrations, associated with a greater interest in food. Nutritional intervention directed towards the management of this eating behavior and the adjustment of the foods with the greatest impact on lipemia may help in the early prevention of health problems in this population.
FundingThis study received funding and support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; financiamento 001), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the members of the Neurodevelopment Center of Universidade Federal de Pelotas for their support and encouragement for the performance of this study, and to Laboratório Novara for subsidizing part of the blood tests.
Please cite this article as: Luçardo JC, Monk GF, Dias MS, Martins-Silva T, Fernandes MP, Maia JC, et al. Interest in food and triglyceride levels in children and adolescents with autistic spectrum disorder. J Pediatr (Rio J). 2020;97:103–8.