To evaluate the influence of gestational and perinatal factors on body composition and birth weight of full-term newborns.
MethodThis was a cross-sectional study, within a prospective cohort, consisting of 124 postpartum women and their newborns. Data included the following: maternal age; ethnicity; pre-gestational body mass index; gestational weight gain; parity; gestational morbidities (hypertension and gestational diabetes mellitus); gestational age at birth; birth weight; and newborn’s gender. Anthropometric and body composition data of the newborns were collected using air-displacement plethysmography (PeaPod® Infant Body Composition System–LMI; Concord, CA, USA). The stepwise technique was applied to a multiple linear regression model.
ResultsThe significant variables in the model that explained 84% of the variation in neonatal fat-free mass were: birth weight; maternal age; newborn’s gender and gestational age. For body fat mass: birth weight; newborn’s gender; gestational arterial hypertension; gestational diabetes; and gestational weight gain. These variables explained 60% and 46% of fat mass, in grams and as a percentage, respectively. Regarding birth weight, the significant factors were gestational age, pre-gestational BMI, and gestational weight gain. Female newborns showed higher body fat mass and male newborns had higher fat-free mass.
ConclusionGestational and perinatal factors influence neonatal body composition. Early identification of these gestational factors, which may be modifiable, is necessary to prevent obesity and chronic noncommunicable diseases in the future.
Avaliar a influência de fatores gestacionais e perinatais na composição corporal e no peso de nascimento de recém-nascidos a termo.
MétodoEstudo transversal, dentro de uma coorte prospectiva, composto por 124 puérperas e seus recém-nascidos. Os dados incluíram: idade materna; etnia; índice de massa corpórea pré-gestacional; ganho de peso gestacional; paridade; morbidades gestacionais, (hipertensão arterial e diabetes mellitus gestacional); idade gestacional do nascimento; peso de nascimento; e sexo do recém-nascido. Os dados antropométricos e de composição corporal dos recém-nascidos, foram coletados com a pletismografia por deslocamento de ar (PeaPod®). Foi aplicada a técnica de stepwise no modelo de regressão linear múltipla.
ResultadosAs variáveis significativas do modelo que explicou 84% da variação da massa livre de gordura neonatal foram: peso de nascimento; idade materna; sexo do recém-nascido; e idade gestacional. Para a massa de gordura corporal: peso de nascimento; sexo do recém-nascido; hipertensão arterial gestacional; diabetes gestacional; e ganho de peso gestacional. Essas variáveis explicaram 60% e 46% da massa de gordura, em gramas e percentual, respectivamente. Em relação ao peso de nascimento os fatores significativos foram: idade gestacional; IMC pré-gestacional; e ganho de peso gestacional. Os recém-nascidos do sexo feminino apresentaram maior massa de gordura corporal e os do sexo masculino maior massa livre de gordura. Conclusão: Fatores gestacionais e perinatais influenciam a composição corporal neonatal. A identificação precoce desses fatores gestacionais, que podem ser modificáveis, é necessária para prevenção de obesidade e de doenças crônicas não transmissíveis no futuro.
In Brazil, the prevalence of obesity in children under 5 years old is increasing in all regions of the country.1 The obesity rate in Brazil in individuals older than 18 years increased from 11.8% in 2006 to 18.9% in 2016, and is considered a relevant public health issue.1,2
The initial period of growth and development of the fetus and the child demonstrates windows of sensitivity, also known as critical periods, where environmental factors may increase the risk of non-communicable chronic diseases, such as obesity.3–6 Changes in neonatal adiposity are partially explained by epigenetic mechanisms occurring in the intrauterine period.5
Several studies have shown that neonatal adiposity may be a better marker of adequate intrauterine growth and, thus, may be a better predictor of future obesity.5–9
Maternal gestational factors, such as pre-gestational body mass index (BMI) and excess gestational weight gain, have been associated with higher birth weight of newborns, but little information is available abouttheir association with neonatal body composition, and the datashow conflicting results.5,9–11
The aim of this study was to evaluate gestational and perinatal factors that influence body composition and birth weight of full-term newborns.
