A review article was carried out, addressing the clinical and epidemiological characteristics of immune system deficiencies, which are associated with or predispose to recurrent infectious processes, autoimmune diseases, auto inflammatory diseases, or neoplasms, and which are classified as inborn errors of immunity (IEI) and secondary immunodeficiencies (SID). Emphasis was placed on the classification of the main signs and symptoms for each organ and system, which will serve as warning signs, to guide the pediatrician in the investigation of the main IEI. In addition, the main secondary changes in the immune system triggered by infections (with emphasis on COVID-19), drugs, chronic diseases, metabolic and nutritional disorders were identified.
Sources of dataThis review included articles published in the last five years and that were identified in the MEDLINE platform (PubMed).
Summary of findingsThe recurrence of infectious processes, associated with the severity of the condition and/or unusual profile of the infectious agent, always related to the age range of symptom onset, are the most important findings for suspected diagnosis.
ConclusionsConsidering this scenario, immunity disorders should be part of the investigation carried out by the general pediatrician, whether they are the innate errors of immunity (primary immunodeficiencies) or secondary immunodeficiencies, so that the diagnosis is attained as early as possible and therapeutic measures are implemented, reducing the morbidity and mortality of these patients.
Immunodeficiencies (IDs) are a group of diseases that present with quantitative and/or functional alterations in the elements that comprise the inborn and adaptive immune response. They are classified as primary when their origin is genetic, also currently called inborn errors of immunity (IEI), and secondary when they are acquired. Both situations are associated with or predispose to recurrent infectious processes, autoimmune or auto inflammatory diseases, typical of immune dysregulation processes, in addition to lymph proliferative and/or neoplastic processes.1
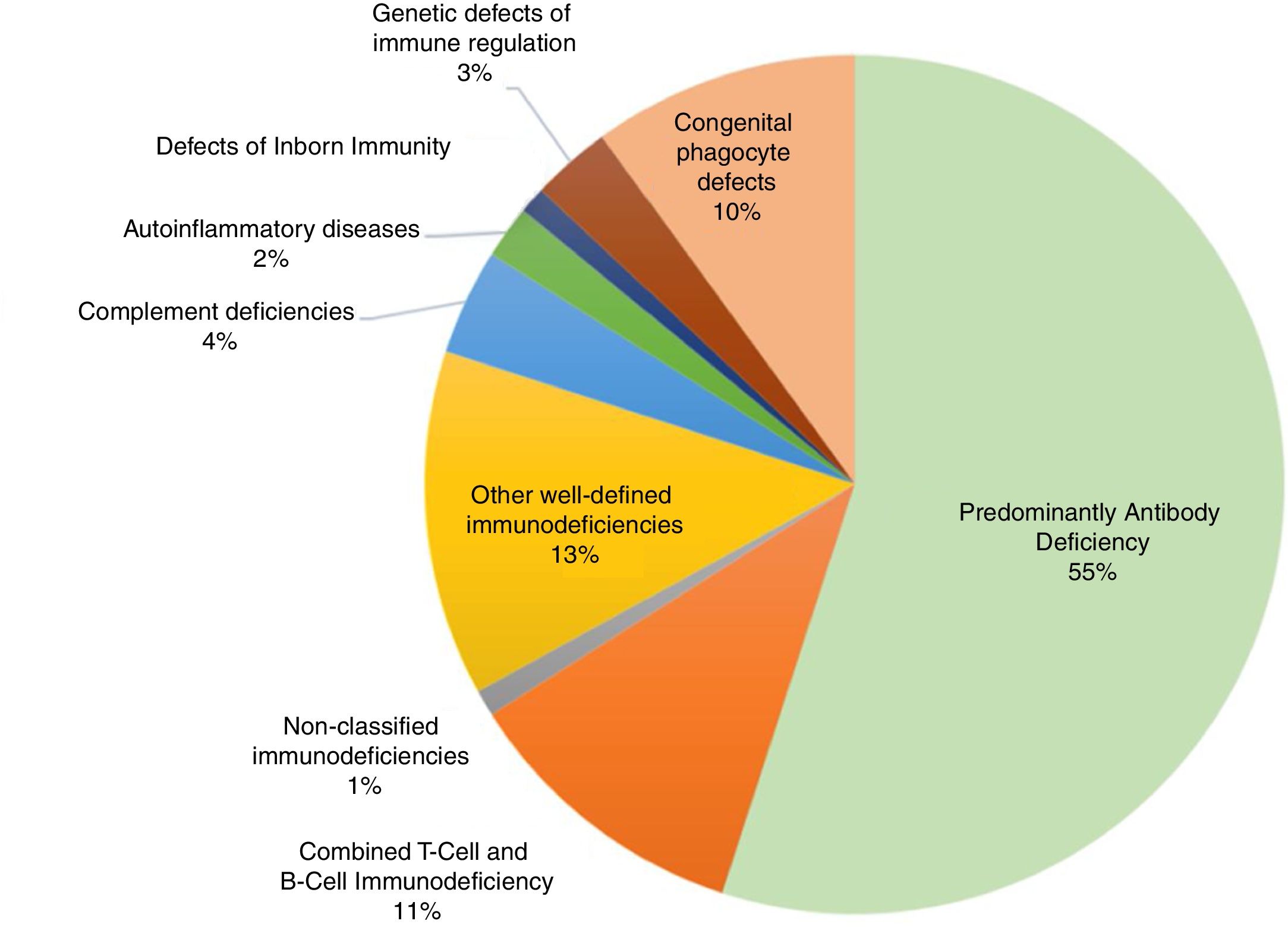
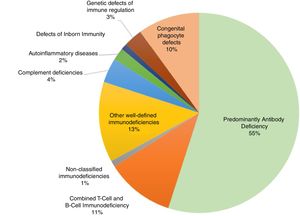
Primary immunodeficienciesPrimary immunodeficiencies (PIDs), more recently called inborn errors of immunity (IEI), include approximately 420 monogenic diseases that are more susceptible to infections, immune dysregulation leading to severe types of allergies, autoimmunity or auto inflammation, susceptibility to cancer, or integrate complex syndromes that affect different organs and systems, including developmental disorders, autism, intellectual disability, epilepsy, gastroenteropathies, pneumopathies, dermatoses, skeletal and renal abnormalities, among other clinical events. The estimated prevalence of IEI worldwide is 1:10,000 individuals. This number is increasing, especially with the advances in knowledge, diagnostic resources, and also in communities with a high rate of inbreeding. In a global context, within the classification of primary immunodeficiencies we found the following frequencies, confirmed by specialization societies (Fig. 1).2,3
Classification of primary immunodeficiencies and their relative frequencies. Data extracted from the main specialized societies (ESID, LASID, USID net) and records from Asia, Africa and Australia.
