Human immunodeficiency virus infection can result in the early impairment of anthropometric indicators in children and adolescents. However, combined antiretroviral therapy has improved, in addition to the immune response and viral infection, the weight and height development in infected individuals. Therefore, the objective was to evaluate the effect of combined antiretroviral on the growth development of human immunodeficiency virus infected children and adolescents.
Source of dataA systematic review was performed. In the study, the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) strategy was used as the eligibility criterion. The MEDLINE-PubMed and LILACS databases were searched using these descriptors: HIV, children, growth, antiretroviral therapy. The objective was defined by the population, intervention, comparison/control, and outcome (PICO) technique. Inclusion and exclusion criteria were applied for study selection.
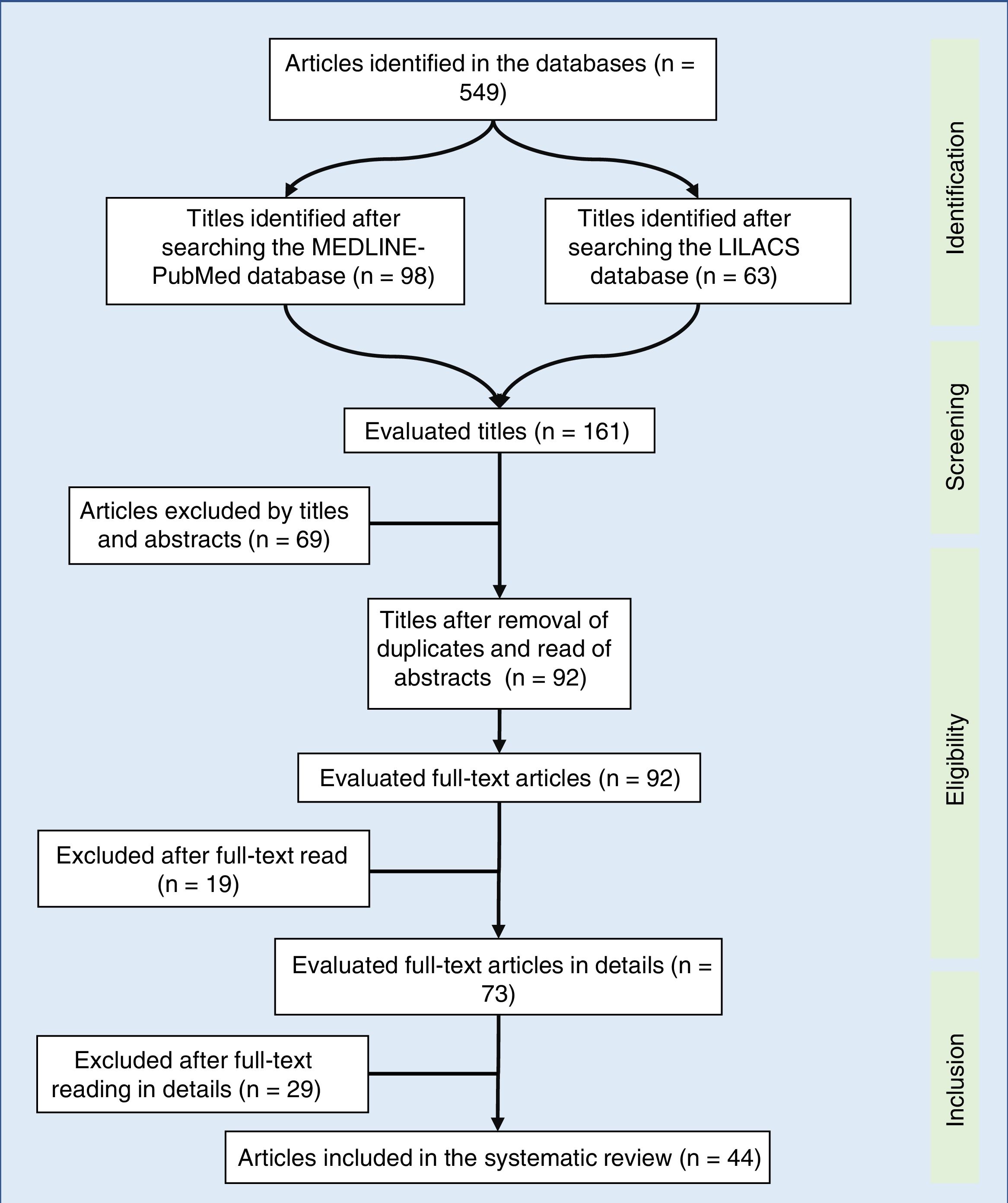
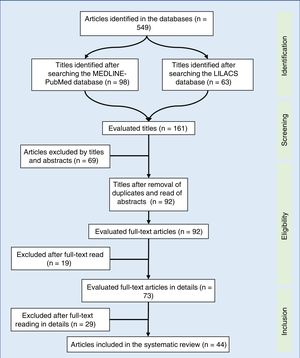
Synthesis of dataOf the 549 studies indexed in MEDLINE-PubMed and LILACS, 73 were read in full, and 44 were included in the review (33 showed a positive impact of combined antiretroviral therapy on weight/height development, ten on weight gain, and one on height gain in children and adolescents infected with human immunodeficiency virus). However, the increase in growth was not enough to normalize the height of infected children when compared to children of the same age and gender without human immunodeficiency virus infection.