Material and methodsThis study is a cross-section of a prospective cohort of newborns, carried out at InstitutoNacional da Saúde da Mulher, da Criança e do Adolescente Fernandes Figueiras. A total of 124 postpartum women and 124 full-term newborns, born from March 2016 to August 2017, admitted to the Institute under rooming-in care and followed after discharge at the Pediatric Outpatient Clinic, were included in the study. The study was approved by the IFF/Fiocruz Research Ethics Committee (CAE 00754612.9.0000.5269) and registered at clinicaltrials.gov (NCT00875251). An informed consent was signed by all participants prior to data collection.
The number of pregnant women and newborns included in the study was calculated considering the results observed by Hull et al.,12 who evaluated the body composition of newborns of pregnant women with excessive weight gain (mean of 11.2±5.3% fat mass for the adequate weight gain group and 12.7±4.6% fat mass for the excess weight gain group), with a difference between groups of at least 2.5%, power of 80%, and 95% confidence level.
Newborns with congenital malformations and genetic syndromes exposed to congenital infections of the TORCH group (toxoplasmosis, rubella, cytomegalovirus, herpes simplex), human immunodeficiency virus, Zika virus, blood incompatible with receiving phototherapy, and twin gestations were excluded from the study.
The maternal factors, collected through medical records and interviews with the mothers, were as follows: maternal age; ethnicity; marital status; working outside the home; history of smoking during the pregnancy; pre-gestational BMI; gestational weight gain; number of prenatal consultations; type of delivery; parity; and gestational morbidities, such as arterial hypertension and gestational diabetes mellitus.
BMI was calculated by dividing pre-gestational maternal weight by squared height (kg/m2), which was used to classify nutritional status: low weight (BMI<18.5); normal weight (BMI=18.5–24.9); overweight (BMI=25.0–29.9); and obesity (BMI≥30.0).13
Gestational weight gain was calculated by subtracting the weight measured at the last prenatal visit (38 weeks ± 2 weeks) from the pre-gestational weight, and was classified as insufficient, adequate, or excess weight. The following ranges were considered adequate, according to the 2009 Institute of Medicine (IOM) recommendations: for underweight women, 12.5 to 18kg; for normal-weight women, from 11.5 to 15.9kg; for overweight women, from 7 to 11.5kg; and for obese women, from 5 to 9kg.13
Gestational arterial hypertension was defined when systolic blood pressure was ≥140mmHg and/or diastolic pressure was ≥90mmHg at two different times of gestation.14 Gestational diabetes mellitus was defined by one of the following criteria: fasting glucose ≥92mg/dL; one hour after oral glucose tolerance test (OGTT) ≥180mg/dL; two hours after OGTT≥153mg/dL, at any time during pregnancy.15
Fat mass and fat-free mass (grams and %) were estimated by air-displacement plethysmography (PeaPod® Infant Body Composition System–LMI;Concord, CA, USA), a validated method for evaluating neonatal body composition.16,17
Weight in grams was obtained using the high-precision PeaPod® scale; length, in centimeters, with the anthropometric ruler recommended by the Brazilian Society of Pediatrics, with the child lying on a flat surface, and head circumference was obtained using a non-extensible measuring tape fitted to the head anteriorly in the supraorbital region and, posteriorly, on the occipital prominence.18
BMI and Z-score indices (calculated for weight/age, height/age, and head circumference/age) were obtained based on the 2006 World Health Organization (WHO) growth curves. The assessment of nutritional status was performed using the WHO Anthros program (WHO Anthro for personal computers, version 3.2.2, 2011: Software for assessing growth and development of the world's children. Geneva: WHO, 2010).
Neonatal variables such as gender, gestational age at birth, and birth weight were obtained from medical records. Anthropometric data and body composition of the newborns were evaluated within 96h of life. Gestational age at birth was calculated using the first trimester ultrasound or by the date of the last menstrual period.