The implementation of neonatal screening for immunodeficiencies with T and B lymphopenias, particularly the group of severe combined immunodeficiencies and agammaglobulinemia, which is now universal in the United States of America, and the implantation in several European countries and in Brazil has contributed to a significant change in the epidemiological profile of PIDs.4
The immune system is anatomically distributed throughout the body, especially in the barrier areas such as the skin, gastrointestinal and respiratory mucosa. This explains why the clinical picture of recurrent infections occurs at these barriers. However, in the case of the most severe immunodeficiencies, they become more invasive and manifest themselves as deep abscesses, sepsis, osteoarticular infections, meningitis, and encephalitis. This brings an interdisciplinary characteristic to the study of immunodeficiencies, since the infection, allergy, and autoimmunity aspects affect the different organs and systems. In practice, the patient with IEI is initially seen by general pediatricians, pulmonologists, otorhinolaryngologists, gastroenterologists, infectologists, hematologists, dermatologists and rheumatologists.3,4
Tables 1–7 show the main signs and symptoms for each organ and system, constituting a warning sign that directs the physician to the investigation of IEI.5
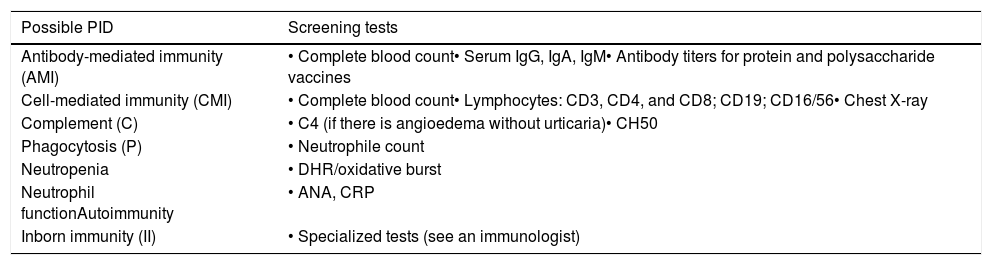
Screening laboratory tests for the non-immunology specialist to detect patients with possible primary immunodeficiency.
| Possible PID | Screening tests |
|---|---|
| Antibody-mediated immunity (AMI) | • Complete blood count• Serum IgG, IgA, IgM• Antibody titers for protein and polysaccharide vaccines |
| Cell-mediated immunity (CMI) | • Complete blood count• Lymphocytes: CD3, CD4, and CD8; CD19; CD16/56• Chest X-ray |
| Complement (C) | • C4 (if there is angioedema without urticaria)• CH50 |
| Phagocytosis (P) | • Neutrophile count |
| Neutropenia | • DHR/oxidative burst |
| Neutrophil functionAutoimmunity | • ANA, CRP |
| Inborn immunity (II) | • Specialized tests (see an immunologist) |
The screening tests should be part of any initial immunological assessment. Only the abbreviations in parentheses will be listed as suggested screening tests in all subsequent tables. The HIV test should be a routine test to exclude AIDS. Abbreviations: ANA, antinuclear antibodies; CBC, complete blood count; DHR, dihydro-rhodamine oxidation test; CRP, C-reactive protein.
