To compare quantitative CT parameters between children with severe asthma and healthy subjects, correlating to their clinical features.
MethodsWe retrospectively analyzed CT data from 19 school-aged children (5–17 years) with severe asthma and 19 control school-aged children with pectus excavatum. The following CT parameters were evaluated: total lung volume (TLV), mean lung density (MLD), CT air trapping index (AT%) (attenuation ≤856 HU), airway wall thickness (AWT), and percentage of airway wall thickness (AWT%). Multi-detector computed tomography (MDCT) data were correlated to the following clinical parameters: forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), forced expiratory flow at 25–75% (FEF 25–75%), FEV1/FVC ratio, sputum and bronchoalveolar lavage analysis, serum IgE levels, and previous hospitalizations due to asthma.
ResultsAsthma patients presented higher mean values of AT% (23.8 ± 6.7% vs. controls, 9.7 ± 3.2%), AWT (1.46 ± 0.22 mm vs. controls, 0.47 ± −735 ± 28 HU vs. controls, −666 ± 19 HU). Mean AT% was 29.0 ± 4.7% in subjects with previous hospitalization against 19.2 ± 5.0% in those with no prior hospitalization (p < 0.001). AT% presented very strong negative correlations with FVC (r = −0.933, p < 0.001) and FEV1 (r = −0.841, p < 0.001) and a moderate correlation with FEF 25–75% (r = −0.608, p = 0.007). AT% correlation with FEV1/FVC ratio and serum IgE was weak (r = −0.184, p = 0.452, and r = −0.363, p = 0.202)
ConclusionChildren with severe asthma present differences in quantitative chest CT scans compared to healthy controls with strong correlations with pulmonary function tests and previous hospitalizations due to asthma.
Asthma is the most prevalent chronic respiratory disease in children. It affected around 8.3 percent of US children in 2016, with up to 20% of children aged 6–7 years experiencing severe wheezing episodes within a year.1 Although most patients respond well to inhaled or oral corticosteroids, around 5% of children with asthma will maintain symptoms even at maximum doses, characterizing severe asthma.2 Despite representing a small portion of cases, this group of patients contributes to the majority of the costs associated with asthma.1
Many studies in adults thrived to identify different phenotypes of this heterogeneous condition that could be targeted by new interventions. Multi-detector computed tomography (MDCT) with images acquired at different lung volumes for the characterization of morphological abnormalities in the lung and airways has shown good correlation to spirometric features in asthmatic adult.3–6 Quantitative MDCT can measure airway wall thickness (AWT) and the percentage of air trapping in the parenchyma, which allows the identification of phenotypes at higher risk for asthma-related hospitalization, intensive care unit admissions, and worse lung function.7,8
Although there are several studies on the use of quantitative CT as a biomarker for asthma severity, its role in children is less evident and results are contradictory.9,10 The correlation of quantitative CT and the severity of the disease in children is not fully understood yet, despite multiple studies in adults. The purpose of our study was to compare quantitative CT parameters in children with severe asthma and healthy control subjects, analyzing also their clinical features.
Material and methodsParticipantsWith the approval of our institutional review board (IRB) (Hospital São Lucas – Pontificia Universidade Catolica do Rio Grande do Sul), we retrospectively evaluated data from one center between 2014 and 2017 to identify school-aged children (5–17 years) with severe asthma. Subjects were considered to have severe asthma if they met the 2014 Global Initiative for Asthma (GINA) for severe asthma after a multidisciplinary evaluation by pediatricians, pediatric pulmonologists, and nurses from the department of pulmonology of our institution.11 Severe asthma is defined as a subset of difficult-to-treat asthma that is uncontrolled despite adherence with maximal optimized therapy with high doses of inhaled corticosteroid (e.g., >400 mcg of beclomethasone for 6–11-year-old children) plus a second controller.11
We included all pediatric patients who had undergone chest MDCT imaging as part of the diagnostic assessment of severe asthma according to the ATS/ESR 2014 guidelines.12 Patients with atypical presentations, such as excessive mucus production, rapid decline in lung function, absence of atopy in a child with difficult-to-treat asthma, and when bronchiectasis was suspected were considered for MDCT.12 Cases were excluded whether they had undergone any previous cardiothoracic surgery as this could influence the quantitative CT analysis.
