To evaluate the therapeutic effect of Saccharomyces boulardii supplementation on jaundice in premature infants undergoing phototherapy.
MethodsIn this article, the authors reviewed 100 hospitalized jaundiced premature infants under 35 weeks of gestational age. All infants were assigned to a control group (n = 45) and a treatment group (n = 55) randomly. The infants in the treatment group received S. boulardii supplementation by undergoing phototherapy and the infants in the control group were only treated by phototherapy. The total serum bilirubin levels were detected before and at the end of phototherapy, and transcutaneous bilirubin levels were measured on the 1st, 4th, 8th and 15th day of treatment. The duration of jaundice resolution and phototherapy, stool frequency, and characteristics were compared after phototherapy.
ResultsThe duration of jaundice resolution and phototherapy were shortened. Total serum bilirubin level was lower than the control group at the end of phototherapy (p < 0.05). Transcutaneous bilirubin levels decreased more significantly on the 8th and 15th day of treatment (p < 0.05), while there were no significant differences on the post-treatment 1st and 4th day (p > 0.05). In addition, bowel movements including stool frequency and Bristol Stool Form Scale ratings of stools also improved after treatment.
ConclusionsS. boulardii in combination with phototherapy is effective and safe in reducing bilirubin levels and duration of phototherapy, accelerating jaundice resolution in premature infants with jaundice. The procedure also provided an ideal therapeutic effect of diarrhea induced by phototherapy to promote compliance and maternal-infant bonding.
Neonatal jaundice is a disease that causes yellowing of the skin, conjunctiva, and sclera due to elevated serum or plasma bilirubin within two to three days of birth. It is typically a mild and brief event, especially in cases of preterm labor. Normally, the liver removes excess bilirubin from the body. However, many premature babies have immature livers that cannot effectively filter substances from the blood.1 This is believed to be the basic cause of neonatal jaundice.2
Nearly all premature infants, less than 35 weeks of gestational age (GA), have elevated serum/plasma total bilirubin (TBILI) levels. After adjustment for gestational and postpartum age, premature infants are more likely than term (or late) infants to develop bilirubin-induced neurological dysfunction (BIND), which may lead to chronic neurological sequelae if not treated promptly or appropriately.3 Due to the delayed and insufficient colonization of the gastrointestinal flora in the early stage of neonates, there are a relative few microbiota involved in bilirubin metabolism, resulting in a decreased conversion of conjugated bilirubin to stercobilin, which is excreted through feces. On the other hand, β-glucuronidase converts conjugated bilirubin to unconjugated bilirubin in the intestine, which is absorbed by the intestinal mucosa and reintroduced back to the liver to be recycled for bile production. The unconjugated bilirubin accumulates in the intestine and recirculated to the blood in a loop of continuous enterohepatic circulation, leading to an increase in serum bilirubin levels.4 In addition, breast milk β-glucuronidase seems also to be an important factor in neonatal hyperbilirubinemia.5
Phototherapy is the most common intervention for the treatment and prevention of severe hyperbilirubinemia. It is considered a safe intervention because it has been widely used in millions of infants although there are concerns about the increased mortality rate of extremely low-birth weight infants due to aggressive phototherapy6 and bronze baby syndrome.7 The mechanism of phototherapy involves photochemically altered excretion of (ZE)-bilirubin and (EZ)-/(EE)-cyclobilirubin.8 The former is unstable and returns to (ZZ)-bilirubin in bile or intestinal lumen, then reabsorbed into the enterohepatic circulation, resulting the high bilirubin levels, while the latter isomer is excreted in the feces. Phototherapy increases the biliary excretion of unconjugated bilirubin (ZZ-bilirubin). In this form, the excreted bilirubin would be susceptible to the enterohepatic circulation and the real efficacy of phototherapy is diminished.9 Moreover, it would appear that jaundiced neonates were likely to develop loose stools undergoing phototherapy due to a high concentration of bile salts found in colonic contents10 instead of lactase malabsorption,11 while accelerated meconium passage did not affect the degree or duration of hyperbilirubinemia.12
Saccharomyces boulardii is a facultative anaerobe, which is not absorbed in the gastrointestinal tract and has natural resistance against gastric acid and bile. After ingestion, S. boulardii is distributed throughout the digestive tract. Its probiotic activity has been linked to multiple pathways, including improvement of the gut barrier function, competitive exclusion of pathogens, production of antimicrobial peptides, immunomodulatory and nutritional effects. If S. boulardii could inhibit the reabsorption of unconjugated bilirubin (ZZ-bilirubin) from the enterohepatic circulation and cause a reduction in phototherapy-induced diarrhea, it may be used to treat jaundice and have positive effects on improving compliance and infant-mother bonding. Therefore, the purpose of this study was to evaluate the therapeutic effects of S. boulardii-assisted phototherapy in premature infants with jaundice and to provide a basis for intervention research.