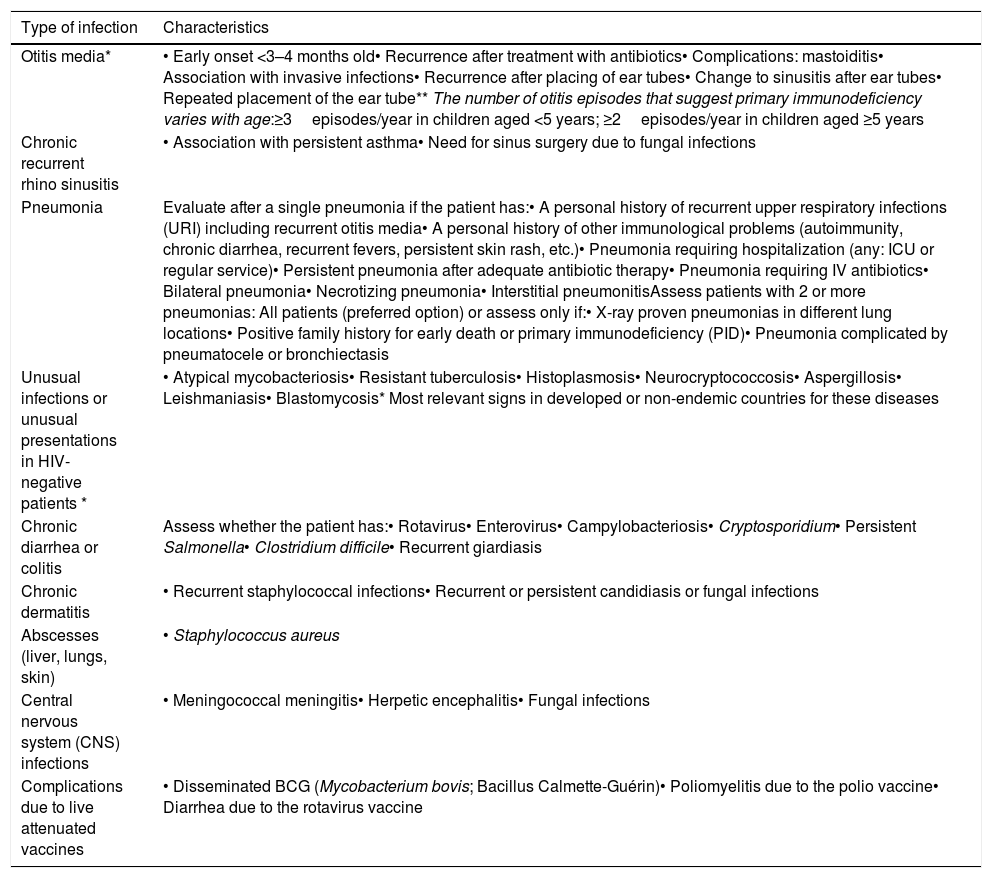
Infections as general warning signs for primary immunodeficiency.
| Type of infection | Characteristics |
|---|---|
| Otitis media* | • Early onset <3–4 months old• Recurrence after treatment with antibiotics• Complications: mastoiditis• Association with invasive infections• Recurrence after placing of ear tubes• Change to sinusitis after ear tubes• Repeated placement of the ear tube** The number of otitis episodes that suggest primary immunodeficiency varies with age:≥3episodes/year in children aged <5 years; ≥2episodes/year in children aged ≥5 years |
| Chronic recurrent rhino sinusitis | • Association with persistent asthma• Need for sinus surgery due to fungal infections |
| Pneumonia | Evaluate after a single pneumonia if the patient has:• A personal history of recurrent upper respiratory infections (URI) including recurrent otitis media• A personal history of other immunological problems (autoimmunity, chronic diarrhea, recurrent fevers, persistent skin rash, etc.)• Pneumonia requiring hospitalization (any: ICU or regular service)• Persistent pneumonia after adequate antibiotic therapy• Pneumonia requiring IV antibiotics• Bilateral pneumonia• Necrotizing pneumonia• Interstitial pneumonitisAssess patients with 2 or more pneumonias: All patients (preferred option) or assess only if:• X-ray proven pneumonias in different lung locations• Positive family history for early death or primary immunodeficiency (PID)• Pneumonia complicated by pneumatocele or bronchiectasis |
| Unusual infections or unusual presentations in HIV-negative patients * | • Atypical mycobacteriosis• Resistant tuberculosis• Histoplasmosis• Neurocryptococcosis• Aspergillosis• Leishmaniasis• Blastomycosis* Most relevant signs in developed or non-endemic countries for these diseases |
| Chronic diarrhea or colitis | Assess whether the patient has:• Rotavirus• Enterovirus• Campylobacteriosis• Cryptosporidium• Persistent Salmonella• Clostridium difficile• Recurrent giardiasis |
| Chronic dermatitis | • Recurrent staphylococcal infections• Recurrent or persistent candidiasis or fungal infections |
| Abscesses (liver, lungs, skin) | • Staphylococcus aureus |
| Central nervous system (CNS) infections | • Meningococcal meningitis• Herpetic encephalitis• Fungal infections |
| Complications due to live attenuated vaccines | • Disseminated BCG (Mycobacterium bovis; Bacillus Calmette-Guérin)• Poliomyelitis due to the polio vaccine• Diarrhea due to the rotavirus vaccine |
Warning signs of primary immunodeficiency for infectious disease specialists.