The control group was composed of healthy school-aged children that had undergone chest CT for preoperative evaluation of pectus excavatum. Controls were excluded whether they had any history of previous pulmonary or airway diseases. The body mass index (BMI) was assessed and were classified according to criteria set by US CDC.13
CT settingsAll subjects underwent inspiratory and expiratory chest CT with 16 × 0.625 mm collimation (LightSpeed 16 Slice Pro, GE Healthcare). CT examinations were conducted to measure total lung capacity (at full inspiration) and functional residual capacity (at the end of normal expiration). Scans were performed in the caudocranial direction, using a helical acquisition. Images were reconstructed with a slice thickness and interval of 1.25 mm. Inspiratory images were acquired at 100 mAs and expiratory images were acquired at 30 mAs; all images were acquired at 120 kVp, using a pitch of 1.375. A standard reconstruction kernel was used to achieve medium- smooth images. A data matrix of 512 × 512 and an FOV was adjusted to patient size, ranging from 15 to 35 cm. Dose length products (DLP) were recorded for each patient and ED was estimated by multiplying DLP and specific coefficients according to age for chest CT.
CT image analysisInspiratory and expiratory CT images were evaluated using proper software for image segmentation (Advantage Workstation 4.6, GE Healthcare, France). Automated segmentation of the right and left lungs from the chest wall and mediastinum was performed. Two blinded chest radiologists decided whether manual editing was necessary.
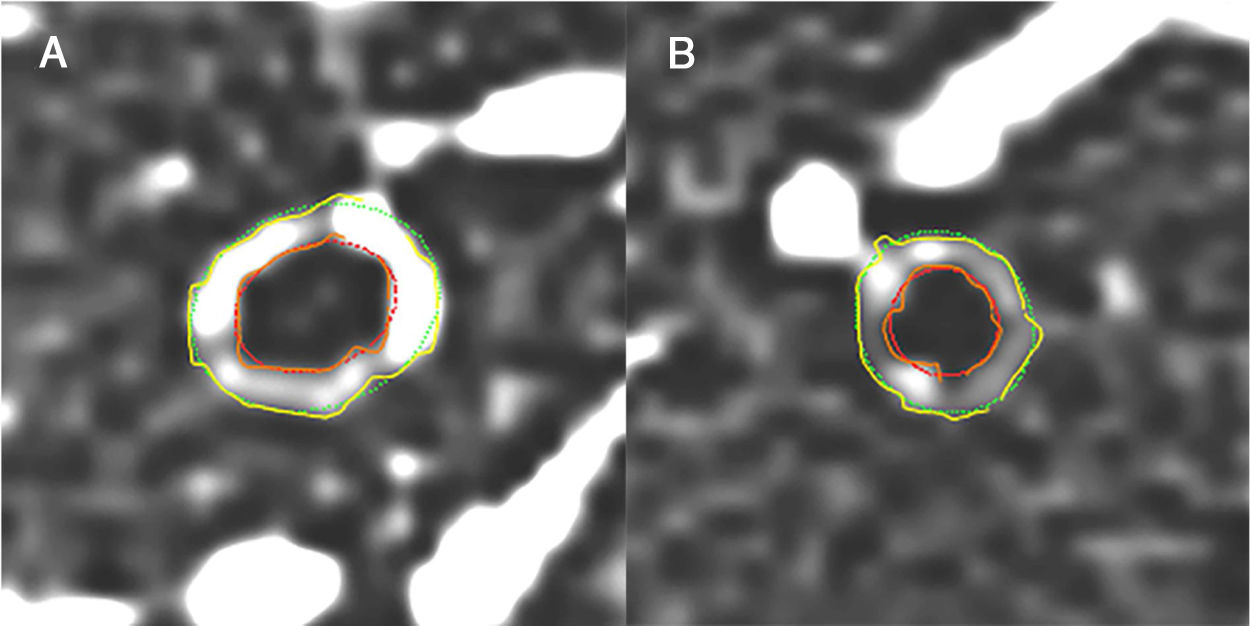
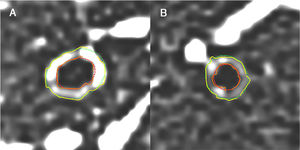
Because studies have shown that CT measures of airway diameter are directly proportional to the severity of asthma, airway disease was quantified using airway wall thickness (AWT) and percentage of AWT (AWT%).4,5,9 Mean AWT values were calculated as the average of values for six segmental bronchi per subject. Airways were measured in cross section on transverse CT images in a lung window setting (level, –600 HU; width, 1600 HU), using Airway Inspector software (Brigham and Women’s Hospital’ [BWH]) and 3D Slicer contributors (Harvard Medical School, Boston, MA, USA).14 The phase congruency edge–detection method was used to locate wall margins. Airways were excluded from measurement in the following situations which were predetermined by the software manufactures as possible limitations: (a) when airway wall circumference appeared to be discontinuous as a result of inadequate resolution; (b) when the long- to short-axis ratio of an ellipse fitting the lumen exceeded 2; (c) and when abutting soft tissues, such as vessels or lymph nodes, obscured more than one-half of the outer wall circumference.14,15
Total lung volume (TLV) and attenuation of all voxels included in the lung segmentation were calculated, and a mean lung density (MLD) histogram was created for each subject.6,16,17 For air trapping assessment, end-expiratory CT data were used, while for the remaining both inspiratory and expiratory phases were used. CT air trapping was calculated as the percentage of voxels on expiratory CT images with attenuation less than -856 HU.3,17
