To describe the impact of the 10-valent pneumococcal conjugate vaccine on the pediatric burden of pneumococcal infections, carriage, serotype replacement, and antimicrobial resistance in Brazil since its introduction in 2010.
Data sourceA narrative review of English, Spanish, and Portuguese articles published in online databases and in Brazilian epidemiological surveillance databases was performed. The following keywords were used: Streptococcus pneumoniae, pneumococcal disease, conjugate vaccine, PCV10, antimicrobial resistance, and meningitis.
Summary of the findingsDeclines in hospitalization rates of all-cause pneumonia occurred in the target age groups and some age groups not targeted by vaccination early after the use of PCV10. Large descriptive studies of laboratory-confirmed pneumococcal meningitis and hospital-based historical series of hospitalized children with IPD have evidenced a significant impact on disease burden, in-hospital fatality rates, and admission to the intensive care unit before and after the inclusion of the vaccine. Impact data on otitis media is limited and inconsistent; the main benefit remains the prevention of complicated diseases. During the late post-vaccine years, a significant and progressive increase in high-level penicillin non-susceptibility pneumococci has been described. Since 2014 serotype 19A has been the leading serotype in all ages and was responsible for 28.2%–44.6% of all IPD in children under 5 yrs.
ConclusionsPCV10 has performed a significant impact on IPD in Brazil since 2010, however, progress has been continuously hampered by replacement. Broader spectrum PCVs could provide expanded direct and indirect protection against ST19A and other additional serotypes of increasing importance if administered to children in the Brazilian National Immunization Program.
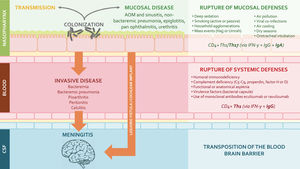
Streptococcus pneumoniae (pneumococcus) is a leading cause of community-acquired pneumonia (CAP), meningitis, and bacteremia (considered high-priority vaccine-preventable diseases) in children, the elderly, and high-risk populations.1,2 According to the World Health Organization, more than 318 000 children under 5 die each year worldwide, with the highest mortality burden in resource-poor regions.1,3 Pneumococcus, a common transient colonizer of the human nasopharynx in young children, has more than 100 serotypes, but a relatively small number are responsible for most of the disease.4,5 Invasive disease (IPD: bacteremic pneumonia, meningitis, and sepsis) and non-invasive/mucosal disease (acute otitis media and non-bacteremic pneumonia) are rare events compared with the frequency of asymptomatic nasopharyngeal colonization – a mandatory precondition for transmission and disease (Figure 1).5–7
Host and environmental factors affecting encapsulated bacteria (including S. pneumoniae) shedding and disease forms. Conjugate vaccines interfere with colonization and disease progression by eliciting robust mucosal immunity (CD4+ Th1/Th17, IgG and IgA) and systemic immunity (CD4+ Th1 and IgG) (developed by the authors, adapted from Supplemental references #60–63). Abbreviations: AOM – acute otitis media; CSF – cerebrospinal fluid.
Following the successful development of the Haemophilus B conjugated vaccine in the 1980 s, the first pneumococcal conjugate vaccines (PCVs) were developed in the 1990s by covalently conjugating capsular serotypes to carrier proteins.8 PCVs have proved to be adequately immunogenic in young children and highly effective against infection: from 2000 to 2015, the use of PCVs has led to near 40% reduction in pediatric cases of pneumococcal pneumonia worldwide.1
However, despite this unquestionable achievement in different settings, successful implementation has been followed by replacement disease – an increase in the incidence of pneumococcal disease caused by non-vaccine serotypes (NVT).6,9,10 In a mature PCV vaccination program, such a phenomenon can erode the impact seen initially after vaccine introduction. Therefore, this review will cover some historical aspects and the essential concepts that significantly reduced the burden of pneumococcal disease in infants and children under 5 years of age in Brazil. Also, by describing the extent of residual IPD in the post-vaccine era, the potential effect of a switch to higher-valency PCVs concerning serotype 19A and other emerging serotypes will be discussed.