| Clinical events | PID | Screening tests (*see Table 2) |
|---|---|---|
| Infections by extracellular bacteria | Antibody deficiencies | Screening for antibody-mediated immunity (AMI) |
| Complement deficiencies | Complement (C), antinuclear antibodies (ANA) | |
| Neutropenia | Screening for phagocytosis (P) defects | |
| IRAK-4, MyD88 | Screening for inborn immunity (II), C-reactive protein (CRP) | |
| Infection by Neisseria meningitidis | Deficiency of terminal components of the complement system (membrane attack complex) | C+AP50 |
| Infection by S. aureus and Gram-negative bacteria:• Serratia marcescens• Burkholderia cepacia and gladioli• Nocardia spp.• Chromobacterium violaceum• Granulobacter bethesdensis | Chronic granulomatous disease (CGD)Hyper IgE syndrome (HIES)Characteristics: S. aureus pneumonia, eczema, fungal infection, joint hypermobility, rough facial features | Serum IgE, eosinophiliaSpecific score |
| Infection by fungi:• Pneumocystis jirovecii• Aspergillus• Candida albicans | T-cell defectsCD40 ligand (L) deficiency | Screening for cell-mediated immunity (CMI) |
| HIES | Serum IgE, eosinophiliaSpecific score | |
| CGD | P | |
| Infection by Candida albicans | Chronic mucocutaneous candidiasis | CMI+lymphocyte proliferation induced by Candida |
| Infection by mycobacteria/atypical Salmonella and/or side effects of Bacillus Calmette-Guérin; Paracoccidioides sp., Leishmania, Cryptococcus | T-cell deficiencies | CMI |
| Severe combined immunodeficiency (SCID) | AMI+CMI | |
| Mendelian susceptibility to mycobacterial diseases | P and/or II | |
| Herpes infections | T and NK cell deficiencies | CMI |
| Fulminant or chronic infection by the Epstein-Barr virus | Familial hemophagocytic lymphohistiocytosis (FHL) syndromeX-linked lymphoproliferative (XLP) syndromes, types 1 or 2 | CBC, triglycerides, ferritin, EBNA serology |
| Infection by Cryptosporidium, recurrent or persistent Isospory | CD40L deficiency | AMI |
| Common variable immunodeficiency (CVID) | AMI | |
| Giardiasis | Antibody deficiencies | AMI |
| Complications due to vaccines for BCG, rotavirus or varicella | SCID, CGD | CMI and/or II and/or P |
| Complications due to oral polio vaccine | Antibody deficiencies | AMI |
| Persistent fever of unknown origin | Autoinflammatory diseases | ANA,CRPblood smear |
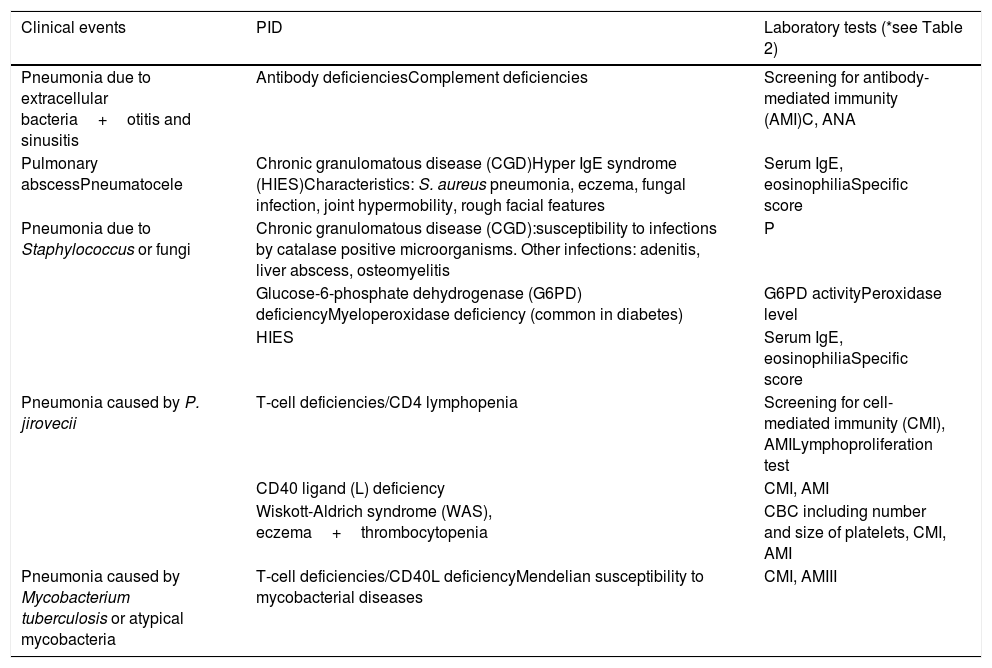
Warning signs of primary immunodeficiency for pulmonologists.