The study data were stored in EpiData (EpiData - Comprehensive Data Management and Basic Statistical Analysis System. V 3.1, Odense Denmark) and analyzed using SPSS. (IBM SPSS Statistics for Windows, Version 22.0. NY, USA). A significance level of 0.05 was adopted for all analyses.
Continuous variables were described as mean and standard deviation, whereas categorical variables were described as absolute frequency. A stepwise multiple linear regression model was used to assess the association of outcomes (fat mass in grams and percentage, fat-free mass, and birth weight) with the set of gestational and perinatal variables. Student's t-test was used to assess significant differences between the newborn’s gender and body composition outcomes.
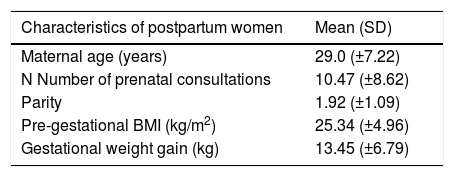
ResultsA total of 124 postpartum women and their newborns were included at the beginning of the cohort and evaluated within the first 96h of life. Of these mothers, 41% had excess weight gain during pregnancy; the prevalence of overweight and obesity was 46%, whereas 30.6% and 16.1% had hypertension and diabetes mellitus, respectively (Table 1).
General characteristics of the postpartum women participating in the study (n=124).
| Characteristics of postpartum women | Mean (SD) |
|---|---|
| Maternal age (years) | 29.0 (±7.22) |
| N Number of prenatal consultations | 10.47 (±8.62) |
| Parity | 1.92 (±1.09) |
| Pre-gestational BMI (kg/m2) | 25.34 (±4.96) |
| Gestational weight gain (kg) | 13.45 (±6.79) |
| Overall characteristics of the postpartum women | n (%) |
|---|---|
| Skin color | |
| White | 48 (39.0%) |
| Brown | 45 (36.6%) |
| Black | 22 (17.9%) |
| Others | 8 (6.5%) |
| Married | 105 (85.4%) |
| Works outside the home | 55 (44.4%) |
| Smoker | 5 (4.0%) |
| Caesarean birth | 61 (49.2%) |
| Gestational arterial hypertension | 38 (30.6%) |
| Gestational Diabetes mellitus | 20 (16.1%) |
| Pre-gestational maternal nutritional status | n (%) |
|---|---|
| Low weight (<18.5kg/m2) | 5 (4.0%) |
| Normal weight (18.5–24.9kg/m2) | 61 (49.2%) |
| Overweight (25–29.9kg/m2) | 41 (33.1%) |
| Obesity (>30kg/m2) | 17 (13.7%) |
| Gestational weight gain (according to IOM 2009 criteria)a | n (%) |
|---|---|
| Insufficient | 31 (25.0%) |
| Adequate | 42 (33.8%) |
| Excess | 51 (41.1%) |
BMI, body mass index; IOM, Institute of Medicine.
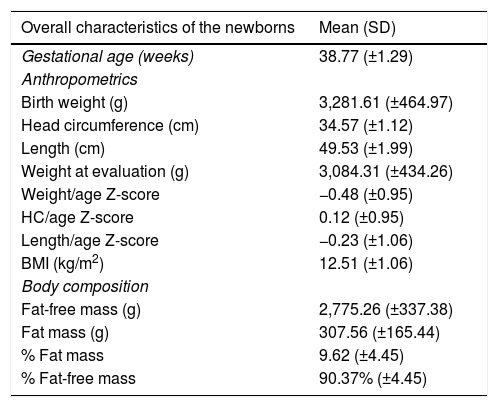
The mean gestational age was 38.7 (±1.29) weeks; 52.4% were males and all newborns had an Apgar score >7 in the fifth minute of life. These newborns had a mean birth weight of 3281.6g (±464.97) and fat mass of 307.56 (±165.44) (Table 2).
