To assess the prevalence of GERD exclusively by means of multichannel intraluminal impedanciometry associated with pH monitoring (MIIpH) and compare it with respiratory symptoms in children with CF. To compare MIIpH with pHmetry alone to perform GERD diagnosis.
MethodsAn analytical cross-sectional study was conducted with children diagnosed with CF who underwent MIIpH. Clinical and laboratory markers, including respiratory and digestive symptoms, were used for comparative analyses. High-resolution chest computed tomography was performed on patients with symptoms of chronic lung disease. Severity was classified according to the Bhalla score.
ResultsA total of 29 children < 10 yo (18 girls) were evaluated; 19 of whom with physiological GER and 10 with GERD. Of the children with GERD, seven had predominantly acid GER, two acid+non-acid GER, and one non-acid GER. Three patients had GERD diagnosed only by MIIpH. Bhalla scores ranged from seven to 17.75 with no significant relationship with GERD. The number of pulmonary exacerbations was associated with a decrease in esophageal clearance regardless of the position in pHmetry and MIIpH.
ConclusionsThe prevalence of GERD was 34% in children with CF. There was no association between respiratory disease severity and GER types. MIIpH detected 30% more patients with GERD than pHmetry.
Gastroesophageal reflux disease (GERD) mainly affects children and adults with cystic fibrosis (CF) compared to healthy individuals and contributes to the worsening of lung disease (LD).1,2 The prevalence of acid gastroesophageal reflux (AGER) in CF ranges from 35-81%.3 To date, it has not been defined whether GERD in CF is a primary or secondary phenomenon to LD.4
Gastroesophageal reflux (GER) is physiological; if the reflux episodes cause symptoms or complications, they evolve into GERD.5 Currently, multi-channel intraluminal impedance associated with pHmetry (MIIpH) and pHmetry has been widely used to perform GERD diagnosis5 with high specificity6 and sensitivity.6-9
Many CF children show early LD symptoms. Most studies evaluating the prevalence and association of GERD and CF provide heterogeneous information about age, and tools used to diagnose GERD and Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) genotype.10-14
This study investigated GERD exclusively by means of MIIpH and aimed: (i) to assess the prevalence of GERD in CF children with and without typical GERD manifestations; (ii) to compare MIIpH and pH monitoring values during 24 hours for GERD diagnosis; and (iii) to analyze the association between episodes of GER and MIIpH and clinical symptoms.
MethodsAn analytical cross-sectional study was conducted in CF children <10 yo, who received follow-up care at the CF Referral Center of a tertiary hospital for two years. Patients with CF detected through newborn screening were included,2,3 namely with two sweat chloride specimens ≥60 mEq/L and/or two pathogenic variants in the CFTR gene.2 After the Ethics Committee's approval, the study was conducted in agreement with the Declaration of Helsinki (CAAE: 60629616.2.0000.5404).
Clinical data were collected using medical records and interviews with parents/guardians. All patients were clinically stable and showed no pulmonary exacerbation at the examination. The following markers were assessed: (i) Clinical markers: sex, age, reported race, family history of CF, chronic cough, recurrent wheezing, number of previous cases of pneumonia, number of episodes of unexplained LD (Apparent life-threatening events/Brief resolved unexplained events), laryngitis, dysphonia, otitis media, number of pulmonary CF exacerbations, regurgitation, vomiting, abdominal pain, diarrhea, low weight gain, anemia, dental erosion, Sandifer syndrome, pancreatic sufficiency or insufficiency, medications used, weight, height, body mass index; and (ii) Laboratory markers: immunoreactive trypsinogen levels (IRT), sweat test, CFTR genotype, sputum culture, high-resolution chest computed tomography (HRCCT) using the Bhalla's scoring system,15 and esophageal monitoring with MIIpH.
Two pediatric pulmonologists and a radiologist analyzed the HRCCT images without knowing the patient's clinical evolution. The modified Bhalla score, the Fleischner Society guidelines, and the Brazilian consensus on HRCCT were utilized for analysis. Discordant screening readings were consensually reviewed by the evaluators to obtain the final score. The scores ranged from zero (no abnormality) to 37 points (severe changes).15 HRCCT was performed in children with a clinical indication from the referral center team.