| Clinical events | PID | Laboratory tests (*see Table 2) |
|---|---|---|
| Pneumonia due to extracellular bacteria+otitis and sinusitis | Antibody deficienciesComplement deficiencies | Screening for antibody-mediated immunity (AMI)C, ANA |
| Pulmonary abscessPneumatocele | Chronic granulomatous disease (CGD)Hyper IgE syndrome (HIES)Characteristics: S. aureus pneumonia, eczema, fungal infection, joint hypermobility, rough facial features | Serum IgE, eosinophiliaSpecific score |
| Pneumonia due to Staphylococcus or fungi | Chronic granulomatous disease (CGD):susceptibility to infections by catalase positive microorganisms. Other infections: adenitis, liver abscess, osteomyelitis | P |
| Glucose-6-phosphate dehydrogenase (G6PD) deficiencyMyeloperoxidase deficiency (common in diabetes) | G6PD activityPeroxidase level | |
| HIES | Serum IgE, eosinophiliaSpecific score | |
| Pneumonia caused by P. jirovecii | T-cell deficiencies/CD4 lymphopenia | Screening for cell-mediated immunity (CMI), AMILymphoproliferation test |
| CD40 ligand (L) deficiency | CMI, AMI | |
| Wiskott-Aldrich syndrome (WAS), eczema+thrombocytopenia | CBC including number and size of platelets, CMI, AMI | |
| Pneumonia caused by Mycobacterium tuberculosis or atypical mycobacteria | T-cell deficiencies/CD40L deficiencyMendelian susceptibility to mycobacterial diseases | CMI, AMIII |
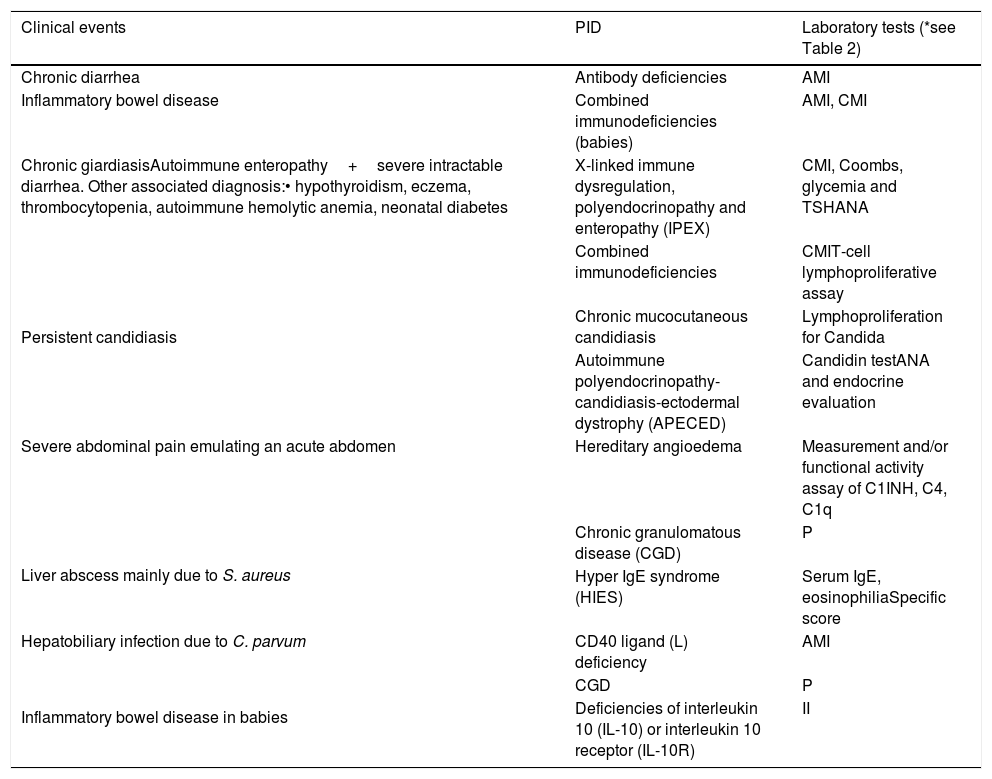
Warning signs of primary immunodeficiency for gastroenterologists.
| Clinical events | PID | Laboratory tests (*see Table 2) |
|---|---|---|
| Chronic diarrhea | Antibody deficiencies | AMI |
| Inflammatory bowel disease | Combined immunodeficiencies (babies) | AMI, CMI |
| Chronic giardiasisAutoimmune enteropathy+severe intractable diarrhea. Other associated diagnosis:• hypothyroidism, eczema, thrombocytopenia, autoimmune hemolytic anemia, neonatal diabetes | X-linked immune dysregulation, polyendocrinopathy and enteropathy (IPEX) | CMI, Coombs, glycemia and TSHANA |
| Persistent candidiasis | Combined immunodeficiencies | CMIT-cell lymphoproliferative assay |
| Chronic mucocutaneous candidiasis | Lymphoproliferation for Candida | |
| Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) | Candidin testANA and endocrine evaluation | |
| Severe abdominal pain emulating an acute abdomen | Hereditary angioedema | Measurement and/or functional activity assay of C1INH, C4, C1q |
| Liver abscess mainly due to S. aureus | Chronic granulomatous disease (CGD) | P |
| Hyper IgE syndrome (HIES) | Serum IgE, eosinophiliaSpecific score | |
| Hepatobiliary infection due to C. parvum | CD40 ligand (L) deficiency | AMI |
| Inflammatory bowel disease in babies | CGD | P |
| Deficiencies of interleukin 10 (IL-10) or interleukin 10 receptor (IL-10R) | II |
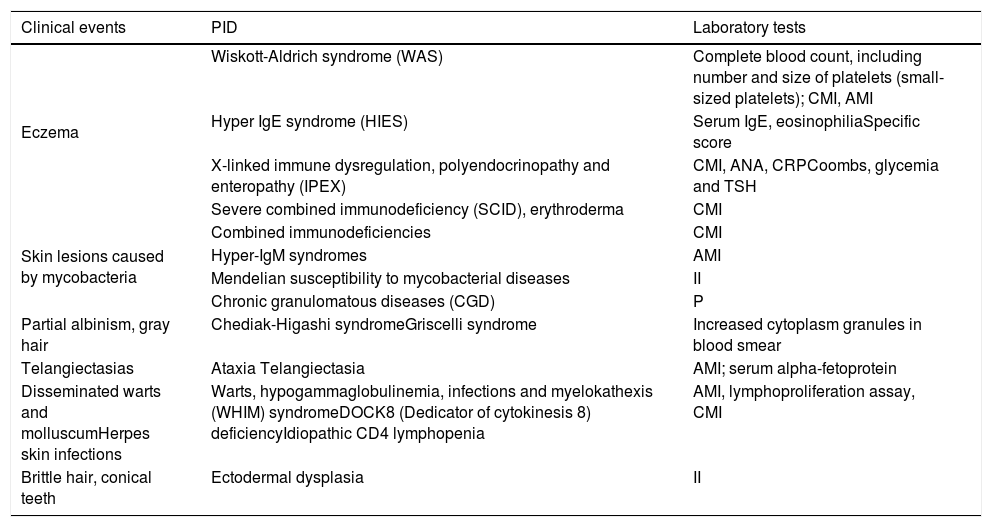
Warning signs of primary immunodeficiency for dermatologists.