Anthropometrics and body composition of full-term newborns (n=124).
| Overall characteristics of the newborns | Mean (SD) |
|---|---|
| Gestational age (weeks) | 38.77 (±1.29) |
| Anthropometrics | |
| Birth weight (g) | 3,281.61 (±464.97) |
| Head circumference (cm) | 34.57 (±1.12) |
| Length (cm) | 49.53 (±1.99) |
| Weight at evaluation (g) | 3,084.31 (±434.26) |
| Weight/age Z-score | −0.48 (±0.95) |
| HC/age Z-score | 0.12 (±0.95) |
| Length/age Z-score | −0.23 (±1.06) |
| BMI (kg/m2) | 12.51 (±1.06) |
| Body composition | |
| Fat-free mass (g) | 2,775.26 (±337.38) |
| Fat mass (g) | 307.56 (±165.44) |
| % Fat mass | 9.62 (±4.45) |
| % Fat-free mass | 90.37% (±4.45) |
BMI, body mass index; HC, head circumference.
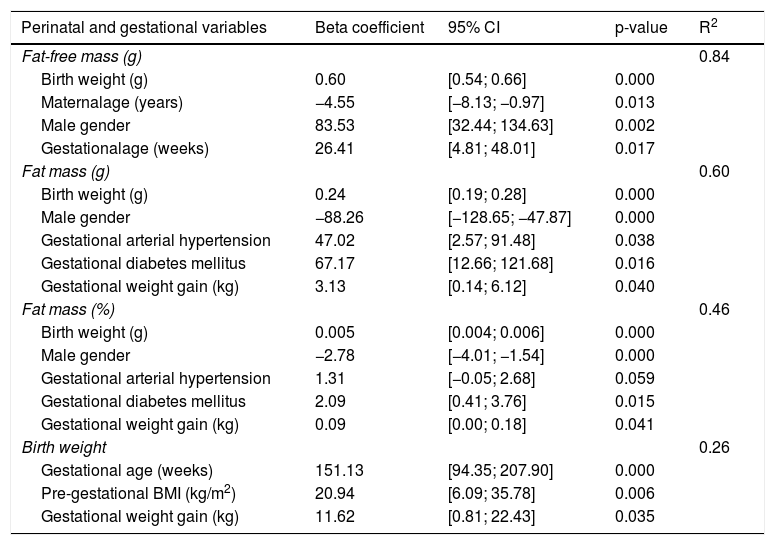
The coefficients of the regression models were estimated considering maternal age, ethnicity, pre-gestational BMI, gestational weight gain, parity, gestational diabetes mellitus, gestational hypertension, gestational age, birth weight, and the newborn’s gender. Table 3 shows the final adjustment of the models considering these variables.
Stepwise multiple linear regression analysis (n=124).
| Perinatal and gestational variables | Beta coefficient | 95% CI | p-value | R2 |
|---|---|---|---|---|
| Fat-free mass (g) | 0.84 | |||
| Birth weight (g) | 0.60 | [0.54; 0.66] | 0.000 | |
| Maternalage (years) | −4.55 | [−8.13; −0.97] | 0.013 | |
| Male gender | 83.53 | [32.44; 134.63] | 0.002 | |
| Gestationalage (weeks) | 26.41 | [4.81; 48.01] | 0.017 | |
| Fat mass (g) | 0.60 | |||
| Birth weight (g) | 0.24 | [0.19; 0.28] | 0.000 | |
| Male gender | −88.26 | [−128.65; −47.87] | 0.000 | |
| Gestational arterial hypertension | 47.02 | [2.57; 91.48] | 0.038 | |
| Gestational diabetes mellitus | 67.17 | [12.66; 121.68] | 0.016 | |
| Gestational weight gain (kg) | 3.13 | [0.14; 6.12] | 0.040 | |
| Fat mass (%) | 0.46 | |||
| Birth weight (g) | 0.005 | [0.004; 0.006] | 0.000 | |
| Male gender | −2.78 | [−4.01; −1.54] | 0.000 | |
| Gestational arterial hypertension | 1.31 | [−0.05; 2.68] | 0.059 | |
| Gestational diabetes mellitus | 2.09 | [0.41; 3.76] | 0.015 | |
| Gestational weight gain (kg) | 0.09 | [0.00; 0.18] | 0.041 | |
| Birth weight | 0.26 | |||
| Gestational age (weeks) | 151.13 | [94.35; 207.90] | 0.000 | |
| Pre-gestational BMI (kg/m2) | 20.94 | [6.09; 35.78] | 0.006 | |
| Gestational weight gain (kg) | 11.62 | [0.81; 22.43] | 0.035 |
BMI, body mass index.