MethodsGeneral informationThis was a prospective open-label study of premature infants admitted to the neonatal intensive care unit (NICU) from February 2013 to March 2016. This study adhered to the tenets of the Declaration of Helsinkin (as revised in 2013) and was approved by the Ethics Committee of The Second Hospital of Tianjin Medical University, China. Informed consent was obtained from all infants’ parents or guardians. All methods were carried out under relevant guidelines and regulations of China. The inclusion criteria were: (1) premature infants born under 35 weeks GA; (2) the laboratory test results of the enrolled children met the indication criteria for phototherapy; (3) the enrolled children had symptoms such as yellowing of the skin and sclera; and (4) the child's family allowed participation in the study. Infants with these criteria were excluded: (1) term or late infants; (2) vital organ diseases; (3) obstructed bile excretion; (4) malformations of the digestive system; and (5) incomplete medical records. They were randomly assigned to the treatment group and the control group by using SPSS Statistics 20.0 (IBM Corp., Armonk, NY, USA) to achieve random grouping.
Treatment methodsThe treatment group was administered one pack of S. boulardii (Florastor®; Biocodex Pharmaceuticals & Healthcare, France) daily for 15 days. S. boulardii is a live microbiota agent. Each sachet contained 765 mg of powder and 250 mg of fungus. The amount of viable microbiota per 1 g of powder was not less than 1.3 × 109 colony forming units (CFU). This was mixed with 10 mL of sterile water and administered orally. The dosage of S. boulardii supplement was determined empirically based on results from the previous study in children13,14 and guidelines of ESPGHAN/ESPID.15 The infants in the control group were fed without S. boulardii. All infants received phototherapy for 12 h daily.
Transcutaneous bilirubin (TcB) was measured on the forehead or the sternum with transcutaneous jaundice meter (JM – 103; Draeger Medical, Inc, USA), out of direct sunlight and away from areas with hair or other skin abnormalities. Venous blood was collected for total serum bilirubin (TSB) to determine whether to discontinue phototherapy if TcB was 34.2 - 51.3 μmol/L (2 - 3 mg/dL)16 below the low risk level (P40) of Hour-Specific Total Serum Bilirubin Percentiles of the local pediatrics society of medical association. The phototherapy lamp (BT-400®; Bistos Co., Ltd, Korea, wavelengths: 450 – 470 nm, irradiation dose: 8 −10 μW(cm2·nm)) was placed 20 −50 cm from the skin. The room temperature was set to 26 - 28 °C, and the humidity was maintained at 55 - 65%.
Nursing measuresThe nursing staff informed the family members about neonatal jaundice, explained its causes, symptoms, and hazards of jaundice, and ensured compliance. Treatment was not withheld if the crying baby was difficult to appease and/or the parents were stressed.
The babies were checked for skin lesions or bleeding from the umbilical cord. Tight gloves were placed on their hands to prevent scratches, a black eye patch was used for protection against blue light, and diapers were used to cover the genitals. The head was kept tilted to one side to avoid vomiting milk. Furthermore, body position was changed every three hours to ensure uniform exposure to blue light.