Search sources and strategyThe authors have performed a narrative review of English, Spanish, and Portuguese articles published in the following online databases: Medline (via PubMed), Web of Science, Scopus, Scientific Electronic Library Online (SciELO), and Latin American & Caribbean Health Sciences Literature (LILACS). Epidemiological surveillance databases were also analyzed, when available, from local surveillance systems such as Sistema de Informação de Agravos de Notificação (SINAN) (via the Brazilian Ministry of Health´s open-access public health database system [DATASUS]),11Centro de Vigilância Epidemiológica da Secretaria de Estado da Saúde de São Paulo (CVE/SP), and Sistema de Informação do Programa Nacional de Imunizações (please refer to Supplemental references).
Historical perspective of IPD in BrazilEarly in the 1980 s, penicillin-resistant pneumococci began to be described in South Africa, Europe, and the USA.12 Taunay et al. serotyped meningitis strains in the early 1990s in Brazil,13 and a few years later, Berezin et al. evaluated pediatric patients with pneumonia and meningitis caused by Spn.14 The most prevalent serotypes in these studies were 1, 5, 6B, and 14 – the former was considered the leading serotype in pediatric IPD in Brazil at that time. Other evaluations performed in the same period resulted in similar serotypes distribution.15
At the end of the decade, led by this public health priority and to strengthen the scientific and technological capacity of the countries of Latin America and the Caribbean, the Pan American Health Organization (PAHO) established the Regional Vaccine System for Latin America (Sistema Regional de Vacunas para América Latina - SIREVA)16 as a source for basic research and to promote the development of affordable vaccines with appropriate serotype formulation for the region. In 1993, with financial support from the Government of Canada, a pilot laboratory surveillance study to evaluate the serotype distribution and antimicrobial resistance of S. pneumoniae causing invasive disease in children under 5 years began.16
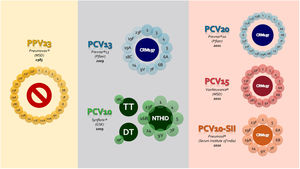
The era of universal pneumococcal vaccination in BrazilThe 23-valent pneumococcal polysaccharide vaccine (PPV23; Pneumovax™, Merck Sharp & Dohme Corp.) was among the first vaccines commercialized to address the pneumococcal disease burden in 1983. This vaccine was able to protect against most of the serotypes associated with pneumococcal infections at that moment. However, due to its T-cell-independent mechanisms of action, it results in poor responses in children under 2 years of age, induces short-lasting immunity, lacks anamnestic/booster response upon subsequent challenge, and has no impact on the pneumococcal carriage (as with all unconjugated vaccines). Despite these limitations, PPV23 has been widely recommended for three decades for the direct protection of persons ≥ 2 years of age with selected chronic comorbidities that increase the risk of IPD, such as immunocompromising conditions, and in adults ≥ 65 years of age (Figure 2).
Schematic of the pneumococcal vaccines available in 2022, including serotype composition (light colored circles), protein carrier (dark colored central circles), and year of licensure (developed by the authors, adapted from products packages insert). Abbreviations: PPV23 – 23-valent pneumococcal polysaccharide vaccine; PCV10 – 10-valent pneumococcal conjugate vaccine; PCV13 – 13-valent pneumococcal conjugate vaccine; PCV15 – 15-valent pneumococcal conjugate vaccine; PCV20 – 20-valent pneumococcal conjugate vaccine; PCV10-SII – 10-valent pneumococcal conjugate vaccine produced by Serum Institute of India; NTHiD – non-typeable Haemophilus influenzae protein D; TT – tetanus toxoid; DT – diphtheria toxoid; CRM197 – diphtheria toxoid variant CRM197 (Corynebacterium diphtheria cross-reactive material 197).