Additionally, semi-quantitative analyses performed for assessment of airway and lung parenchyma. For AWT, a semi-quantitative scoring (BT, bronchial thickening) evaluation was done including only third (segmental) and fourth (sub-segmental) generation bronchi that could be clearly identifiable as previously reported.9 A separate score was given to each of the six pulmonary lobes (including the lingula): 0, normal wall thickness; 1, minimal wall thickening; 2, AWT equivalent to 50% of the diameter of the adjacent blood vessel; 3, AWT equivalent from 50% to 100% of the diameter of the adjacent vessel; and 4, AWT greater than 100% the diameter of the adjacent vessel. Air trapping extent was scored as the absolute number of lobes involved as defined by the Fleischner Society’s Glossary of Terms – areas of lung tissue with a lower increase in attenuation than expected at end-expiratory CT images in normal lung tissue with lack of volume reduction.18
Pulmonary function testsSpirometry and flow volume curves were obtained according to the American Thoracic Society/ European Respiratory Society guidelines (Gould 9000; Sensormedics; Dayton, Ohio), using a Master-Screen spirometer (Jaeger, Germany).19 Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and forced expiratory flow at 25–75% (FEF 25–75%) were expressed in liters and predicted percentage values. The FEV1/FVC ratio was also obtained.
Laboratory testsPatients were classified according to the dominant inflammatory phenotype of the airways presented on histological reports as: (a) eosinophilic, (b) paucigranulocytic, (c) neutrophilic inflammation, and (d) mixed.20 This classification has been demonstrated to adequately correlate with asthma severity in children.21 Transbronchial biopsy was considered for patients presenting atypical features of severe asthma in order to rule out differential diagnosis.22 A cutoff of 900 units/mL was used to discriminate low and high serum total IgE as previously described in children with asthma.23
Statistical analysisThe Shapiro-Wilk test was used to assess data distribution. Continuos variables are described as mean values and standard deviations or median (interquartile range), whereas nominal data is described as proportions. We performed associations between variables by exact Fisher’s test or Chi-square test and unpaired Student t test were used for comparison between groups. Spearman's rank and Pearson’s correlation test was used for assessment of linear association. Coefficients were interpreted using the following parameters: 0.00 to 0.20 were considered very weak, >0.20 to 0.40 weak, >0.40 to 0.70 moderate, > 0.70 to 0.89 strong and ≥0.90 very strong.24 Statistical significance was accepted at p ≤ 0.05. All statistical analyses were performed using the SPSS v.18 (IBM, Chicago, IL, USA).