Increased skin water loss in preterm infants is imperceptible due to increased mass surface area and thin dermis. Phototherapy increases water loss from the skin in infants.17 Therefore, it is crucial to assess dehydration by some of the following indicators: (1) weight loss: mild dehydration (4 - 5% loss of body weight); moderate (6 - 9% loss of body weight); severe (greater than 10%); (2) urinary excretion; (3) mental status (restlessness or irritability); and (4) sunken eyes, dry lips, wrinkled skin and other clinical features. Timely feeding was ensured to prevent dehydration. If the child was dehydrated, fluid and electrolyte replacement was provided. The head was raised, and the proper lateral position for feeding was maintained. The feeding amounts, stool frequency, and vomitus volumes were recorded.
As the thermoregulatory centers of premature infants are immature, an appropriate temperature of the phototherapy box was maintained. An abnormal body temperature may occur during treatment. The nursing staff recorded the body temperature every hour, adjusted the temperature in the box according to the temperature change, and recorded it every four hours after stabilization. If the body temperature exceeded 38 °C, body cooling was performed. The child's consciousness level and emotions were observed, vomiting, apnea, and hypo-responsiveness were monitored, any abnormal situation was promptly reported, and the parents were reassured.
Observation indices- (1)
The durations of jaundice resolution and phototherapy were recorded.
- (2)
TSB values were compared before and after phototherapy.
- (3)
TcB values were recorded on the post-treatment 1st, 4th, 8th and 15th day. Calibrate the jaundice meter before each measurement according to the manufacturer's instructions to ensure measurement accuracy.
- (4)
Stool frequency and characteristics were compared after phototherapy. Stool characteristics were assessed using the Bristol Stool Form Scale (BSFS), which rates stool type on a scale of one to seven. The standard score is four; the lower the rating, the more severe the constipation, and the higher the rating, the more severe the diarrhea.
All data were analyzed using SPSS Statistics 20.0 (IBM Corp., Armonk, NY, USA), with t and “ࣥx ± s” to represent the measurement data. P < 0.05 indicated statistically significant differences.
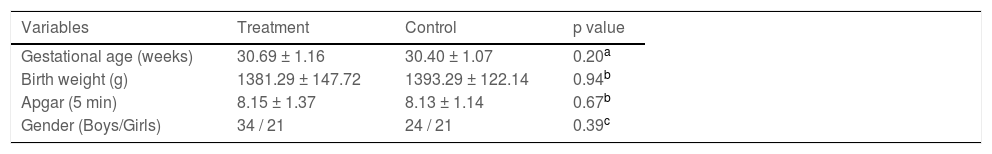
ResultsA total of 100 premature infants (58 males and 42 females) were enrolled in this study. The sample included 39 cesarean sections and 61 natural deliveries. The characteristics of premature infants in this study are listed in Table 1 including gestational age, birth weight, Apgar (5 min) and gender. The data and procedure variables were analyzed by using t-test, Mann-Whitney U test or chi-square test, and did not indicate any statistically significant difference.
Baseline characteristics of the included infants (X¯ ± s).
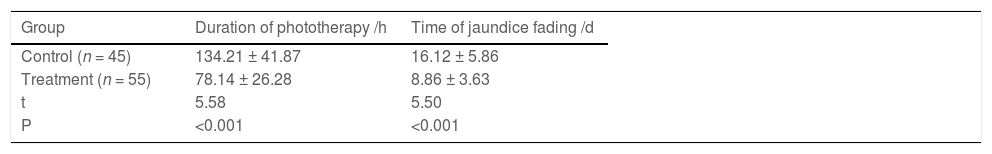
In this study, jaundice faded on (8.86 ± 3.63) days in the treatment group. Additionally, the duration of phototherapy was (78.14 ± 26.28) hours, which were (16.12 ± 5.86) days and (134.21 ± 41.87) hours respectively in the control group (p < 0.05) (Table 2).