Based on the success of the recently developed vaccine conjugation system against Haemophilus influenzae b, the reports of increasing frequency of penicillin non-susceptible pneumococci, and the necessity to address the unmet needs of PPV23 in the pediatric population, the first pneumococcal conjugate vaccine was licensed.8 It included serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F (PCV7; Prevenar™, Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.) and was first authorized in the USA for use in children in 2000. In Brazil, PCV7 was introduced into its National Immunization Program (NIP) in 2007 to high-risk groups only, offering no or limited herd protection.
A decade after PCV7 approval, two second-generation, higher-valent conjugate vaccines that included additional serotypes were licensed based on immunogenicity data and replaced PCV7: a 10-valent (PCV10, Synflorix™, GlaxoSmithKline) and a 13-valent vaccine (PCV13, Prevnar™13, Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.). They diverge in the number of serotypes included (ten serotypes in PCV10 and thirteen serotypes in PCV13) (Figure 2), polysaccharide concentration (more than double in PCV13 for seven of the ten shared serotypes), conjugation methods, and carrier protein – eight of the serotypes in PCV10 are conjugated to protein D, a surface lipoprotein of non-typeable Haemophilus influenzae (NTHi), and tetanus and diphtheria toxoids for the remaining two. In contrast, all serotypes in PCV13 are conjugated to diphtheria toxoid variant CRM197 (Corynebacterium diphtheria cross-reactive material 197).8
In March 2010, Brazil became the first country to introduce PCV10 into its routine childhood immunization program, free of charge, to all children under 2 years old. The recommended schedule included a 3+1 dose schedule (at 2, 4, and 6 months of age plus a booster at 12–18 months),17 which shifted to a 2+1 program (at 2 and 4 months plus a booster at 12–18 months) in 2016. Following the routine PCV vaccination, those at increased risk for IPD were additionally offered PPV23 after their second birthday (PCV-prime and PPV-boost schedule), and since September 2019, PCV13 was also provided to selected high-risk populations that did not receive PCV10: patients living with HIV/AIDS, hematopoietic stem cell transplant recipients, solid organ transplant recipients, and cancer patients).
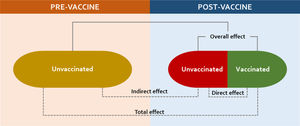
Pneumococcal vaccine uptake in BrazilPost-routine PCV introduction studies have demonstrated a substantial direct effect on vaccinated children but also on unvaccinated age groups.9 Such a highly desirable additional benefit from PCVs comprises an extension of this effect to those not targeted to receive the vaccine.18,19 Observational studies suggest that significant indirect (herd) effects can be achieved at 60%–75% coverage,20,21 while elimination or near-elimination of vaccine-type (VT) carriage and disease can only be achieved when PCV is given at sustained high rates (usually greater than 90%).22,23 By reducing VT carriage in the nasopharynx among vaccinees, conjugate vaccines interrupt the transmission of pneumococci to vaccine-ineligible groups, including the very young, older adults, and high-risk population7,24,25 (Figure 3).
Schematic of the varied vaccine effects in two populations, before and after national introduction, if high uptake is sustained through time (developed by the authors, adapted from Supplemental reference #64).
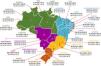
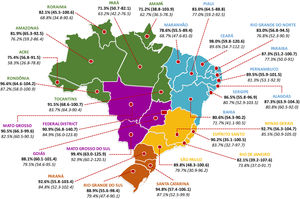
In Brazil, comprehensive national data on vaccine uptake are available on persons who receive publicly funded healthcare. These accounted for approximately 80% of the population, with heterogenous regional distribution.26 Early in the post-vaccine period in Brazil (the first 2-3 years after the implementation of PCV10), for example, significant declines in hospitalizations for all-cause pneumonia were described in major capitals but not in others with lower vaccination rates.27 After this period the average primary series uptake was 93.1% (range: 88.4–95.2%) and 85.3% (range: 76.3–93.1%) for the booster dose until 2018 nationally (http://tabnet.datasus.gov.br/cgi/deftohtm.exe?pni/cnv/cpniuf.def).11 However, since 2019 and fueled by the COVID-19 pandemic, fake news, politically-driven misinformation, and weakening of public health leadership, overall vaccine uptake declined dramatically in relation to previous years (Figure 4).28,29 Until the acceptance of this review, the year 2022 has reached the lowest historical vaccination rates for PCV10 following the first transition years: 53.6% for primary series and 45.5% for booster dose (http://tabnet.datasus.gov.br/cgi/deftohtm.exe?pni/cnv/cpniuf.def).11
Average PCV10 uptake (with range) for primary doses (in bold) and booster doses (in Italic), per Brazilian state, during late post-vaccine period (January 2012 – August 2022) National coverage data for full PCV10 vaccination among children (developed by the authors, data obtained from reference11 and adapted from reference29).