MIIpH was monitored during 20-24 hours. A software-based analysis was made and two MIIpH probes (ZIN: ≤2 yo and ZPN: >2 yo) from Sandhill Scientific-ComforTec Z/pH, Inc., Highlands Ranch, CO, USA were used. After calibration, the probes were placed transnasally according to the modified Strobel formula: [(height×0.252)+5]×0.8718.6 The probe position was checked using radiographic scanning.6
All parents/guardians were instructed to follow their children's daily routine and report symptoms, times of meals, and position (orthostatic: sitting or standing; supine: lying; overall: orthostatic+supine). Data were analyzed with BioVIEW Analysis version 5.6 (Sandhill Scientific). A software-based analysis was conducted, followed by a manual review of each test in pairs, i.e., by the leading author and another author – a pediatric gastroenterologist. A reflux episode was considered GER when the impedance wave was retrograde in at least two distal channels, with a drop of 50% or more from baseline, and duration ≥5 seconds;6 AGER and non-acid GER (NAGER) episodes, if the distal pH electrode remained below or above 4.0, respectively.6 The refluxate was considered to be proximal if it reached either or both of the proximal channels (channels 1 and/or 2).6,16 In pHmetry alone, the reflux index (RI) was used to define GERD, representing pH <4.0. When RI ≥10% in children <1 year or ≥5% in children >1 year, the episode was considered GERD.5
The parameters evaluated were: (i) pHmetry: RI, GER episodes, mean acid clearance time, GER >5 minutes, duration and position of the longest episode of GER, and patient's final score according to age (Boix-Ochoa <2 years and Johnson/DeMeester 2-10 years); (ii) MIIpH: monitoring period in hours and minutes; proximal and distal episodes of GER, AGER, and NAGER; percentage of reflux exposure time; and bolus contact time. The parameters were evaluated in total number, and in the orthostatic and supine positions and correlation with symptoms.6,8
The relationship between GER episodes and symptoms was considered positive in the presence of two or more of the following indexes: symptom index (SI) >50% and/or symptom sensitivity index (SSI) ≥10% and symptom association probability index (SAP) >95%.6,8,16 SAP values >95% for all symptoms, including cough, were considered altered.
For MIIpH, the normality values by Mousa et al. (2014)8 were considered for both pediatric age groups: ≤12 months and 1-17 yo (Supplementary material 1).
The authors considered the presence of GERD in MIIpH when altered parameters occurred in pHmetry and/or MIIpH correlated with symptoms.5,8
Statistical analysisThe descriptive analysis was performed according to data distribution using a number of individuals (N) and percentage for categorical data; means (standard deviation) or medians (interquartile range) for parametric and non-parametric data, respectively. Normality was assessed using descriptive measures for central tendency, graphical method (Normal Q-Q plot, Q-Q plot without trend and Boxplot), and statistical tests (Kolmorov-Smirnov and Shapiro-Wilk tests).
Intergroup comparisons of numerical data were performed with the Mann-Whitney test; the Chi-square and Fisher's Exact tests, for categorical data. Additionally, the Spearman correlation was applied for clinical and laboratory markers and MIIpH parameters. Alpha was set at 0.05, and no techniques were employed to impute missing data values. All statistical analyses were performed with Statistical Package for the Social Sciences version 24.0 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp).