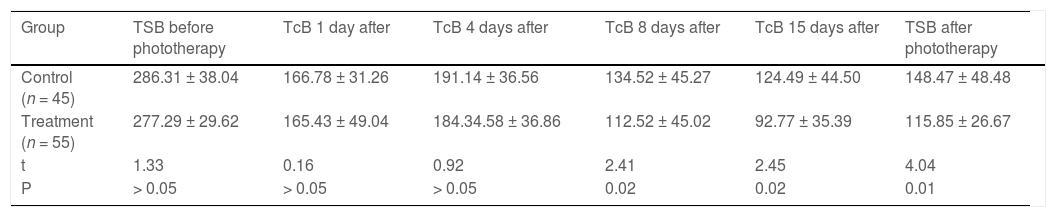
Total serum bilirubin levelsTable 3 shows TSB levels of infants. Although there were no significant differences before treatment (p > 0.05), TSB level was much lower than the control group at the end of phototherapy (p < 0.05).
Total Serum bilirubin (TSB) and Transcutaneous bilirubin (TcB) levels during the treatment (X¯ ± s, μmol/L).
TcB levels of the two groups were similar at the beginning of treatment (p > 0.05). TcB levels decreased significantly on post-treatment 8th and 15th day than the control group (p < 0.05), but with no significant differences on the 4th day of treatment (p > 0.05) (Table 3).
Defecation statusAfter phototherapy, the rating of stool characteristics based on BSFS in the treatment group was (4.53 ± 0.43), the mean stools per day were (1.43 ± 0.41), which were significantly better than the control group (p < 0.05) (Table 4).
DiscussionsEarly research elucidated that the intestinal tract in the early stage of neonates is rich in oxygen, the first to colonize are aerobe and facultative anaerobe, such as Escherichia coli and Staphylococcus spp.18 Over time, there is a shift from aerobe and facultative anaerobe to anaerobe in the infant's intestinal flora species, such as Bifidobacterium and Lactobacillus.19S. boulardii can directly adsorb flagellated bacteria and consume oxygen in the intestine, resulting in a decrease of aerobe and facultative anaerobe such as E. coli20 and Staphylococcus spp,21 while an increase in the content of obligate anaerobe such as Bacteroides.22 Wallace et al.23 found that β-glucuronidases produced by these intestinal florae can be divided into two types: loop-containing β-glucuronidase (L-GUS) and no-loop β-glucuronidase (NL-GUS). GUS enzymes from these categories are also shown to exhibit different functional capacities. E. coli and Staphylococcus spp mainly produce L-GUS in vivo, while Bacteroides mainly produce NL-GUS. L-GUS with longer loops processes the glucuronide substrate. Bilirubin glucuronides are deconjugated by L-GUS, thereby resulting in the reabsorption of unconjugated bilirubin into the blood.24 Moreover, S. boulardii is able to reduce the pH of the intestine, suppressing the activity of the β-glucuronidase to decrease the enterohepatic circulation.25 Therefore, S. boulardii was suspected to affect the enterohepatic circulation of bilirubin by altering the content, structural differences and activity of β-glucuronidase in the intestine, thus causing changes in serum bilirubin levels.
A total of 100 premature infants were investigated in this study. Out of these 55 infants received S. boulardii supplementation and 45 were taken as controls. Research data indicate that S. boulardii shortened the duration of phototherapy (78.14 ± 26.28) hours compared with the control group (134.21 ± 41.87) hours, and had effects on accelerating jaundice resolution (8.86 ± 3.63) days in premature infants of the treatment group, which were (16.12 ± 5.86) days in the control group (p < 0.05). Perhaps differences in the duration of phototherapy cannot alter the length of hospitalization period in premature infants with longer hospital stays. But when considering the complications of phototherapy, the duration is shorter the better.