ConclusionsCombined antiretroviral therapy, which is known to play a role in the improvement of viral and immunological markers, may influence in the weight and height development in children infected with human immunodeficiency virus. The earlier the infection diagnosis and, concomitantly, of malnutrition and the start of combined antiretroviral therapy, the lower the growth impairment when compared to healthy children.
A infecção pelo vírus da imunodeficiência humana pode comprometer, precocemente, os indicadores antropométricos de crianças e adolescentes. No entanto, a terapia antirretroviral combinada tem melhorado, além da resposta imunológica e da infecção viral, o ganho pôndero-estatural dos infectados. Dessa forma, nosso objetivo foi avaliar o efeito da terapia antirretroviral combinada no crescimento, de crianças e adolescentes, infectadas pelo vírus da imunodeficiência humana.
Fonte dos dadosFoi realizada uma revisão sistemática. No estudo, adotou-se como critério de elegibilidade dos artigos, a estratégia PRISMA (preferred reporting items for systematic reviews and meta-analyses). Foram consultadas as bases de dados MEDLINE-PubMed e LILACS pelos descritores: HIV (vírus da imunodeficiência humana), children, growth, antiretroviral therapy. O objetivo foi definido pela estratégia PICO (population, intervention, comparison/control, outcome). Critérios de inclusão e exclusão foram aplicados na seleção dos estudos.
Síntese dos dadosDos 549 estudos indexados no MEDLINE-PubMed e LILACS, 73 foram lidos na íntegra – 44 incluídos na revisão (33 demonstraram impacto positivo da terapia antirretroviral combinada no ganho pôndero-estatural, dez no ganho de peso e um no de estatura, em crianças e adolescentes, infectados com vírus da imunodeficiência humana). No entanto, o incremento no crescimento não foi o suficiente para normalizar a estatura de crianças infectadas, quando comparado com crianças da mesma idade e sexo, sem infecção pelo vírus da imunodeficiência humana.
ConclusõesA terapia antirretroviral combinada que, conhecidamente, atua na melhora de marcadores virais e imunológicos, pode influenciar no ganho pôndero-estatural de crianças infectadas com vírus da imunodeficiência humana. Quanto mais precoce o diagnóstico da infecção e, concomitante, desnutrição e início da terapia antirretroviral combinada, menores serão os prejuízos no crescimento, quando comparado às crianças saudáveis.
Acquired immunodeficiency syndrome (AIDS) is an infectious disease caused by the human immunodeficiency virus (HIV), which qualitatively and quantitatively affects the CD4+ T lymphocytes (CD4+ TL).1 Vertical HIV transmission in childhood can occur at three distinct moments: (i) intrauterine-transplacental transmission; (ii) during labor and/or delivery; and (iii) through breastfeeding. Most cases (about 65%) occur during labor and delivery, with the remaining 35% occurring through intrauterine transmission, especially in the last weeks of gestation and when breastfeeding. Breastfeeding, as a transmission risk, is responsible for 7% to 22% of infection cases.1
Global data for 2016 estimate that 36.7 (30.8–42.9) million individuals, of whom 2.1 million are children, are infected with HIV.2 In Brazil, from 1980 to 2016, 842,710 cases of HIV infection were identified, of which approximately 24,900 represent children under 14 years old – a number that is possibly underestimated.3,4
Individuals infected with HIV have a reduction in the number of CD4+ TL – a hallmark characteristic of the immunological system status. The mechanism that results in failure to reconstitute CD4+ TL has not been fully elucidated, but it increases the risk of opportunistic infections. In addition to immunological changes, there are nutritional and endocrine-metabolic problems in AIDS, with HIV infection being associated with growth impairment.5
Growth deficiency in AIDSChildren and adolescents with HIV infection show a greater prevalence of growth disorders, ranging from growth difficulties – resulting in weight and height below the normal range for age – to growth interruption and wasting syndrome.6 Growth retardation is a disease severity progression indicator and a risk factor for death in individuals with AIDS.7 Moreover, growth deficiency is multifactorial in AIDS, resulting from the effect of antiretroviral therapy, as well as other factors described below:
- (i)
malnutrition: may occur mainly due to inadequate nutritional intake secondary to anorexia, oral or upper digestive tract lesions (infectious cause), intake reduction (psychological or economic cause), or nutrient malabsorption (chronic diarrhea).8 Another important aspect is the acceleration of protein catabolism and increased metabolic expenditure secondary to uncontrolled viral replication and the resulting inflammatory response (including cytokine network dysregulation), as well as opportunistic infections or neoplasms secondary to immunosuppression.2 HIV-associated malnutrition was termed wasting syndrome9;
- (ii)
gastrointestinal disorders (malabsorption): may result from the HIV infection, opportunistic infections (enteric parasites, such as Cryptosporidium, Mycobacterium avium-intracellulare, and cytomegalovirus, among others) or neoplasms. Diarrhea, abdominal pain, and dysphagia are commonly observed in HIV-infected patients; however, intestinal malabsorption may occur in children with or without diarrhea.10 Damage to mucosal integrity and increased permeability favor intestinal dysfunction, leading to the malabsorption of fats, carbohydrates, and proteins, interfering with weight and height development. Intestinal dysfunction may adversely interfere with the ability to absorb oral medications, including zidovudine (AZT, C10H13N5O4)11;