| Clinical events | PID | Laboratory tests |
|---|---|---|
| Eczema | Wiskott-Aldrich syndrome (WAS) | Complete blood count, including number and size of platelets (small-sized platelets); CMI, AMI |
| Hyper IgE syndrome (HIES) | Serum IgE, eosinophiliaSpecific score | |
| X-linked immune dysregulation, polyendocrinopathy and enteropathy (IPEX) | CMI, ANA, CRPCoombs, glycemia and TSH | |
| Severe combined immunodeficiency (SCID), erythroderma | CMI | |
| Skin lesions caused by mycobacteria | Combined immunodeficiencies | CMI |
| Hyper-IgM syndromes | AMI | |
| Mendelian susceptibility to mycobacterial diseases | II | |
| Chronic granulomatous diseases (CGD) | P | |
| Partial albinism, gray hair | Chediak-Higashi syndromeGriscelli syndrome | Increased cytoplasm granules in blood smear |
| Telangiectasias | Ataxia Telangiectasia | AMI; serum alpha-fetoprotein |
| Disseminated warts and molluscumHerpes skin infections | Warts, hypogammaglobulinemia, infections and myelokathexis (WHIM) syndromeDOCK8 (Dedicator of cytokinesis 8) deficiencyIdiopathic CD4 lymphopenia | AMI, lymphoproliferation assay, CMI |
| Brittle hair, conical teeth | Ectodermal dysplasia | II |
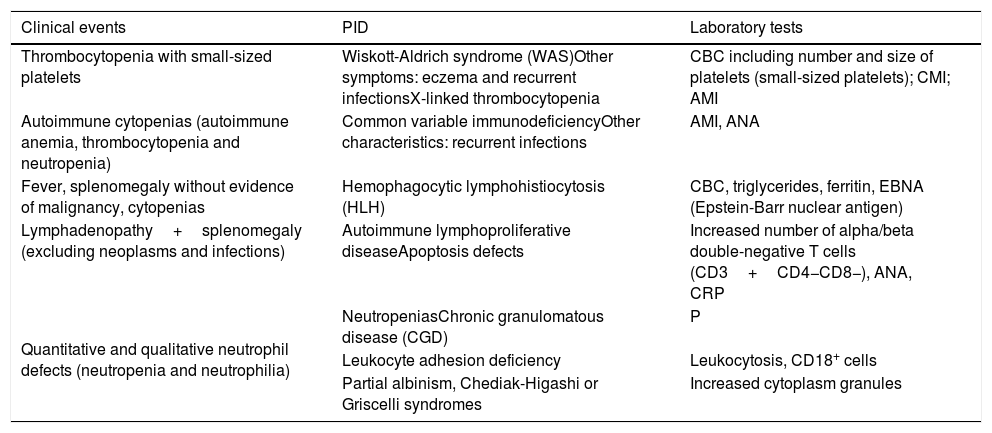
Warning signs of primary immunodeficiency for hematologists.
| Clinical events | PID | Laboratory tests |
|---|---|---|
| Thrombocytopenia with small-sized platelets | Wiskott-Aldrich syndrome (WAS)Other symptoms: eczema and recurrent infectionsX-linked thrombocytopenia | CBC including number and size of platelets (small-sized platelets); CMI; AMI |
| Autoimmune cytopenias (autoimmune anemia, thrombocytopenia and neutropenia) | Common variable immunodeficiencyOther characteristics: recurrent infections | AMI, ANA |
| Fever, splenomegaly without evidence of malignancy, cytopenias | Hemophagocytic lymphohistiocytosis (HLH) | CBC, triglycerides, ferritin, EBNA (Epstein-Barr nuclear antigen) |
| Lymphadenopathy+splenomegaly (excluding neoplasms and infections) | Autoimmune lymphoproliferative diseaseApoptosis defects | Increased number of alpha/beta double-negative T cells (CD3+CD4−CD8−), ANA, CRP |
| Quantitative and qualitative neutrophil defects (neutropenia and neutrophilia) | NeutropeniasChronic granulomatous disease (CGD) | P |
| Leukocyte adhesion deficiency | Leukocytosis, CD18+ cells | |
| Partial albinism, Chediak-Higashi or Griscelli syndromes | Increased cytoplasm granules |
There are several causes of secondary immunodeficiencies (SIDs), among which we can highlight: chronic protein-losing diseases (e.g. nephrotic syndrome); autoimmune and onco-hematological diseases, associated or not with the use of immunosuppressant drugs; nutritional alterations, whether malnutrition or obesity/metabolic syndrome; post-trauma splenectomy or autosplenectomy (sickle-cell anemia),which increase the risk of sepsis and chronic or prolonged viral infections, such as HIV, HTLV, cytomegalovirus, mononucleosis virus and, more recently, the SARS-CoV-2 that has caused immunological alterations both in the short term and in the medium and long terms.1,6
Nutritional alterationsMalnutrition has traditionally been the focus of nutrition agendas in underdeveloped or developing countries. However, rapid economic growth and urbanization have given rise to a nutritional transition where energy-dense diets have replaced traditional diets and sedentary lifestyles prevail. In this sense, childhood obesity rates have increased dramatically. The global prevalence of overweight or obesity has increased in all regions, from 4.2% in 1990 to 6.7% in 2010. Although the prevalence is higher in developed countries, developing countries have a higher absolute number of affected children and higher relative increases. It is estimated that these growing trends will continue to increase, now in 2020, with 60 million overweight or obese children under the age of five.7
In malnourished children, common infections are the main causes of death, which shows a probable underlying, inborn and adaptive immune deficiency, even in mild forms of malnutrition. The altered mechanisms of immune function include: alteration of the cutaneous and intestinal epithelial barrier; reduced granulocyte microbicidal activity; quantitative decrease in circulating dendritic cells, B lymphocytes and complement system proteins; reduced levels of soluble IgA in saliva and tears; atrophy of lymphoid organs; impaired late hypersensitivity responses; lymphocyte hyper responsiveness with a predominance of Th2 response. Despite that, most malnourished children seem to respond adequately to vaccination, although the timing, quality and longevity of specific vaccine responses may be impaired.8