Systematic reviews have shown no evidence for differences in the effects of PCV10 and PCV13 against hospital admissions due to radiologically confirmed pneumonia and clinical pneumonia.17,30,31 In a recent systematic review including data from six Latin America and Caribbean countries, for example, PCV effectiveness against hospitalizations ranged from 7.4–20.6% for clinical pneumonia, 8.8–37.8% for radiographic confirmed pneumonia, 13.3–87.7% for meningitis, and 56–83.3% for IPD hospitalization.32 Attention should be drawn to the width of the 95% confidence intervals, as they may reveal imprecise estimates. Population-based studies using the Brazilian Unified Health System database (DATASUS) demonstrated significant declines in hospitalization rates of all-cause pneumonia, X-ray confirmed pneumonia and non-respiratory causes in children under 2 years of age in the early post-vaccination period.27,33–35 Five years after PCV10 introduction, similar reductions were described in the target age groups (17.4–26.5%; p < 0.01) and some age groups not targeted by vaccination (11.1–27.1%; p < 0.01), but no impact was seen among elderly over 65 years.36,37
From 1980 and 2010, pneumonia mortality in children younger than 5 years decreased by 10 times in Brazil (from 150 to 15 deaths per 100 000 children), a period during which the national Human Development Index rose substantially.38–40 Because randomized clinical trials generally do not have the power to detect significant mortality reductions from pneumococcal diseases, there are limited post-marketing PCV10 effectiveness studies in the country with such an endpoint.
Two long time-series studies used publicly available mortality data among Brazilian children younger than 5 years. The first was further stratified by socioeconomic status to address PCV-associated reduction in childhood pneumonia mortality.38 Although the introduction of PCV10 resulted only in a modest additional reduction at a national level (10%), substantially higher vaccine-related benefits occurred in the subpopulation living in extreme childhood poverty and low maternal education (point estimates of 16–24%) – particularly in younger children. Another research demonstrated consistent PCV10-associated declines in all-cause pneumonia hospitalizations in low- and high-income Brazilian populations.36
Finally, a third study compared the impact on pneumonia hospitalization rate following PCV10 implementation among the five administrative Brazilian regions (North, Northeast, Midwest, Southeast, and South)41 (Figure 4). The authors identified the largest hospitalization rate variation in the post-vaccine period in the North region of the country, where more significant increases were seen in the Human Development Index (very low in 2003; medium in 2010; high in 2017) and PCV10 coverage (from 58.79% in 2011 to 84.28% in 2017). In this administrative region, a significant decline that could be explained by vaccination was described only among age groups 0–4 years (12.5%; p = 0.01), 5–9 years (-38.5%; p < 0.01), and 10–14 years (-10.7%; p = 0.03).41 Putting these apparently low reductions in pneumonia mortality in perspective, even a modest benefit of the vaccine would translate into a large number of hospitalizations and deaths prevented annually in low-income and highly populated regions of Brazil.36,39–41
Like pneumonia, no randomized, head-to-head studies assessing the impact of both vaccines on IPD are available. Recent reports from Latin America have addressed the impact of PCV10 on IPD:42 Chile reported a significant decrease in invasive infections and mortality in children – attributed almost exclusively to meningitis cases43 but despite this initial success, PCV13 replaced PCV10 based on the increase of IPD caused by serotype 19A. A Brazilian group evaluated more than 14 400 episodes of laboratory-confirmed pneumococcal meningitis (PM) occurring between 2007 and 2021.44 In this descriptive study, PM incidence per 100 000 inhabitants was reduced from 2.5 during the pre-vaccine period (2007) to 1.5 in 2015, remained stable from 2016 through 2019 (1.11 cases/100 000 inhabitants), and then sharply declined to 0.33-0.39 during the COVID19 pandemic.