- (iii)
stress: in AIDS, the expression of cytokines, including interleukins (1 and 6), tumor necrosis factor-alpha, and interferon is altered, promoting metabolic stress, which may lead to loss of appetite, anorexia, and catabolism9;
- (iv)
chronic disease: HIV infection leads to a progressive reduction of the immune function, with a reduction in the number of CD4+ TL, concomitant to changes in the function of these cells and the main immune regulatory pathways. Therefore, the susceptibility to repeat infections and antibody deficiencies alter metabolism through infection and/or intake reduction and, thus, patient nutritional monitoring is required8;
- (v)
endocrine disorders: hormonal changes are probably caused by the viral infection itself or are secondary to endocrine gland involvement, due to opportunistic infections and/or medications used to treat infections and/or their complications.12
Hence, it is important to assess factors associated with growth deficit, reinforcing the need to broaden their understanding in order to minimize nutritional status losses.11 Adequate monitoring by the multidisciplinary team contributes to the early detection of opportunistic infections, metabolic and hormonal changes, decreased food intake, nutrient malabsorption, and socioeconomic problems, probably resulting in benefits to weight and height development.12
Antiretroviral therapy – general aspectsThe 1990s were a milestone in the treatment of AIDS, with the use of more effective antiretroviral regimens – highly active antiretroviral therapy. Initially, antiretroviral therapy was based on AZT monotherapy, but it showed low efficacy, requiring a progressive combination of drugs. Subsequently, dual therapy was introduced, with the use of new nucleoside analog reverse-transcriptase inhibitors. Subsequently, protease inhibitors and non-nucleoside reverse-transcriptase inhibitors appeared – a three-drug regimen that was referred to as combined antiretroviral therapy (cART). cART reduced HIV mortality, mainly in developed countries.6,13
Since 1996, cART has been used in childhood to minimize disease progression. It is estimated that without the use of antiretroviral therapy, one-third of HIV-infected children would die before one month, and more than half before two years of age.14
Antiretroviral therapy – association with anthropometric and other dataAntiretroviral therapy in childhood and adolescence can result in metabolic disorders, mitochondrial toxicity, and adverse effects on nutritional status, especially in the first months of treatment. Patients may develop nausea or vomiting, lipodystrophy, and reduced bone mineralization.2 Diagnosis and treatment of malnutrition is necessary, since micronutrient deficiency, especially of vitamins A, C, E, D, and minerals (e.g., selenium and iron), alters the immune system (which is already altered in AIDS) and reduces growth.2
In adults, the duration of cART increases the risk of cardiovascular disease (stroke, infarction, and dilated cardiomyopathy). Thus, it is suggested that children using antiretrovirals are likely to have a higher risk of morbidity and premature cardiovascular mortality.3 Moreover, immune activation, with persistent chronic inflammation in HIV-infected individuals, also increases the risk of cardiovascular disease and other diseases of inflammatory origin. Increased levels of inflammatory markers (C-reactive protein, interleukin 6, D-dimer, fibrinogen) associated with the risk of atherosclerosis and cancer, for instance, are also found in AIDS, and remain elevated regardless of the response to cART.15 Finally, treatment adherence, which includes the use of cART, constitutes a challenge, mainly in childhood and adolescence, due to the chronic drug use, esthetic alterations related to lipodystrophy, and psychosocial aspects.16
Growth in childhood and adolescence is crucial; hence, in the presence of AIDS, the positive and negative impact of cART should be assessed. Thus, the aim of this systematic review was to evaluate the association between antiretroviral therapy and growth in HIV-infected children and adolescents.
MethodA systematic review was performed, using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) strategy as eligibility criteria. In the PRISMA strategy, the population, intervention, comparison/control, outcome (PICO) technique was used to establish the study objective, as follows: P, children and adolescents from 0 to 19 years of age; I, individuals using cART; C, child growth; O, change in growth pattern.
In the PRISMA strategy, the inclusion of studies published from 1996 (year of cART start) to September 2017 was selected. Original articles published in journals indexed in the English language were included. It was decided to include all types of studies, and among the selected articles, the classification was made by degree of recommendation and level of evidence.
The searches were carried out in the Medical Literature Analysis and Retrieval System Online (MEDLINE-PubMed) and Latin American and Caribbean Health Sciences Literature (LILACS) databases, using the following descriptors: antiretroviral therapy, children, growth, HIV. The following filters were used: (i) presence of the descriptors in the title and/or abstract; (ii) publications in the last 21 years; (iii) being a scientific article. The excluded articles were: (i) those that had a duplicate in another electronic database of the bibliographic search; (ii) those about the cost of AIDS treatment, without addressing growth; (iii) those including children exposed to HIV, but not infected; (iii) those on perinatal growth; (iv) those about cART use in pregnant women; (v) those about the exclusive use of one or two antiretroviral drugs.