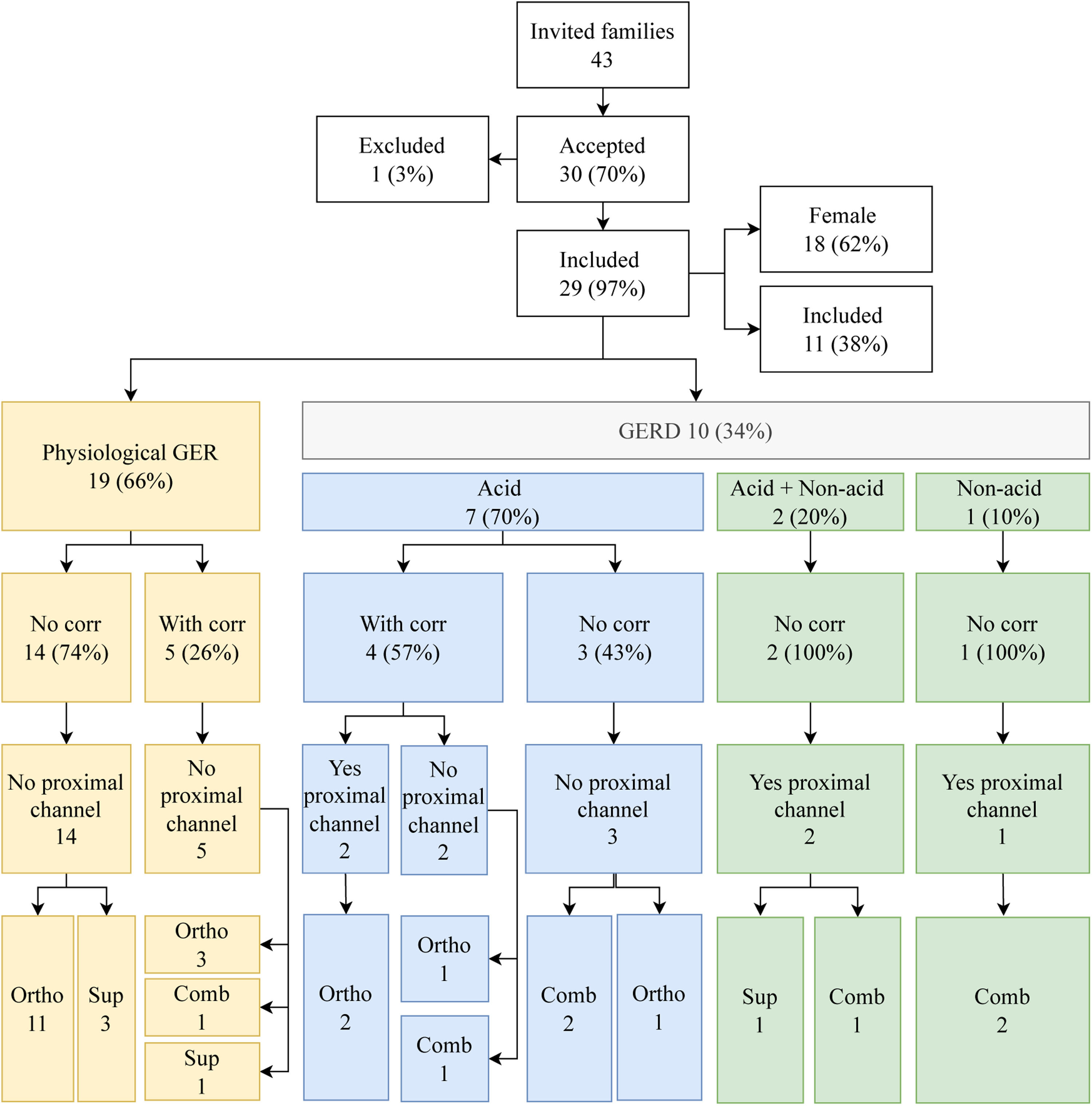
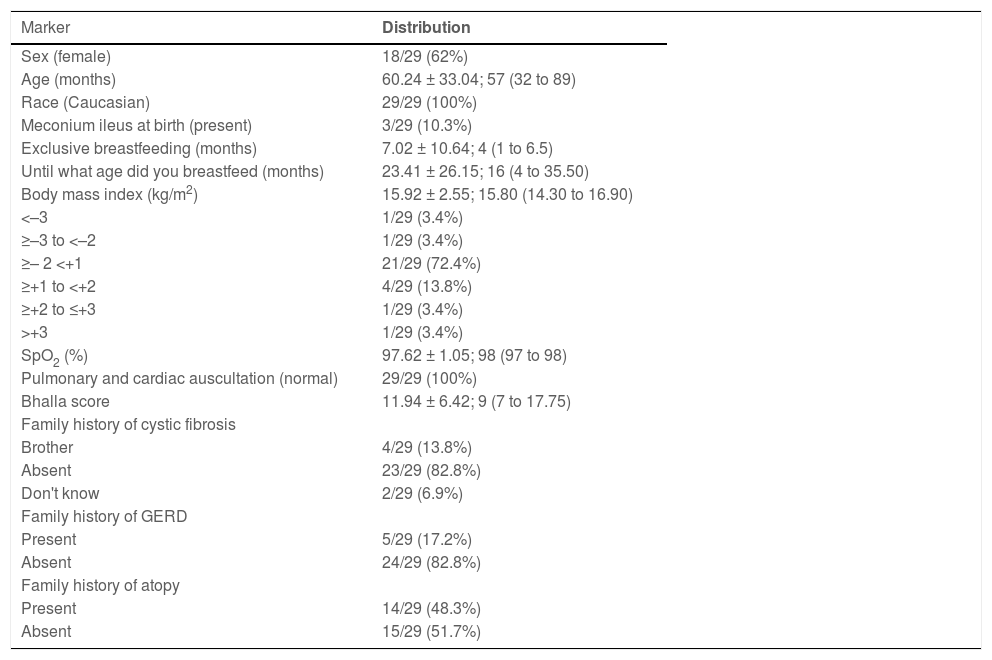
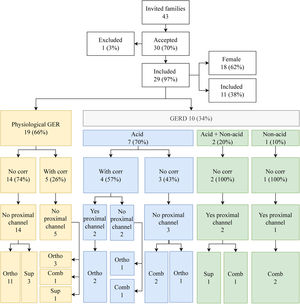
ResultsOf the 43 children invited to participate in the study, 30 accepted and underwent MIIpH. One patient was excluded due to an artifact caused by monitoring probe problems. Eighteen of 29 (62%) patients were females; 19/29 (65.5%) were 2 years or older, and 28/29 (96.6%) had pancreatic insufficiency (Table 1). The descriptive analysis of the demographic data of the CF children is described in Table 1.
Descriptive analysis of demographic data, peripheral oxygen saturation (SpO2) and Bhalla score, and family history of patients with cystic fibrosis.
GERD, Gastroesophageal reflux disease. We presented the data by absolute and relative frequency or mean ± standard deviation; median (25th to 75th percentile).
A total of 25/29 (86.20%) patients were identified with the p.Phe508del (F508del) variant of the CFTR [19/29 (65.61%) heterozygotes and 6/29 (20.68%) homozygotes]; 3/29 (10.3%) patients did not have the CFTR genotype, and 1/29 (3.44%) patient had p.Gly542X/p.Gln1100Pro (G542X/Q1100P) genotype.
Analyses of clinical and laboratory markers showed that 28/29 (96.5%) patients had one or more pulmonary exacerbations; 25/29 (86.2%) had chronic colonization by Staphylococcus aureus or non-mucosal Pseudomonas aeruginosa; 7/29 (24.13%) by Burkholderia cepacia and 6/29 (20.08%) by mucoid Pseudomonas aeruginosa.
The authors found 10/29 (34%) children with GERD further classified into AGER (7/10; 70%), AGER+NAGER (2/10; 20%), and NAGER (1/10; 10%). Four patients, all with AGER, showed a correlation with symptoms. Regarding the extension to the proximal channels, 5/10 (50%) had proximal reflux 2/5 (40%) AGER, 2/5 (40%) AGER+NAGER, and 1/5 (20%) NAGER (Figure 1).
Flow chart with the results of impedanciopHmetry, physiological GER (gastroesophageal reflux) and GERD (Gastroesophageal reflux disease), correlation with symptoms, extension to the position of the proximal channel associated with reflux of the patients with cystic fibrosis included in the study. Comb, combined; corr, correlation; ortho, orthostatic; sup, supine.