Jaundice usually first appears on the face and progresses to the tail of the trunk and extremities, but since visual bilirubin levels can be erroneous based on the degree of jaundice, the best documented method to assess the risk of subsequent hyperbilirubinemia is to measure TSB or TcB levels. However, both visual assessment of jaundice and TcB measurements in infants undergoing phototherapy are unreliable because phototherapy can "bleach" the skin.26 TSB is required as the gold standard for the diagnosis of neonatal jaundice, with few interference factors and high accuracy. Nonetheless, frequent venous blood collection will carry an increased risk of skin damage, bleeding, infection and places great stress on frail babies and their parents. Therefore, in most infants with TSB levels of less than 257 μmol/L (15 mg/dL), noninvasive TcB meter can provide a valid estimate of TSB levels.27 In this study, TSB and TcB levels were combined to use. TSB values were measured before and after phototherapy as a criterion for the beginning and end of treatment. TcB values were measured with a non-invasive device to assess the dynamic changes of TSB levels undergoing phototherapy. The present data demonstrated that TcB levels of post-treatment 8th and 15th day and TSB levels after phototherapy have more marked reductions (p < 0.05) although there was no statistical significance before and on the 1st and 4th day of treatment than the control group. Some researchers had pointed out that S. boulardii did not affect hyperbilirubinemia significantly, the reason possibly attributed to the short period of time that TSB levels were measured after administration of S. boulardii. Serce et al. did a prospective randomized controlled trial and they found no statistically significant difference in the data between TSB levels at the beginning and within 96 h,28 lacking of a long-term observation of changes in TSB levels.
Moreover, data are conflicting on the association between phototherapy and diarrhea. John29 pointed out that 9.5% of term infants appeared loose green stools, whereas most preterm infants in this study developed mild to moderate diarrhea (BSFS rating: 6.05 ± 1.26, stool frequency: 4.37 ± 1.28) undergoing phototherapy in the control group. This phenomenon can be attributed to various reasons, maybe not only phototherapy induced, including infections or indigestion, or preterm infants were more likely to develop diarrhea than term infants undergoing phototherapy. After phototherapy, stool frequency of the treatment group was 1.43, with the BSFS standard type stool after phototherapy, better than the control group (p < 0.05). This indicated improvement in the bowel movements of premature infants. It should be emphasized that premature infants with jaundice are different from that in adults, or even full-term infants. Although phototherapy-induced diarrhea is not a serious clinical problem, frequent loose stools often present a challenge for parents and families. They may be suffering from great psychological distress to withdraw from the treatment. Compliance is a major challenge because most infants are irritable and uncomfortable, which affects treatment outcomes. S. boulardii effectively resolved phototherapy-induced diarrhea and improved compliance.
Re-admissions due to high levels of unconjugated bilirubin require phototherapy and incur additional costs to the family and health facilities. They also pose emotional and breastfeeding problems, and are one of the reasons for early weaning.30 A combined regimen improved outcomes and reduced the influence of phototherapy on maternal-infant interaction.
In conclusion, the combination of orally administered S. boulardii and phototherapy was rapid, safe, and effective in the treatment of premature infants with jaundice, without noticeable side effects. The authors performed blood cultures and observed no infections associated with the probiotic strain. Additionally, the authors also observed that premature infants might be more likely to develop diarrhea undergoing phototherapy, and S. boulardii supplementation well solved the clinical problem. Therefore, this approach may be useful in clinical practice.
LimitationLimitations included the study was not analyzed in preterm infants with different gestational ages and different severities of jaundice. Moreover, a more comprehensive interpretation requiring a comparison of the safety of S. boulardii used in premature infants considered immunocompromised in the NICU is still under investigation.
Ethics approval and consent to participateThis study adhered to the tenets of the Declaration of Helsinkin (as revised in 2013) and was approved by the Ethics Committee of The Second Hospital of Tianjin Medical University, China. Informed consent was obtained from all infants’ parents or guardians. All methods were carried out under relevant guidelines and regulations of China.
Availability of data and materialsAll the data supporting the present findings is contained within the manuscript.
The authors thank LetPub (www.letpub.com) for linguistic assistance and pre-submission expert review.