At the other end of nutritional disorders, obese individuals have a higher number of infections, which are more severe. This fact is corroborated by recent studies that show a strong association between obesity and the severity of SARS-CoV-2 infection, in the absence of other comorbidities. The dysfunctional hypertrophic adipocytes present in obesity produce an excessive amount of proinflammatory cytokines, such as IL-6, IL-8, monocyte-1 chemo attractant protein, leptin and plasminogen activator inhibitor-1, which lead to an increase in the recruitment of macrophages, especially polarized M1 macrophages. These cells, together with free fatty acids, maintain a high production of pro-inflammatory molecules. This cumulative effect generates a state of chronic inflammation and hypercytokinemia, which leads to defective inborn immunity and creates a breeding ground for the hyper inflammatory response mediated by macrophage activation in severe cases of COVID-19.9
Nephrotic syndromePrimary nephrotic syndrome (NS) in childhood is a disease characterized by the presence of high proteinuria, clinically significant edema, hypoalbuminemia and hyperlipidemia. It occurs at the proportion of 2–7 cases for every 100,000 children per year. Inside the spectrum of possible complications of NS are recurrent infections, which vary in incidence between 8 and 84% and occur mainly due to defects in cell-mediated immunity, use of immunosuppressive therapy, malnutrition and specific urinary protein losses, such as immunoglobulins, properdin factor B and complement factors.10
Sickle-cell anemiaUntil the 1990s, in the United States, up to 30% of young children with sickle-cell anemia (SCA) died of infections, mainly caused by encapsulated bacteria, due to the deficiency in the immune response to polysaccharide antigens, exacerbated by the deficient blood elimination of these bacteria, since there is functional asplenia. Prophylactic penicillin is a safe and beneficial strategy in patients under five years of age, and its introduction reduced the incidence of pneumococcal bacteremia associated with impaired splenic function by 85%. However, the universal use of anti-pneumococcal vaccines has also greatly helped reduce mortality from these infectious diseases. With the introduction of the first pneumococcal conjugate vaccine, there was a decrease in the rate of pneumococcal bacteremia in children under 3 years of age by 93.4%, in addition to further protection for those patients who have low adherence to prophylactic therapy with penicillin.11
MedicationsIn the last decades, we have seen an increase in the use of drugs to suppress the body's undesirable immune responses, mainly in the control of autoimmune diseases, allergic diseases and in the prevention of the rejection of transplanted organs. Such drugs are able to control the exacerbated immune response; however, they leave the individual more susceptible to infections, through several mechanisms.
Glucocorticoids are widely used in clinical practice to treat immune-mediated diseases, either as monotherapy or combined with other immunosuppressants. Their effects on the immune system are secondary to two mechanisms: immunosuppression with increased susceptibility to viral, fungal and bacterial infections, and the anti-inflammatory action, masking infectious signs and symptoms, delaying the diagnosis and the start of treatment.12,13 The mechanism of action occurs mainly in T cells, especially with decreased cytokine production, reduced lymphocyte chemotaxis, cell adhesion and phagocytosis.14 Therefore, interference with the tuberculin skin test is also observed, in addition to restrictions regarding the use of vaccines consisting of live attenuated microorganisms.13
Another class of drugs widely used in immunosuppressive treatment are calcineurin inhibitors (Cyclosporine, Tacrolimus), which inhibit T-cell activation and IL-2 production. They are considered glucocorticoid-sparing drugs and have the advantage of not interfering with macrophage and neutrophil action, but they increase susceptibility to skin infections and viral airway infections.14,15
As for cytotoxic drugs (Azathioprine, Cyclophosphamide, Methotrexate, Leflunomide, and Mycophenolate mofetil), they are highly effective in suppressing the immune response, inhibiting the proliferation and activation of T and B cells. Additionally, these drugs lead to cytopenia, further contributing to secondary immunosuppression and susceptibility to infections.12,14
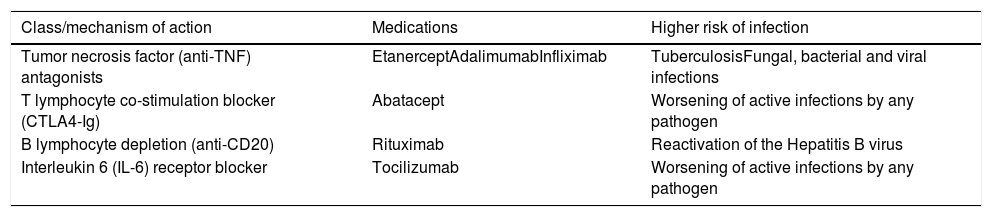
In recent years, immunosuppressive agents with more specific targets have been developed, seeking to obtain less harmful and highly effective drugs in the treatment of autoimmune diseases and in the prevention of transplanted organ rejection (Table 8).12,16,17
Immunobiological agents and risk of infection.