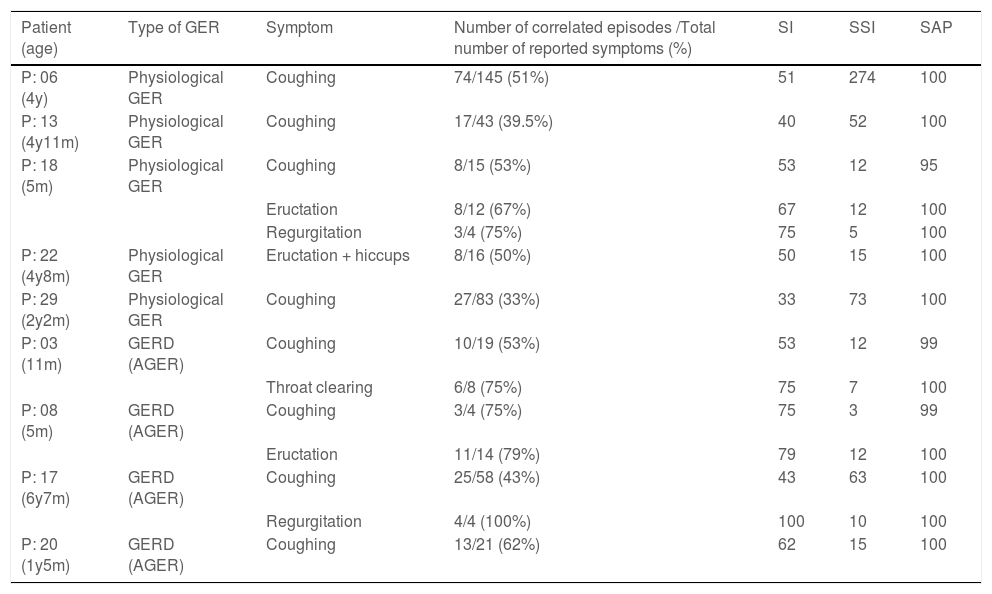
Table 2 summarizes the reported symptoms and the correlation with GER episodes based on two or more altered SI or SSI and SAP values, including tests, such as physiological GER and GERD at MIIpH. The authors observed that 9/29 (31.03%) children showed a correlation between GER episodes and symptoms. Among the children with physiological GER, the authors found a 5-month-old infant with cough in 8/15 (53%) records; eructation in 8/12 (67%) and regurgitation in 3/4 (75%); three children between two and four year olds with correlation only with cough, in 27/83 (33%), 74/145 (51%) and 17/43 (39.5%) records, respectively; and a 4-yo child with a correlation of GER with eructation+hiccups in 8/16 (50%) records.
Symptoms recorded during monitoring and correlation with GER episodes, based on two or more altered SI or SSI, and SAP score values, including tests analyzed as physiological GER and GERD by MIIpH.
AGER, gastroesophageal acid reflux; GER, gastroesophageal reflux; GERD, gastroesophageal reflux disease; m, months; MIIpH, multi-channel intraluminal impedance associated with pHmetry; P, number equivalent to each patient in the sample; SI, symptom index (positive if >50%)6,8; SSI, symptom sensitivity index (positive if ≥10%)6,8; SAP, symptom association probability (positive if >95%)6,8; y, year.
The description of the respiratory and digestive symptoms correlated with GER episodes in the patients with physiological GER and GERD does not show any association between these variables (Supplementary material 2).
The markers for identification, quantification, and localization of GER episodes in pHmetry and MIIpH are outlined in (Supplementary material 3). In pHmetry, there was a positive association between the number of episodes ≥5 minutes (p = 0.05) and the duration of the longest episode (p = 0.002) with GERD. In impedanciometry, GERD was positively associated with the total number of AGERs (orthostasis, p = 0.002) and acidic episodes in the proximal channels (overall and orthostasis, p = 0.021).