A total of 549 articles were identified in the literature review, of which the following were selected by the title: 98 in MEDLINE-PubMed and 63 in LILACS. In the second evaluation, carried out by reading the abstract and by the exclusion of duplicate articles (69 articles), 92 articles were included – read in full. After being read in full, 44 studies were included in the systematic review (Fig. 1). The selected articles were classified by three authors (two physicians and one nutritionist), comprising the main items of the methods and results of each selected study (author, year, country of study origin, sample size, main results). All articles were evaluated and read by at least three authors, and the full-text reading was performed by drawing lots among the authors. Based on the data concordance, these were arranged in Tables 1 and 2.17–57 Therapeutic interventions performed with at least three antiretroviral drugs were included, without considering the cART type.
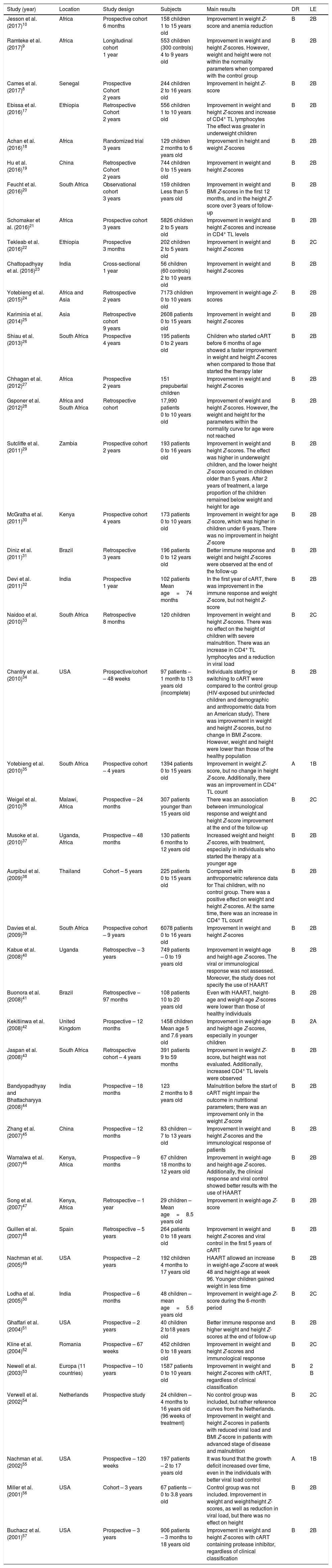
Data obtained from the studies according to year of publication, study location, study design, assessed sample, main results obtained, degree of recommendation, and level of evidence.
| Study (year) | Location | Study design | Subjects | Main results | DR | LE |
|---|---|---|---|---|---|---|
| Jesson et al. (2017)10 | Africa | Prospective cohort 6 months | 158 children 1 to 15 years old | Improvement in weight Z-score and anemia reduction | B | 2B |
| Ramteke et al. (2017)9 | Africa | Longitudinal cohort 1 year | 553 children (300 controls) 4 to 9 years old | Improvement in weight and height Z-scores. However, weight and height were not within the normality parameters when compared with the control group | B | 2B |
| Cames et al. (2017)8 | Senegal | Prospective Cohort 2 years | 244 children 2 to 16 years old | Improvement in height Z-score | B | 2B |
| Ebissa et al. (2016)17 | Ethiopia | Retrospective Cohort 2 years | 556 children 1 to 10 years old | Improvement in weight and height Z-scores and increase of CD4+ TL lymphocytes The effect was greater in underweight children | B | 2B |
| Achan et al. (2016)18 | Africa | Randomized trial 3 years | 129 children 2 months to 6 years old | Improvement in height and weight Z-scores | B | 2B |
| Hu et al. (2016)19 | China | Retrospective Cohort 2 years | 744 children 0 to 15 years old | Improvement in weight and height Z-scores | B | 2B |
| Feucht et al. (2016)20 | South Africa | Observational cohort 3 years | 159 children Less than 5 years old | Improvement in weight and BMI Z-scores in the first 12 months, and in the height Z-score over 3 years of follow-up | B | 2B |
| Schomaker et al. (2016)21 | Africa | Prospective cohort 3 years | 5826 children 2 to 5 years old | Improvement in weight and height Z-scores and increase in CD4+ TL levels | B | 2B |
| Tekleab et al. (2016)22 | Ethiopia | Prospective 3 months | 202 children 2 to 5 years old | Improvement in weight and height Z-scores | B | 2C |
| Chattopadhyay et al. (2016)23 | India | Cross-sectional 1 year | 56 children (60 controls) 2 to 10 years old | Improvement in weight and height Z-scores | B | 2B |
| Yotebieng et al. (2015)24 | Africa and Asia | Retrospective 2 years | 7173 children 0 to 10 years old | Improvement in weight-age Z-scores | B | 2B |
| Kariminia et al. (2014)25 | Asia | Retrospective cohort 9 years | 2608 patients 0 to 15 years old | Improvement in weight and height Z-scores | B | 2B |
| Shiau et al. (2013)26 | South Africa | Prospective 4 years | 195 patients 0 to 2 years old | Children who started cART before 6 months of age showed a faster improvement in weight and height Z-scores when compared to those that started the therapy later | B | 2B |
| Chhagan et al. (2012)27 | Africa | Prospective 2 years | 151 prepubertal children | Improvement in weight and height Z-scores | B | 2B |
| Gsponer et al. (2012)28 | Africa and South Africa | Retrospective cohort | 17,990 patients 0 to 10 years old | Improvement of weight and height Z-scores. However, the weight and height for the parameters within the normality curve for age were not reached | B | 2B |
| Sutcliffe et al. (2011)29 | Zambia | Prospective cohort 2 years | 193 patients 0 to 16 years old | Improvement in weight and height Z-scores. The effect was higher in underweight children, and the lower height Z-score occurred in children older than 5 years. After 2 years of treatment, a large proportion of the children remained below weight and height for age | B | 2B |