For fat mass, in grams and percentage, the multivariate models identified the following significant variables: birth weight, gender, arterial hypertension, gestational diabetes, and gestational weight gain. These variables explained 60.5% of the variation of the fat mass in grams and 46.8% as a percentage.Birth weight, arterial hypertension, gestational diabetes, and gestational weight gain contributed to the increase in fat mass, whereas the male gender contributed to the decrease (Table 3).
In the multivariate model for the analysis of fat-free mass, significant variables were identified: gender, birth weight, maternal age, and gestational age, explaining 84% of the variation in fat-free mass. Maternal age contributed to the reduction in fat-free mass, while birth weight, gestational age, and male gender contributed to the increase in fat-free mass in grams (Table 3).
Gestational age, pre-gestational BMI, and gestational weight gain together explained 26% of the birth weight variation (Table 3).
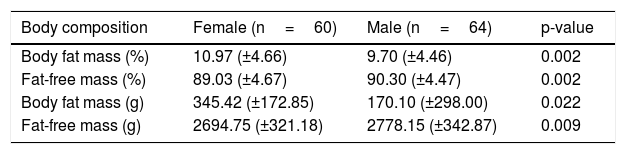
A significant difference was observed regarding the assessed components of body composition in relation to the newborn gender (p-value <0.01). Female newborns had greater body fat mass at birth (Table 4).
Fat mass and fat-free mass of full-term newborns categorized by gender, mean, and standard deviation.
| Body composition | Female (n=60) | Male (n=64) | p-value |
|---|---|---|---|
| Body fat mass (%) | 10.97 (±4.66) | 9.70 (±4.46) | 0.002 |
| Fat-free mass (%) | 89.03 (±4.67) | 90.30 (±4.47) | 0.002 |
| Body fat mass (g) | 345.42 (±172.85) | 170.10 (±298.00) | 0.022 |
| Fat-free mass (g) | 2694.75 (±321.18) | 2778.15 (±342.87) | 0.009 |
The present study showed that perinatal factors, such as birth weight, gender, gestational age, and the pregnant woman’s characteristics, such as age, gestational weight gain, pre-gestational BMI, and the presence of morbidities (hypertension and diabetes mellitus) significantly contributed to the variation in body composition of full-term newborns.
In the studybyAu et al.,10 using a methodology similar to that of the present study, a 19% variation in the percentage of body fat mass was demonstrated, with female gender, Caucasian ethnicity, and excess gestational weight gain being the main factors associated with body fat increase. These authors did not observe an association between gestational diabetes and neonatal adiposity, and described other factors that influenced the variation in body fat percentage, such as gestational age, pre-gestational BMI, parity, and maternal hypertension.10 The model of the present study showed a 46% variation in the percentage of body fat mass, with the following associated factors: birth weight, gender, arterial hypertension, gestational diabetes, and gestational weight gain.
Similar to the study of Au et al.,10 it was identified that female gender and gestational weight gain were factors associated with increased body fat mass. However, in contrast to this study, gestational arterial hypertension was one of the model variables associated with increased body fat mass. This may be explained by the fact that the group of pregnant women with this morbidity also had excess weight gain during pregnancy.
Logan et al., in a systematic review and meta-analysis, showed that newborns of mothers with gestational diabetes had a higher body fat mass when compared to newborns of non-diabetic mothers.19 In the present study, gestational diabetes was also a variable that was positively correlated with neonatal adiposity. In contrast, the study by Au et al.20 showed that newborns, children of pregnant women with diabetes and good glycemic control, showed no difference in the percentage of body fat mass when compared to those born to non-diabetic mothers.
Other studies have shown that the newborn’s body fat mass percentage increases with gestational age and higher pre-gestational BMI.5,10,21 In the current study, pre-gestational BMI was positively associated with birth weight, but not with body composition.