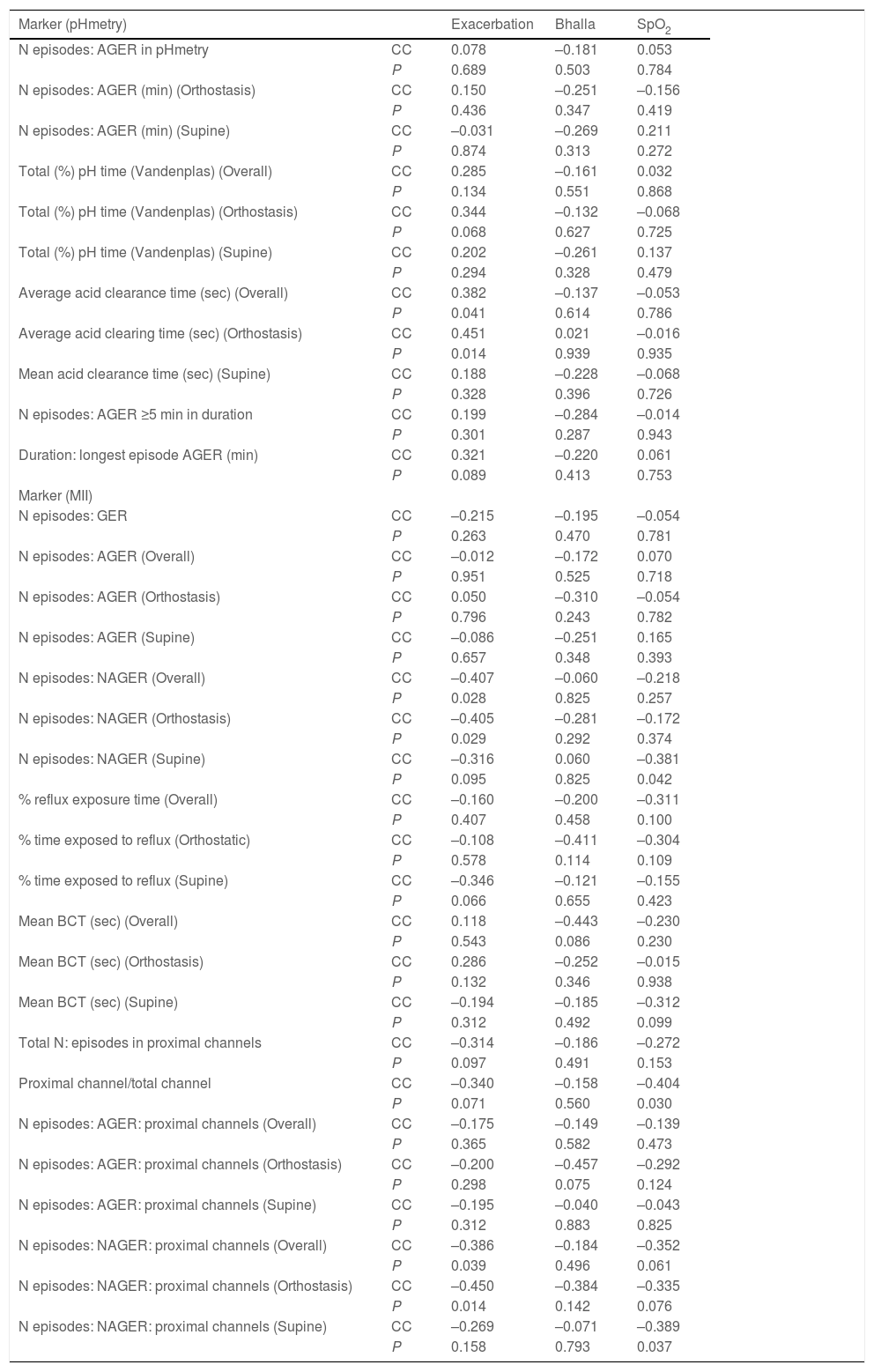
Table 3 displays the relationship of MIIpH markers with clinical and laboratory features in patients with CF. In pHmetry, the mean acid clearance time positively correlated with the number of exacerbations (P=0.014). In impedanciometry, there was an inverse correlation between total NAGER and the episodes reaching the proximal channels with exacerbations. An inverse relationship was observed between peripheral oxygen saturation (SpO2) and NAGER episodes in the supine position (total and proximal channels) (Table 3).
Correlation of pHmetry (pH) and multi-channel intraluminal impedance (MII) imaging markers with clinical and laboratory features in patients with cystic fibrosis.
AGER, acid gastroesophageal reflux; BCT, Bolus contact time; CC, Correlation Coefficient; GER, gastroesophageal reflux; min, minutes; N, number; NAGER, non–acid gastroesophageal reflux; SpO2, peripheral oxygen saturation; sec, seconds. We presented the data by absolute frequency and relative frequency or mean ± standard deviation; median (25th to 75th percentile). We performed the statistical analysis using the Spearman correlation test, and we adopted an alpha error of 0.05.
The MIIpH results did not have any statistically significant association with demographic, clinical, and laboratory markers (Supplementary materials 4 and 5). The values of IRT and chloride in the ST of the first and second specimens showed no statistically significant relationship with the evaluated parameters of MIIpH and pHmetry (Supplementary material 5).