| McGratha et al. (2011)30 | Kenya | Prospective cohort 4 years | 173 patients 0 to 10 years old | Improvement in weight for age Z-score, which was higher in children under 6 years. There was no improvement in height Z-score | B | 2B |
| Diniz et al. (2011)31 | Brazil | Retrospective 3 years | 196 patients 0 to 12 years old | Better immune response and weight and height Z-scores were observed at the end of the follow-up | B | 2B |
| Devi et al. (2011)32 | India | Prospective 1 year | 102 patients Mean age=74 months | In the first year of cART, there was improvement in the immune response and weight Z-score, but not height Z-score | B | 2B |
| Naidoo et al. (2010)33 | South Africa | Retrospective 8 months | 120 children | Improvement in weight and height Z-scores. There was no effect on the height of children with severe malnutrition. There was an increase in CD4+ TL lymphocytes and a reduction in viral load | B | 2C |
| Chantry et al. (2010)34 | USA | Prospective/cohort – 48 weeks | 97 patients – 1 month to 13 years old (incomplete) | Individuals starting or switching to cART were compared to the control group (HIV-exposed but uninfected children and demographic and anthropometric data from an American study). There was improvement in weight and height Z-scores, but no change in BMI Z-score. However, weight and height were lower than those of the healthy population | B | 2B |
| Yotebieng et al. (2010)35 | South Africa | Prospective cohort – 4 years | 1394 patients 0 to 15 years old | Improvement in weight Z-score, but no change in height Z-score. Additionally, there was an improvement in CD4+ TL count | A | 1B |
| Weigel et al. (2010)36 | Malawi, Africa | Prospective – 24 months | 307 patients younger than 15 years old | There was an association between immunological response and weight and height Z-score improvement at the end of the follow-up | B | 2C |
| Musoke et al. (2010)37 | Uganda, Africa | Prospective – 48 months | 130 patients 6 months to 12 years old | Increased weight and height Z-scores, with treatment, especially in individuals who started the therapy at a younger age | B | 2B |
| Aurpibul et al. (2009)38 | Thailand | Cohort – 5 years | 225 patients 0 to 15 years old | Compared with anthropometric reference data for Thai children, with no control group. There was a positive effect on weight and height Z-scores. At the same time, there was an increase in CD4+ TL count | B | 2B |
| Davies et al. (2009)39 | South Africa | Prospective cohort – 9 years | 6078 patients 0 to 16 years old | Improvement in weight and height Z-scores | B | 2B |
| Kabue et al. (2008)40 | Uganda | Retrospective – 3 years | 749 patients – 0 to 19 years old | Improvement in weight-age and height-age Z-scores. The viral or immunological response was not assessed. Moreover, the study does not specify the use of HAART | B | 2B |
| Buonora et al. (2008)41 | Brazil | Retrospective – 97 months | 108 patients 10 to 20 years old | Even with HAART, height-age and weight-age Z-scores were lower than those of healthy individuals | B | 2B |
| Kekitiinwa et al. (2008)42 | United Kingdom | Prospective – 12 months | 1458 children Mean age 5 and 7.6 years old | Improvement in weight-age and height-age Z-scores, especially in younger children | B | 2A |
| Jaspan et al. (2008)43 | South Africa | Retrospective cohort – 4 years | 391 patients 9 to 59 months | Improvement in weight Z-score, but height was not evaluated. Additionally, increased CD4+ TL levels were observed | B | 2B |
| Bandyopadhyay and Bhattacharyya (2008)44 | India | Prospective – 18 months | 123 2 months to 8 years old | Malnutrition before the start of cART might impair the outcome in nutritional parameters; there was an improvement only in the weight Z-score | B | 2B |
| Zhang et al. (2007)45 | China | Prospective – 12 months | 83 children – 7 to 13 years old | Improvement in weight and height Z-scores and the immunological response of patients | B | 2B |
| Wamalwa et al. (2007)46 | Kenya, Africa | Prospective – 9 months | 67 children 18 months to 12 years old | Improvement in weight-age and height-age Z-scores. Additionally, the clinical response and viral control showed better results with the use of HAART | B | 2B |