Catalano et al. observed that the best predictor for higher birth weight was gestational age, followed by gestational weight gain, pre-gestational maternal weight, gender, and parity. These factors together explained 29% of birth weight variation.22 In the present study, gestational age, pre-gestational BMI, and gestational weight gain were also important factors, which contributed to 26% of birth weight variation. However, in contrast to previous studies,10,23 parity was not a factor influencing body composition and birth weight variation.
Gender is described as a determining factor for body composition in full-term newborns.11,21 The study by Simon et al. showed that full-term, male newborns had more lean mass than females, assuming that the difference in body composition was explained by the action of intrauterine sex steroids.24 Fields et al. evaluated the body composition of 1-month-old infants and observed that girls had a higher percentage of body fat mass and lower fat-free mass than boys. At 6 months of age, however, this difference was not observed.25 In the present study, gender was a factor that contributed to the increase in body fat mass (female gender) and fat-free mass (male gender).
Another relevant study finding is the high prevalence of pre-gestational overweight/obesity (46%), similar to the study by Starling et al., which showed a 45% prevalence of pre-gestational overweight/obesity.26 The literature already emphasizes the short- and long-term consequences of this morbidity for both pregnant women and their children.5,27,28 The hypothesis of fetal overnutrition proposes that excess glucose, free fatty acids, and triglycerides cross the placenta, resulting in increased fetal insulin secretion, which promotes adipogenesis and fat cell hypertrophy. Other factors, such as the hypothalamic endocrine system deregulation — which regulates appetite and satiety — as well as epigenetic alterations, are mechanisms that increase the risk of obesity in the future.27
However, it was observed that despite the adequate number of prenatal consultations, excess gestational weight gain was observed in 41.1% of the study participants, and it was also an important factor in the newborn’s body fat mass variation, similar to previous studies.12,29,30 Goldstein et al., in a systematic review and meta-analysis, showed that 47% of women showed weight gain above the 2009 IOM recommendations, and that their newborns had a higher risk of being born large for gestational age, with macrosomia, and through a caesarean birth.9
These results regarding gestational weight gain may indicate that prenatal care is not being effective with regard to raising awareness and providing recommendations about adequate nutritional control. Moreover, the study site is a tertiary referral hospital for fetal risk; this fact may also explain the high number of individuals with overweight and obesity in the included population.
The effects of long-term alterations in the newborn’s body mass of overweight/obese mothers are not yet well established. However, studies indicate that newborn adiposity may be associated with a higher risk of metabolic syndrome at later ages.5,6,30 Thus, early detection of gestational and perinatal factors related to increased body fat mass in newborns has become relevant.
In the present study, preventable gestational factors were more influential on the amount of neonatal fat mass, whereas demographic characteristics (maternal age, gestational age, and newborn’s gender) were more influential on the amount of fat-free mass at birth. This shows that modifiable factors, such as obesity and gestational weight gain, must be highlighted. Additionally, although the assessment was carried out in only one moment (up to 4 days of life), it had the advantage of being close to birth, allowing the evaluation of the association of perinatal factors with body composition.
A better focus on adequate nutritional control, in addition to glycemic and blood pressure control during prenatal care, can modify the newborns’ body composition, resulting in lower future risks of obesity and chronic noncommunicable diseases. Further studies are needed to clarify the long-term effects of excess neonatal adiposity. Other factors such as breastfeeding, complementary feeding, microbiome, xenobiotic exposure, and activities that promote adequate child development, among others, may influence this outcome and deserve to be included in acohort study.
FundingConselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) - Brasil ID: 305090/2016-0. Fundação Carlos Chagas de Amparo à Pesquisa do estado do Rio de Janeiro (Faperj) - Brasil ID: E-26/202.979/2017.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Nehab SR, Villela LD, Abranches AD, Rocha DM, Silva LM, Amaral YN, et al. Influence of gestational and perinatal factors on body composition of full-term newborns. J Pediatr (Rio J). 2020;96:771–7.