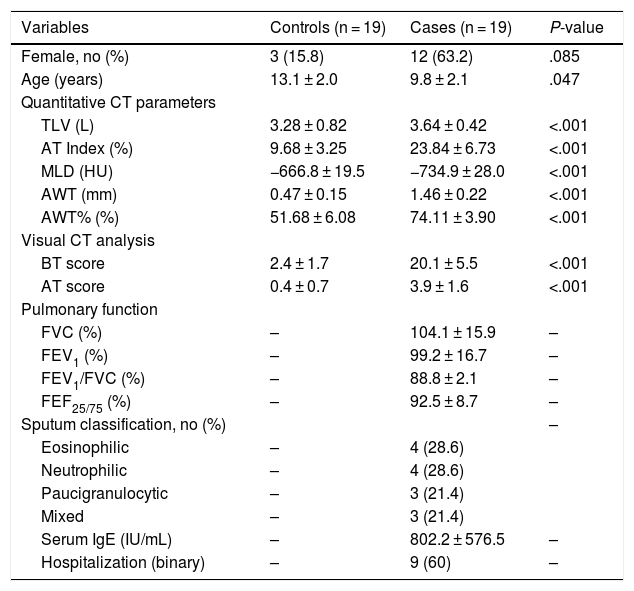
ResultsBaseline demographic characteristics are included in Table 1. In total, 19 patients with severe asthma and 19 control subjects were included. Most patients with asthma were female (n = 12, 63.2%) with a mean age of 9.84 ± 2.01 years. Most patients in the control group were male (n = 16, 84.2%; p = 0.85) with a mean age of 13.1 ± 2.0 years (p = 0.47). BMI of 31 subjects were classified as healthy weight (p5 to < p85) and 7 as overweight (p85 to < p95) or obese (≥p95), being 3 in the asthma group and 4 in the control group. Mean effective radiation dose was 0.42 ± 0.16 mSv and median DLPs were higher according to higher age ranges.
Clinical characteristics and imaging findings.
| Variables | Controls (n = 19) | Cases (n = 19) | P-value |
|---|---|---|---|
| Female, no (%) | 3 (15.8) | 12 (63.2) | .085 |
| Age (years) | 13.1 ± 2.0 | 9.8 ± 2.1 | .047 |
| Quantitative CT parameters | |||
| TLV (L) | 3.28 ± 0.82 | 3.64 ± 0.42 | <.001 |
| AT Index (%) | 9.68 ± 3.25 | 23.84 ± 6.73 | <.001 |
| MLD (HU) | −666.8 ± 19.5 | −734.9 ± 28.0 | <.001 |
| AWT (mm) | 0.47 ± 0.15 | 1.46 ± 0.22 | <.001 |
| AWT% (%) | 51.68 ± 6.08 | 74.11 ± 3.90 | <.001 |
| Visual CT analysis | |||
| BT score | 2.4 ± 1.7 | 20.1 ± 5.5 | <.001 |
| AT score | 0.4 ± 0.7 | 3.9 ± 1.6 | <.001 |
| Pulmonary function | |||
| FVC (%) | – | 104.1 ± 15.9 | – |
| FEV1 (%) | – | 99.2 ± 16.7 | – |
| FEV1/FVC (%) | – | 88.8 ± 2.1 | – |
| FEF25/75 (%) | – | 92.5 ± 8.7 | – |
| Sputum classification, no (%) | – | ||
| Eosinophilic | – | 4 (28.6) | |
| Neutrophilic | – | 4 (28.6) | |
| Paucigranulocytic | – | 3 (21.4) | |
| Mixed | – | 3 (21.4) | |
| Serum IgE (IU/mL) | – | 802.2 ± 576.5 | – |
| Hospitalization (binary) | – | 9 (60) | – |
Data are means with standard deviations.