| Song et al. (2007)47 | Kenya, Africa | Retrospective – 1 year | 29 children – Mean age=8.5 years old | Improvement in weight-age Z-score | B | 2B |
| Guillen et al. (2007)48 | Spain | Retrospective – 5 years | 264 patients 0 to 18 years old | Improvement in weight and height Z-scores and viral control in the first 5 years of cART | B | 2B |
| Nachman et al. (2005)49 | USA | Prospective – 2 years | 192 children 4 months to 17 years old | HAART allowed an increase in weight-age Z-score at week 48 and height-age at week 96. Younger children gained weight in less time | B | 2B |
| Lodha et al. (2005)50 | India | Prospective – 6 months | 48 children – mean age=5.6 years old | Improvement in weight-age Z-score during the 6-month period | B | 2C |
| Ghaffari et al. (2004)51 | USA | Prospective – 2 years | 40 children 2 to18 years old | Better immune response and higher weight and height Z-scores at the end of follow-up | B | 2B |
| Kline et al. (2004)52 | Romania | Prospective – 67 weeks | 452 children 0 to 18 years old | Improvement in weight and height Z-scores and immunological response | B | 2C |
| Newell et al. (2003)53 | Europa (11 countries) | Prospective – 10 years | 1587 patients 0 to 10 years old | Improvement in weight and height Z-scores with cART, regardless of clinical classification | B | 2 B |
| Verwell et al. (2002)54 | Netherlands | Prospective study | 24 children – 4 months to 16 years old (96 weeks of treatment) | No control group was included, but rather reference curves from the Netherlands. Improvement in weight and height Z-scores in patients with reduced viral load and BMI Z-score in patients with advanced stage of disease and malnutrition | B | 2C |
| Nachman et al. (2002)55 | USA | Prospective – 120 weeks | 197 patients – 2 to 17 years old | It was found that the growth deficit increased over time, even in the individuals with better viral load control | A | 1B |
| Miller et al. (2001)56 | USA | Cohort – 3 years | 67 patients – 0 to 3.8 years old | Control group was not included. Improvement in weight and weight/height Z-scores, as well as reduction in viral load, but there was no effect on height | B | 2B |
| Buchacz et al. (2001)57 | USA | Prospective – 3 years | 906 patients – 3 months to 18 years old | Improvement in weight and height Z-scores with cART containing protease inhibitor, regardless of clinical classification | B | 2B |
USA, United States of America; DR, degree of recommendation; LE, level of evidence; BMI, body mass index; CD4+ TL, CD4+ T lymphocytes; HAART, highly-active antiretroviral therapy; cART, combined antiretroviral therapy.
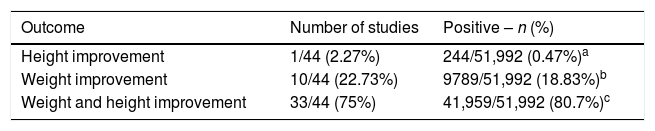
Outcome related to weight/height development in HIV-infected patients (total number of infected individuals=51,992 children and adolescents), submitted to therapy with cART.
| Outcome | Number of studies | Positive – n (%) |
|---|---|---|
| Height improvement | 1/44 (2.27%) | 244/51,992 (0.47%)a |
| Weight improvement | 10/44 (22.73%) | 9789/51,992 (18.83%)b |
| Weight and height improvement | 33/44 (75%) | 41,959/51,992 (80.7%)c |
cART, combined antiretroviral therapy; HIV, human immunodeficiency virus.
Ramteke et al., 20179; Ebissa et al., 201617; Achan et al., 201618; Hu et al., 201619; Feucht et al., 201620; Schomaker et al., 201621; Tekleab et al., 201622; Chattopadhyay et al., 201623; Kariminia et al., 201425; Shiau et al., 201326; Chhagan et al., 201227; Gsponer et al., 201228; Sutcliffe et al., 201629; Diniz et al., 201131; Naidoo et al., 201033; Chantry et al., 201034; Weigel et al., 201036; Musoke et al., 201037; Aurpibul et al., 200938; Davies et al., 200939; Kabue et al., 200840; Buonora et al., 200841; Kekitiinwa et al., 200842; Zhang et al., 200745; Wamalwa et al., 200746; Guillen et al., 200748; Nachman et al., 200549; Ghaffari et al., 200451; Kline et al., 200452; Newell et al., 200353; Verweel et al., 200354; Miller et al., 200156; Buchacz et al., 2001.57
The data obtained are summarized in Tables 1 and 2. After the summation of the assessed studies, a population of 51,992 HIV-infected individuals was included. In the studies, the positive response to cART, with growth improvement, was observed in 34 articles. However, concurrent improvement in weight and height development occurred in 33 studies. Most studies emphasized the importance of early diagnosis of HIV infection in children and adolescents, aiming to delay AIDS progression and start the use of cART as early as possible.