| Class/mechanism of action | Medications | Higher risk of infection |
|---|---|---|
| Tumor necrosis factor (anti-TNF) antagonists | EtanerceptAdalimumabInfliximab | TuberculosisFungal, bacterial and viral infections |
| T lymphocyte co-stimulation blocker (CTLA4-Ig) | Abatacept | Worsening of active infections by any pathogen |
| B lymphocyte depletion (anti-CD20) | Rituximab | Reactivation of the Hepatitis B virus |
| Interleukin 6 (IL-6) receptor blocker | Tocilizumab | Worsening of active infections by any pathogen |
Taking into account the innumerable immunosuppression pathways secondary to the use of medications to control autoimmune diseases, the immunization recommendations should be followed, updating the vaccination schedule before the start of treatment whenever possible. It is important to recommend follow-up at Special Immunobiological Reference Centers (CRIE), keeping the immunization against Influenza, pneumococcal infections, meningitis, HPV, and Hepatitis B updated and individually assessing the risk of vaccines consisting of live attenuated microorganisms, according to the drug and doses used.18,19
InfectionsSince the last century, it has been observed that the measles virus leads to a condition of transient immunosuppression with anergy and greater susceptibility to infections by other microorganisms, due to changes in the function of T cells and dendritic cells.14 Since then, several microorganisms have been implicated in transient immunosuppression, such as cytomegalovirus (due to changes in T cells) and the Epstein-Barr virus (due to B-cell depletion). Other agents that cause frequent diseases in some Brazilian regions may also increase the risk of opportunistic infections with increased morbidity and mortality, such as: Leishmaniasis, which leads to macrophage dysfunction, decreased production of cytokines and cytopenias, and Malaria, by altering the function of Tcells.20
In the 1980s, the human immunodeficiency virus (HIV) was recognized as a cause of acquired and permanent immunodeficiency, where there is progressive involvement of CD4+ T cells, leading the infected individual to death by opportunistic infections if not treated. This occurs when the peripheral CD4+ T cell count is less than 200cells/mL, and thus the patient can acquire any of the various opportunistic infections present in acquired immunodeficiency syndrome (AIDS), such as pneumonia caused by Pneumocystis jirovecii, histoplasmosis, toxoplasmosis, and tuberculosis.14
Recently, after the pandemic caused by the new Coronavirus (SARS-CoV-2), the association between secondary infections and increased mortality in patients with the severe form of COVID-19 has been demonstrated. The greater susceptibility to these infections is probably due to dysregulation of the immune system and alterations in the intestinal microbiota, in addition to direct tissue damage caused by the SARS-CoV-2 virus.6,21
In this context of COVID-19, unfortunately, there are no studies that analyze the immune response in asymptomatic infected individuals and compare them with individuals with mild, moderate or severe symptoms. Therefore, the information on the following immunological alterations is based on the symptomatic ones, as follows:
- •
Cytopenia: The most evident cell decrease would be lymphopenia, which is more marked in severe symptomatic patients and mainly includes a decrease in T cells (CD8, which when present exhibits abnormal functional phenotype; CD4, especially subtypes Th1 and Treg). NK cells and monocytes may also show decreased rates, especially in moderate and severe cases.22
- •
Alterations in cytokine levels: There is an increase in cytokine levels, especially in severe cases of SARS-CoV-2 infection, especially of: IL-2, IL-2r, IL-6, IL-7, IL-8, IL-10, MP-10, MP-1A, and TNF-α. Of all these cytokines, IL-6 has shown the highest correlation with severity and therefore can be used as a good biomarker to monitor the evolution of the clinical picture.22
Clearly, the immunological alterations described above directly influence the evolution of the disease from a mild or even asymptomatic profile to severe situations with a high mortality rate. Moreover, these immunological alterations caused by the virus favor the onset of secondary infectious processes in the short term and have favored the emergence of autoimmune and inflammatory dysregulations, such as the Kawasaki-like Pediatric Multisystem Inflammatory Syndrome (PMIS), in the medium and long terms.22,23
In conclusion, considering the recurrent infectious and/or inflammatory clinical conditions, especially those that escape the age range normality, immunity disorders, whether primary (inborn errors of immunity) or secondary (secondary immunodeficiencies) alterations, should be part of the investigation of the general pediatrician so that the diagnosis can be made as soon as possible and therapeutic measures can be implemented, thus preventing the repetition of events and functional sequelae as well as promoting an adequate quality of life for these patients.
Conflicts of interestThe authors declare no conflicts of interest.