SIREVA, the largest and more reliable Brazilian database on IPD,16 relies on a passive surveillance network not designed to evaluate pneumococcal disease burden, disease incidence calculations, or vaccine impact. Alternatively, hospital-based historical series of hospitalized children with the pneumococcal disease have been used to assess the effect of PCV10 in severe disease.45 Berezin et al. described the changes in IPD episodes, in-hospital fatality rates, and admission to the intensive care unit before and after the inclusion of PCV10 in the Brazilian immunization program.45 Hospitalizations decreased from 20 cases to 5 cases per 10 000 pediatric admissions (p < 0.0001) and fatalities from 6.6 to 2.0 cases per 10 000 admissions (p < 0.0001) (driven mainly by meningitis and pneumonia). In contrast, 30% of cases required intensive care, with no percentual changes during the evaluation period. In addition, while IPD cases due to vaccine serotypes were reduced, infection rates caused by NVT increased.45
Another study evaluated the effect of PCV10 on hospitalizations, the need for intensive care, and outcomes in more than 700 cases of laboratory-confirmed pneumococcal infection at a large tertiary teaching hospital in Sao Paulo, Brazil.46 From 2000 to 2022, the annual IPD incidence rates in children under 5 years declined 69% (from 5.0 to 1.16 per 1 000 pediatric hospitalizations in the late post-vaccine period), while increases were evidenced in severe diseases among the high-risk population (from 36.3% to 47.2%) and case fatality rates also among healthy children (unpublished data).
Vaccine impact in non-invasive pneumococcal disease (nIPD)Early and dense nasopharynx colonization with S. pneumoniae markedly increases the risk of acute otitis media (AOM).47 Following the completion of their primary PCV immunization, systemic immune responses are induced, and serotype-specific IgA and IgG antibodies are detected in saliva.9,48 Even higher antibody levels following subsequent booster vaccination lead to reduced vaccine-serotype-specific carriage.49 By reducing or eliminating nasopharyngeal colonization by VT pneumococcus, PCVs may ultimately reduce mucosal infections such as AOM.50 Additionally, by preventing early mucosal disease caused by VT, conjugate vaccines have the potential to disrupt the continuum of evolution from pneumococcal‐associated otitis media towards recurrent and chronic otitis media, thereby reducing the progression to subsequent and more complex disease caused by NVT and NTHi.51,52
Before the nationwide implementation of PCVs, the three leading bacterial pathogens isolated from the middle ear fluid of children with AOM were Streptococcus pneumoniae (up to 39%), non-typeable H. influenzae (up to 23%), and Moraxella catarrhalis (up to 15%).53,54 Despite limited and inconsistent data and extensive debate, recent evidence shows that successful implementation of pneumococcal vaccination has led to substantial reductions in ambulatory care visit rates for AOM, greater efficacy for more severe outcomes (such as complex otitis, tympanostomy tube placement, and recurrent otitis media), and near-elimination in the occurrence of such otopathogens included in the vaccine.52,55–60 In addition, as expected for PCVs, colonization by vaccine serotypes decreased in the post-vaccine period among toddlers in Brazil, while, surprisingly, colonization by NTHi increased from 26% at baseline to 43.6%.61