In the literature, cART is associated with improvement in anthropometric and survival indexes. In the review, it was observed that 42,203/51,992 (81.17%) children showed improvement in the growth pattern with the use of cART.
Of the articles included in the review, 42 have a degree of recommendation B ([i] one with level of evidence 2A; [ii] 35 with level of evidence 2B; [iii] six with level of evidence 2C) and two articles have level of evidence 1B (degree of recommendation A). Article classification for the level of evidence was performed according to recommendations of the Oxford Centre for Evidence-based Medicine.
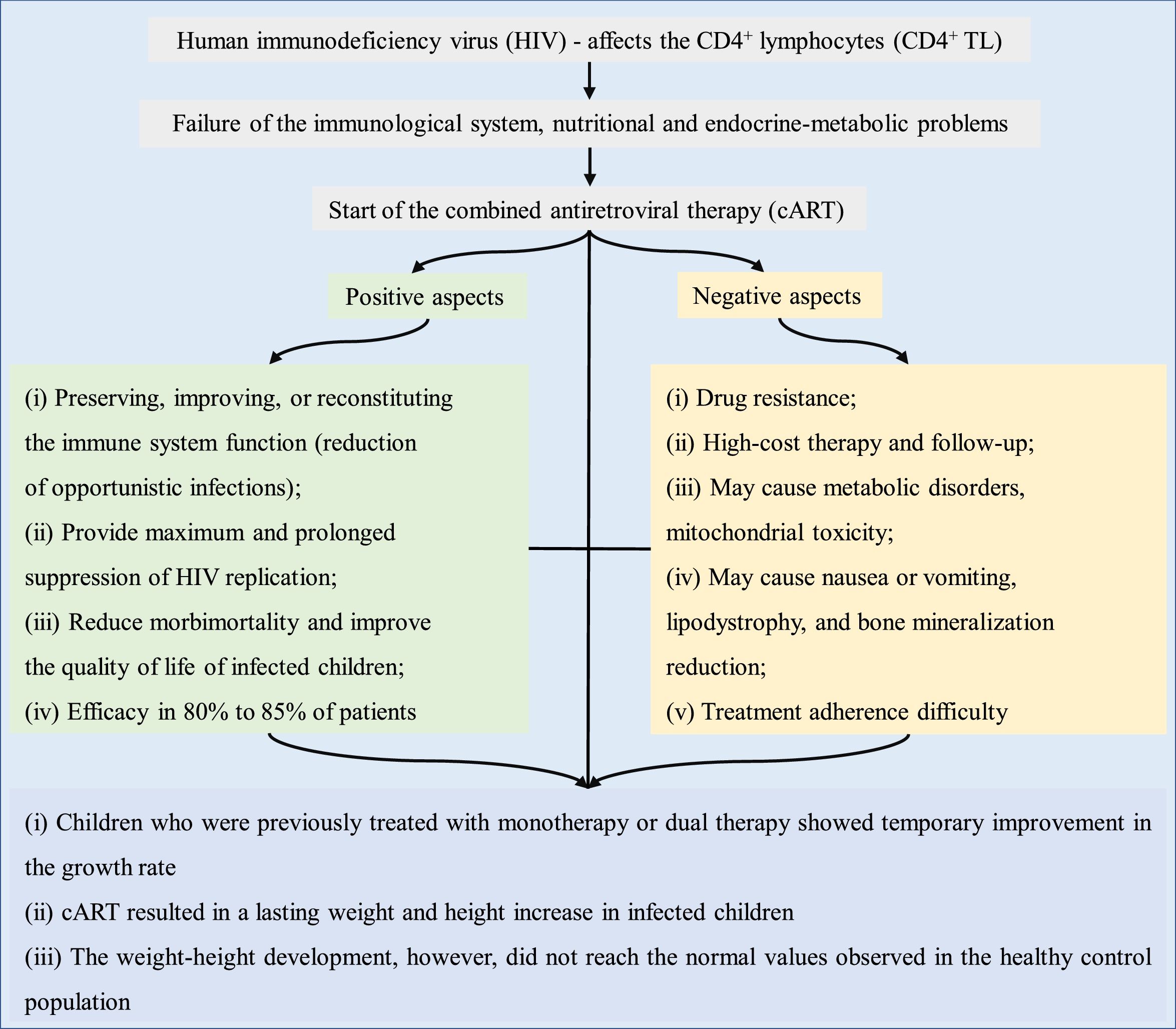
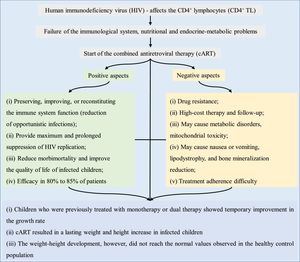
Fig. 2 briefly shows the growth-related outcomes regarding the use of cART in HIV-infected children and adolescents.