AT, air trapping; AWT, airway wall thickness; BT, bronchial thickening; BWA, bronchial wall area; CT, computed tomography; FEF25/75, forced expiratory flow at 25–75 %; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MLD, mean lung density; TLV, total lung volume.
The main clinical and radiological data are summarized in Table 1. There was a statistically significant difference for all parameters analyzed in the CT images between the asthma and control groups. In the quantitative analysis, asthma patients presented higher mean values of AT% (23.8 ± 6.7% vs. controls, 9.7 ± 3.2%), AWT (1.46 ± 0.22 mm vs. controls, 0.47 ± 0.15 mm), and AWT% (74.1 ± 3.9% vs. controls, 51.7 ± 6.1%), and a lower MLD (−735 ± 28 HU vs. controls, -666.8 ± 19.5 HU). Likewise, BT and AT assessed in the semi-quantitative analysis were higher for patients with asthma (mean BWT score, 20 ± 6 vs. 2 ± 2; mean AT score, 4 ± 2; p < 0.001).
As spirometry is not mandatory as part of the preoperative evaluation for surgical correction of pectus excavatum and carinatum, we could only include pulmonary function data for asthmatic patients (Table 1). Laboratorial data was available in 14 of the 19 patients. Of these, 14 patients had sputum, 3 were submitted to BAL and sputum and 2 performed all three methods. Sputum distribution was homogeneous with a slightly higher prevalence (57.2%) of eosinophilic and neutrophilic predominant patterns. Serum IgE levels were available in 14 of the 19 subjects with asthma. Median serum IgE was 529.5 (interquartile range (IQR), 360–1060) UI/mL. Most patients (n = 9; 64.3%) presented high serum IgE levels (>900 UI/mL).
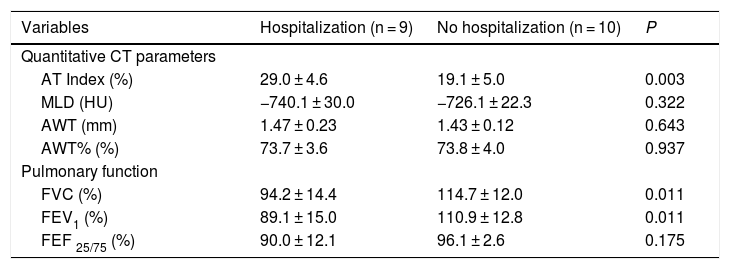
Nine asthmatic patients (47.4%) had previous hospitalizations and their main parameters in CT imaging and pulmonary function tests differed significantly from patients without previous hospitalization (Table 2). Hospitalized patients had a significantly higher AWT% (29.0 ± 4.7% vs. controls, 19.2 ± 5.0%; p < 0.001). Likewise, FEV1, FVC and FEF 25–75 were worse in patients with previous hospitalization.
Imaging and pulmonary function of patients with a history of hospitalization.
| Variables | Hospitalization (n = 9) | No hospitalization (n = 10) | P |
|---|---|---|---|
| Quantitative CT parameters | |||
| AT Index (%) | 29.0 ± 4.6 | 19.1 ± 5.0 | 0.003 |
| MLD (HU) | −740.1 ± 30.0 | −726.1 ± 22.3 | 0.322 |
| AWT (mm) | 1.47 ± 0.23 | 1.43 ± 0.12 | 0.643 |
| AWT% (%) | 73.7 ± 3.6 | 73.8 ± 4.0 | 0.937 |
| Pulmonary function | |||
| FVC (%) | 94.2 ± 14.4 | 114.7 ± 12.0 | 0.011 |
| FEV1 (%) | 89.1 ± 15.0 | 110.9 ± 12.8 | 0.011 |
| FEF 25/75 (%) | 90.0 ± 12.1 | 96.1 ± 2.6 | 0.175 |
Data are means with standard deviations.