No studies have evaluated the impact of PCV10 in pneumococcal OM in the Brazilian population, mainly due to limitations in obtaining middle ear fluid cultures for microbiologic diagnosis.52 Alternatively, case-based electronic data from the Outpatient Visits Information System of the Unified Health System have been used to measure the impact of PCV10 on all-cause pediatric OM. In an interrupted time-series analysis conducted in Goiania, Brazil, the estimated impact of PCV10 on all-cause OM was 43.0% (95% CI: 41.4–44.5%).62 When PCV10 was given to healthy infants during early infancy, the all-cause AOM risk varied from 6% (95% CI: −6% to 17%) to 15% (95%CI: −1% to 28%) – neither of these estimates reaching significance.50 Despite the encouraging data regarding the impact of PCV10 on the overall burden of otitis media, the main benefit of these vaccines remains the prevention of complicated disease.60
Vaccine impact in pneumococcal carriage, serotype replacement, and residual IPD burdenConjugated vaccines have demonstrated a marked impact on the acquisition of vaccine-type serotypes in the nasopharynx.63 Significant geographical differences and redistribution of serotypes in the late post-vaccine period, driven by the selective pressure of PCVs (serotype replacement), have reduced the prevalence of serotypes included in the vaccine, but also a concerning emergence of NVT causing residual disease.6 All commercially available PCVs can result in a replacement. Still, there is insufficient evidence to direct comparisons of whether the changes in pneumococcal dynamics are more pronounced with one product or the other.55,64 After more than two decades of serotype replacement following the introduction of PCVs worldwide, the authors have learned that the population structure of pneumococci is reshaped due to the expansion of a limited number of NVT in carriage and disease.22
Pneumococcal carriage data can also predict reductions in VT and NVT following PCV introduction. Data on PCV10 impact on the nasopharyngeal carriage is limited in Brazil. An extensive carriage assessment among children living in São Paulo reported a 91% reduction in colonization by VT and a significant increase in NVT – mainly driven by serotype 6C – after 3 years of PCV10 use.61 An updated analysis compared the prevalence of nasopharyngeal colonization by VT between the pre-PCV10 survey (2010) and post-PCV10 survey (2017): carriage by vaccine type decreased 95.5% compared to that at baseline (19.8%), while NVT colonization increased by 185% in the late post-PCV10 survey – driven by serotype 6C (883.3% increase), NVT isolates (501% increase), non-PCV10 types (273.9% increase), and serotype 19A (233.3% increase).65
Despite the importance of bacterial carriage in the pathogenesis of the pneumococcal disease (Figure 1), data from the mucosa should not be fully extrapolated to symptomatic infection. For example, complete nasopharyngeal replacement of VT by non-VT after PCV implementation leads to incomplete replacement with less virulent serotypes in disease.55,66,67 While no randomized, head-to-head studies became available, a subnational Swedish study comparing regions using PCV10 and PCV13 in equivalent populations revealed a similar overall residual IPD burden. However, in areas using PCV10, the serotype share was taken up by 6A and 19A, while in those using PCV13, it was taken up by non-PCV13 serotypes.68
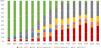
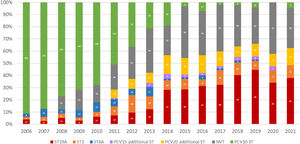
Similarly, in Brazil, serotype 19A in the vaccine-eligible group increased from an average of 3.5% (range: 1.4%–5.0%) in the pre-PCV10 period and early vaccine period to 39% on average from 2019 and 2021 (Figure 5). Serotype 3 (considered unique due to its abundant capsule, vaccine escape capabilities, and enhanced virulence69) showed a similar trend jumping from a 3.2% average in 2016 to 8.0% later in 2017, reaching two historical records of 10.2% in 2020 and 2021.70
Distribution of IPD cases caused by additional PCV13 serotypes (3, 6A and 19A), PCV15 unique serotypes (22F and 33F), PCV20 exclusive serotypes (8, 10A, 11A, 12F, and 15B), and non-vaccine serotypes (NVT) in children under 5 years in Brazil, 2006-2021 (developed by the authors, adapted from Ref.70). Abbreviations: ST – serotype.