DiscussionTo the best of our knowledge, this is the first study to assess the characteristics of physiological GER and GERD in CF children <10 yo in a single center using MIIpH. Previous research on MIIpH included children, adolescents, and adults, and, in some cases, it was performed in multiple centers.10-14,17,18
The pathophysiological relationship between esophagus reflux and the worsening of LD in CF is still poorly understood, in comparison with other LD, such as asthma,18-21 bronchopulmonary dysplasia,22,23 and chronic obstructive LD in adults.21
GER episodes are more common in CF patients, regardless of age, compared with their respective healthy counterparts. Moreover, GER is present in almost all end-stage LD CF patients.1
The present study showed a prevalence of GERD of 34% in CF children. This prevalence is high when compared with the prevalence of GERD in patients without CF, i.e., 3.5-6.2% in children and adolescents24 and 9-28% in adults.25 On the other hand, the 34% prevalence of GERD here is lower than that of most studies about GERD in CF, with values ranging from 35-81%.2,26 Furthermore, the authors have not found studies in the literature that included the age range to allow for a comparison of the prevalence of GERD in CF patients. One hypothesis suggested for the lower prevalence of GERD in the patients is the younger age.1 In addition, in CF, lung function (LF) decreases considerably with age and worsens the esophagogastric function.1
The population included children <10 yo, caucasians, eutrophic, and without other CF-related comorbidities, such as diabetes mellitus and liver disease. All children showed positive neonatal screening and molecular CF diagnosis, with CFTR class I and II mutations with at least one allele of the p.Phe508del variant in 86.2% of patients. These features have not been described in previous studies.10-14
Blondeau et al. (2010)10 performed a multi-center study using MIIpH to screen GERD in 24 CF children/adolescents of 0.3-13 yo. The authors confirmed a 67% prevalence of GERD; all cases were AGER, and 16 cases had no association with symptoms.
Dziekiewicz et al. (2015) analyzed GERD by MIIpH in a multi-center study of 44 children/adolescents aged 3-17 yo. The prevalence of GERD was 54%, with AGER predominance (77%), and 44% of reflux episodes reached the proximal channels.13
In a prospective study, Hauser et al. (2016) investigated the relationship between GER and gastric emptying in 56 CF children/adolescents, aged 1-17 yo, divided into two groups. Group 1 included 28 patients with GERD symptoms who performed a gastric emptying test and MIIpH; Group 2 had 28 patients with and without GERD symptoms who performed a gastric emptying test. The authors found a 46% prevalence of AGER, with 21.4% delayed gastric emptying, and no relationship between GER and gastric emptying.14
The analysis of laboratory markers of chronic infection in the patients is similar to that described in the literature about GERD in CF. Palm et al. (2012) investigated a group of 7-19 yo patients and found that reflux was common in CF children/adolescents, and AGER and NAGER were related to greater reductions in forced expiratory volume in the first second.11
The high prevalence of GERD is described in all international CF registries. It correlates directly with the deterioration of LF; and both increase with age,1,3,11,26 probably due to the vicious cycle between GERD and chronic LD.3,11,21 There is no consensus on whether GERD is a primary or secondary phenomenon to LD in CF.4
The relationships between GERD and LD are complex, and controversies about their interaction abound in the literature. A classic example of this controversy is asthma: some studies show a positive relationship between GERD and asthma;18 others yield diverging results for this association.19,20 Here, this relationship shows a clear positive association between acid clearance and pulmonary exacerbations.
In the present study, of the 19 patients classified with physiological GER, five (26%) showed a significant relationship with symptoms; however, they did not meet other criteria for GERD diagnosis by pHmetry and/or MIIpH and were not considered GERD. Some questions need to be answered: Is the relationship between reflux episodes and the symptoms real? Can the high cough frequency alone define GERD? Is it a coincidence that children who already cough a lot due to chronic LD in CF had a simultaneous cough and GER episodes, suggesting a relationship between them? These data should be thoroughly investigated, preferably with longitudinal studies, to provide a better understanding of the relationship between LD symptoms in CF patients and the presence or absence of GERD.