DiscussionChanges in growth are common in HIV-infected children.58 Growth disorders may be associated with: (i) opportunistic infections associated with the disease; (ii) absence of immunological and viral control at the follow-up; (iii) metabolic changes associated with HIV.50 However, after the start of cART, changes in growth pattern have been used as effectiveness parameters in the treatment of children and adolescents with HIV.
Based on this review carried out over the last 21 years, it can be observed that most of the studies showed a positive association in the height-age and weight-age Z-score with the use of cART (Table 1). In addition to the anthropometric improvements, the literature demonstrates that cART has shown a decrease in morbidity, mortality, and hospitalization levels of patients infected with HIV. Treatment with cART increases CD4+ TL counts, reduces viral replication, and partially restores the immune system. As a result, it reduces the incidence of opportunistic infections.54,58 In 2003, Benjamin et al. reported that children using cART had fewer alterations in the immune system, a better response to the virus, and a better height-age Z-score curve.4 Verweel et al., in 2002, showed decreases of 63.69% in hospitalizations after the introduction of cART.54
The literature suggests that children who were treated in the past with monotherapy or dual therapy showed a temporary improvement in the growth rate. However, cART results in a lasting increase in the weight and height of infected children. This increase, however, did not reach the values close to those of the general population. In 2008, Buonora et al. demonstrated that, even with cART, infected patients had lower growth parameters than the non-HIV infected population.41 Another study assessed that, after the introduction of cART, weight-age Z-score was close to that of the population without HIV infection, regardless of the time of treatment start. In turn, the height-age Z-score remained low.26 Similar findings were observed in Europe, where after comparing growth curves of infected and non-infected children for ten years, it was concluded that HIV infection results in slower growth rates and that the differences increase with age.53
Younger children may show greater weight and height recovery after the start of cART. Nachman et al. reported in 2005 that children younger than two years of age had a higher height Z-score during therapy.49 Weight gain was also predominantly higher in younger patients.49 In 2013, Shiau et al. evaluated a four-year follow-up of 195 AIDS patients and showed that patients who started cART before 6 months of age showed a faster improvement in weight-age and height-age Z-scores than those who started therapy later.26 Therefore, the earlier the cART is initiated, the lower the growth deficit.26,37
Another important finding indicated in the studies refers to the nutritional status before treatment, as it can influence the anthropometric outcomes after the introduction of cART. Bandyopadhyay and Bhattacharyya showed in 2008 that malnourished children have worse results in the evolution of weight-age and height-age Z-scores compared to those who started therapy under adequate nutritional conditions.44
In the review, one of the main limitations was the inclusion of many studies conducted in Africa, where nutritional deficiency may indirectly influence growth control and may represent a bias in the use of cART. Malnutrition and growth retardation occur early in HIV vertical transmission, and the negative impact of chronic infection and nutritional deficiency on growth may be irreversible.32 However, the lack of growth pattern improvement occurred in studies carried out during a short period of time. For instance, in the 2011 study by Devi et al., who assessed 102 patients only in the first year of cART use.32
Furthermore, the review did not compare the impact of different combined antiretroviral therapies with each other. Furthermore, it was not possible to analyze growth by age group. None of the studies compared groups undergoing cART, with and without virologic failure, to assess growth.
Some studies have also highlighted the need to assess drug interactions in children and the potential adverse effects of cART on metabolism, but studies are still scarce in this age group. It is noteworthy that the possible metabolic effects of cART include insulin resistance, dyslipidemia, hyperlactatemia, and that such changes may lead to an increased risk for cardiovascular diseases and diabetes. The elevation of total cholesterol and lipodystrophy associated with the use of cART remains a challenge in the treatment of these patients. Factors associated with children's adherence to cART showed that low adherence was strongly associated with the caregivers’ profile (low level of schooling and/or degree of poverty).
Based on the data described herein, the possible adverse effects of cART do not outweigh its benefits in terms of weight and height development, morbidity, and mortality in HIV-infected children. cART is known to play a role in the improvement of viral and immunological markers and may influence the growth of HIV-infected children. However, the increase in anthropometric markers (weight and height) did not normalize the growth when compared to the healthy population. The earlier the diagnosis of the HIV infection and, concurrently, of malnutrition and the initiation of cART, the lower the growth deficit when compared to healthy children. Patients with AIDS and without previous treatment for antiretroviral therapy show better anthropometric response to cART than previously treated patients. However, unfavorable socioeconomic and intrafamilial conditions influence treatment adherence, representing a limiting factor for more significant and long-lasting results of the use of cART in HIV infection treatment. Finally, mainly in countries with low economic resources to quantify the viral load, the improvement of anthropometric data during the use of cART as a parameter of therapeutic efficacy is more evident.
FundingFALM, research support provided by Fundação de Amparo à Pesquisa do Estado de São Paulo – post-doctoral fellowship (# 2015/12858-5).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Golucci AP, Marson FA, Valente MF, Branco MM, Prado CC, Nogueira RJ. Influence of AIDS antiretroviral therapy on the growth pattern. J Pediatr (Rio J). 2019;95:7–17.
Study conducted at the Faculdade de Ciências Médicas, Universidade Estadual de Campinas, Campinas, SP, Brazil.