Antimicrobial-resistant pneumococci were documented in humans as early as 1917 when optochin resistance was first described. In 1939, treatment-acquired sulfonamide resistance was reported in a case of meningitis, but it was not until 1965 that the first clinical penicillin-resistant pneumococci were reported in humans. During the 1970 s and 1980 s, pneumococcal resistance to penicillin, trimethoprim-sulfamethoxazole, and erythromycin increased and spread rapidly worldwide,12,18 while tetracycline and chloramphenicol resistances were also described with varied rates. In addition, multidrug-resistant strains (defined as reduced susceptibility to three or more chemical classes of antimicrobials) have become increasingly prevalent and are particularly associated with carriage and disease among young children.18
Consequently, pneumococcus has been a prominent cause of antibiotic consumption globally.71 As previously described, PCVs are considered a powerful strategy to reduce resistance,71–73 mainly by preventing the horizontal spread of non-susceptible strains within a community and reducing antibiotic use (thus alleviating selection pressure from broad-spectrum antibiotics).18,72 Multiple pneumococcal strains can concurrently colonize the same host (a phenomenon known as co-colonization or multiple serotype carriage).65 When associated with biofilm formation, it favors horizontal gene transfer – the primary evolutionary mechanism of Streptococcus species.5,74 Of the seven serotypes frequently associated with resistance (6A, 6B, 9V, 14, 19A, 19F, and 23F), serotype 19A is the only clinically significant, globally prevalent, highly multidrug-resistant, and not at all affected by PCV10.75
In Brazil, like other countries that have not incorporated PCV13 into their NIP, a combination of clonal expansion of NVT and capsule switch led to the development of serotype 19A lineages with high-level penicillin non-susceptibility.18,76,77 In a large study that evaluated 11 380 isolates from the SIREVA project,76 a reduction of infections due to penicillin (MIC ≥ 0.125 mg/l) and ceftriaxone (MIC ≥ 1.0 mg/l) non-susceptible pneumococcal strains was observed during the early post-vaccine period versus pre-PCV10 years in Brazil. From 2014 to 2019, this trend was followed by a significant and progressive increase in the proportion of Spn with higher MIC break points to penicillin (39.4%) and ceftriaxone (19.7%) – driven mainly by serotypes 19A, 6C, and 23A (Table 1). In addition, multidrug resistance reached an alarming 75% from 2018 to 2021.70
Penicillin susceptibility profile among meningeal and non-meningeal S. pneumoniae strains in children under 5 years in Brazil, 2006-2021. Prevalence of highly resistant serotype 19A (ST19A) is provided (adapted from reference70).
The constant shift in serotype distribution and pneumococcal disease led to the development of new vaccine platforms and products to solve unmet needs in varied settings. A new 10-valent PCV (PCV10-SII; Pneumosil™, Serum Institute of India, Pvt. Ltd) includes serotype 19A and other prevalent disease-causing serotypes in Latin America and the Caribbean, Africa, and Asia, conjugated to CRM197. The comparable performance to PCV10 and PCV13, unprecedented low cost, and WHO prequalification could make it an affordable and accessible option for LMICs. Broader spectrum PCVs have also been developed, and more formulations are in the pipeline for the next decade. The serotypes covered in these new PCVs are associated with a more significant disease burden in countries with mature PCV13 vaccination programs:78 a 15-valent vaccine (PCV15; VaxNeuvance™, Merck Sharp & Dohme Corp.) adds unique serotypes 22F and 33F and is approved for children and adults; a 20-valent vaccine (PCV20; Prevnar™20, Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc.) adds 5 additional serotypes compared to PCV15 (8, 10A, 11A, 12F, and 15B) and has been licensed for adults only, with approval to infants expected shortly.