In CF, it is also not well established whether GERD worsens LD or vice versa.4,10,11,14 However, GERD may be further exacerbated in CF patients due to chronic cough. Cough increases the gastroesophageal pressure gradient, causes alteration in the diaphragm with impaired lower esophageal sphincter (LES) function, increases the frequency of transient LES relaxations, and prolongs gastric emptying time.3 Both cough and regurgitation are frequent symptoms in CF patients' first years of life. One should be careful about using a single parameter for the GERD diagnosis in the pediatric age group where physiological GER is more frequent than in the adult population.24,25
Blondeau et al. (2010) evaluated 24 CF children/adolescents between 0.3 and 13 yo and found that AGER was prevalent in comparison with NAGER and previous to the onset of respiratory symptoms.10 Van der Doef et al. (2009) showed that treatment of AGER did not change bacterial acquisition, but it might improve LF. The authors associated reduced LF and earlier P. aeruginosa and S. aureus infection with GERD diagnosis; this provided evidence that diagnosis and treatment of GERD should be performed as early as possible in CF.27 Palm et al. (2012) evaluated CF children/adolescents and GERD and found MIIpH tracings showed a positive association between GERD and a higher number of pulmonary exacerbations, worsening LF, and earlier infection by P. aeruginosa.11
Characteristics of chronic LD in CF, such as cough, increased gastroesophageal pressure gradient, impaired LES function, increased frequency of transient LES relaxations, and prolonged gastric emptying time result in upper digestive tract physiology changes, which are most likely to be aggravated with age.1,3 Woodley et al. (2019) demonstrated that aging is associated with increased acid exposure in a cohort study with CF patients.1
Most of the patients with GERD (70%) had AGER, which is in line with the literature.9,16 The presence of 30% NAGER here is noteworthy. Studies with larger sample sizes would provide a better understanding of the harmful effects of reflux in the airways and esophagus in CF. Palm et al. (2012) found that AGER and NAGER are common in CF children and related to the worsening of LF.11
Importantly, most studies on GERD evidence that GER episodes occur more frequently in the orthostatic position.9,16 The authors found various GER prevalence rates in different decubitus positions: 5/10 (50%) overall, 4/10 (40%) orthostatic, and 1/10 (10%) supine.
Some studies used pHmetry to evaluate GERD in CF children and adolescents, demonstrating an AGER prevalence of 55-76%.28,29 Most studies show higher specificity and sensitivity outcomes for MIIpH than for pHmetry alone.7,10,16,17 In the present study, among the 10 patients with GERD, three patients were diagnosed by combined MII and pHmetry: 2/10 AGER+NAGER and 1/10 NAGER. The prevalence of 30% of patients with GERD confirmed by MIIpH and not pHmetry reiterates the importance of evaluating the esophageal content with MIIpH.
An intriguing aspect of pediatrics is the physiological differences between infants, preschoolers, and schoolchildren, whose body systems show rapid and progressive development at different paces and stages of maturation, including the digestive and respiratory tracts.30
Therefore, unlike the classification for adults, these parameters should be stratified by age for the pediatric population, as it is well established that GERD increases with age in CF.1
The authors applied the reference values proposed by Mousa et al. (2014),8 who assessed two age groups (<12 months and 1.3-17 yo) to define the normality parameters for MII values. In contrast to MII, pHmetry has specific parameters for infants, preschoolers, schoolchildren, and adults, being able to characterize acid refluxes and define GERD.5
Several suggestions for future research can be proposed: to evaluate the interactions between GERD and CF; to carry out multi-center studies; to design studies according to the specific age range for pediatrics; to correlate CF severity determined by genotypic and phenotypic characteristics with GERD; to evaluate bronchial hyperreactivity by acid stimulation of the esophagus; to correlate GERD determined by MIIpH with anatomical pathological markers obtained by esophageal biopsy; to conduct studies to identify which children would benefit from surgical treatments for GERD and devices that increase contraction and muscle competence of the LES;, and standardizes type and timing of treatment in CF children and GERD.
In conclusion, the prevalence of GERD was 34% in CF children ̶ which is high compared to children without CF in the same age group, and yet at the lower limit of the prevalence described for CF adults. There was no association between LD severity and type of GER acid or non–acid. Most episodes of GERD occurred in the overall position. MIIpH detected 30% more patients with GERD than pHmetry.
JDR: FAEPEX (Fundo de Apoio ao Ensino, à Pesquisa e à Extensão) Unicamp: #519.294; CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico): #407364407364/2016-1.