In Brazil, the potential benefits of broader serotype coverage can be extrapolated from the distribution of such serotypes during post-PCV10 years (Figure 5). PCV15 unique serotypes accounted for 0.5% during the years 2011-2012, increasing to 2.5% on average (range: 0.3–3.8%) from 2013 to 2021. PCV20 additional serotypes were responsible for 7.5% of invasive diseases during the early vaccine period, increasing to 11.9% on average (range: 8.4–15.3%) during the late post-vaccine years. While PCV20 aims for wider coverage, PCV15 new conjugation technique resulted in improved immunologic responses against serotype 3 compared to PCV13. Real-world data is required to address the impact of this laboratory advantage on disease.
Further prospects on pneumococcal vaccinationIn order to alleviate some of the limitations of the PCVs currently in use – particularly the issue of serotype replacement and the reduced immunogenicity and efficacy for serotype 3 – a new generation of pneumococcal vaccines is under development. New vaccine platforms aim for distinct pneumococcal proteic antigens (such as pneumococcal surface proteins A [PspA] and C [PspC], pneumolysin [Ply], pneumococcal histidine triad protein D [PhtD], elongation factor Tu [EF-Tu], and pneumococcal peptide 27 [Pep27]), new delivery system technologies, and enhanced immunogenicity (through conjugation to Toll-like receptors and reformulation into nanoparticle). Whole-cell vaccines can express all protein antigens without purification of individual proteins, conferring broader protection. Refer to Supplemental references for further reading.
Future perspectives for PCV vaccination in BrazilDespite the significant impact of PCV10 on the pneumococcal disease since 2010, progress has been continuously hampered by serotype replacement. Given that (1) the once hypothesized PCV10 cross-protection against vaccine-related serotypes (mainly 19A) was not confirmed in clinical and epidemiologic studies from Brazil,45,46,65,79 and (2) PCV10 and PCV13 (and presumably PCV10-SII, PCV15, and PCV20) have a comparable impact on IPD, the authors believe that all PCVs that include serotype 19A can perform dramatic changes to the landscape of local serotype epidemiology. Moreover, considering the already discussed indirect effect of PCVs, these new vaccines can add additional protection to unvaccinated populations against other relevant serotypes if given to young children. That said, the country's choice between the available products will ultimately depend on vaccine cost, supply, and logistical factors.
LimitationsThis manuscript was not intended as an exhaustive review PCVs comparability but rather as an overview of the impact of PCV10 in Brazil using local data. Consequently, a comprehensive systematic search was not performed, therefore some of the available evidence may have been missed. Although hospitalizations and PCV10 coverage data were obtained from administrative databases, the burden of community-acquired pneumonia shows similar estimates when compared to hospital primary data.80 Therefore, these sources are reliable to evaluate PCV10 vaccination as a pneumonia intervention. Similarly, surveillance information such as SIREVA can be used to assess circulating strains, support decision-making on vaccine introduction, and provide guidelines for antibiotic use. However, as reporting to laboratory-based systems is not compulsory in Latin America and the Caribbean (except for pneumococcal meningitis), such national passive surveillance systems are subject to under-reporting and lack of representativeness in IPD occurrence. The proportion of SIREVA-reported isolates, for example, in relation to the estimated expected cases varies considerably between the countries and is estimated to be less than 10% in Brazil.81 Therefore, such data require cautious interpretation due to timely and limited data availability, as well as heterogeneous case reporting and laboratory diagnosis of IPD.42,81 Additional references are provided as Supplemental references for further reading.
ConclusionIn a scenario of consistent improvements in healthcare delivery, socioeconomic status, and health interventions impacting childhood mortality in Brazil, PCV10 adds relatively modest reductions (but expressive in absolute terms) of hospitalization and mortality from pneumococcal diseases, especially in lower-income regions of the country. However, progress has been continuously hampered by replacement. Broader spectrum PCVs could provide expanded direct and indirect protection against ST19A and other additional serotypes of increasing importance if administered to children in the Brazilian NIP.
DisclaimerThe authors' findings and conclusions are those of the authors and do not necessarily represent the views of the institutions cited in the article.