AT, air trapping; AWT, airway wall thickness; BWA, bronchial wall area; CT, computed tomography; FEF25/75, forced expiratory flow at 25–75 %; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; MLD, mean lung density; TLV, total lung volume.
AWT% presented very strong negative correlations with FVC (r = −0.933, p < 0.001) and FEV1 (r = −0.841, p < 0.001) and a moderate correlation with FEF 25–75 % (r = −0.608, p = 0.007). On the other hand, AT% correlation with FEV1/FVC ratio and serum IgE was not statistically significant (r = −0.184, p = 0.452, and r = −0.363, p = 0.202). TLV presented positive moderate correlations with FVC and FEV1 (respectively, r = 0.554, p = 0.014; r = 0.572, p = 0.013). Among the parameters of the semi-quantitative analysis, only AT and FVC presented a moderate correlation (r = −0.572, p = 0.011).
Fig. 1 illustrates an example of a case of a severe asthmatic (A) and a healthy patient (B) using quantitative CT.
DiscussionThis study analyzed MDCT parameters in children with severe asthma and their correlation with clinical findings. Using quantitative and semi-quantitative parameters, it was possible to identify differences in the CT scans of patients with severe asthma compared to healthy controls. Children with severe asthma presented higher mean values of AT%, AWT, AWT%, and a lower MLD. In addition, AT% and TLV strongly correlated with parameters of pulmonary function test.
Children with severe asthma contribute to a high portion of the costs associated with asthma and phenotyping patients could help to identify patients at higher risk for asthma-related hospitalization, intensive care unit admissions, and worse lung function.7,8 Currently, serum total IgE and blood eosinophil counts are examples of biomarkers used to characterize specific phenotypes in asthma to identify the appropriate patient for specific treatments and monitor response to treatments such as immunomodulatory and biological therapies.25 Imaging has also been used for this purpose, and parameters such as AT% and AWT have been demonstrated as potential outcomes related to disease control after both pharmacological (anti-IgE, IL-5, IL-4a and IL-13) and non-pharmacological (bronchial thermoplasty) therapies.26,27 In our study, subjects with previous hospitalizations had a significant higher mean value of AT% and worse lung function with lower values of FEV1, FVC and FEF 25–75. This could be helpful to phenotype asthmatic children at higher risk and use imaging as a biomarker to monitor targeted interventions.
One should always weight the benefits of submitting children to CT scans against the risks associated to radiation. Some studies have tried to use magnetic resonance imaging (MRI) as an ionizing radiation-free alternative but use of conventional MRI in asthma is still limited to research and often needs sedation to be performed. CT is widely available, and some of the software we used in the quantitative analysis are available for free, such as the ones herein used.
For quantitative analysis we used validated CT parameters such as TLV, MLD, AT% AWT, and AWT%. Patients with asthma often have lower attenuation (i.e., MLD) and higher TLV compared to controls, and these variables have shown to correlate well with FEV1/FVC.6,25 AT%, AWT and AWT% are used to measure the degree of small airway obstruction, while AT% indicates the magnitude of air trapping. Those variables provide an indirect measure of changes in the caliber of peripheral airways; thus, they can be seen as indicators of airflow impairment and hyperreactivity.3
Our study has several limitations. First, further studies should be conducted prospectively including bigger sample sizes. Chest CT is not routine in the management of children with asthma, what limited patient inclusion in our retrospective analysis. Obtaining controls was also a limitation as it would be inappropriate to expose healthy children to radiation, and most of the previous studies analyzing imaging in children with asthma lack in control groups. Although patients with pectus excavatum present a mechanical compression of the thorax, the chest wall disease does not lead to airway disease.28 Considering most of the variables herein analyzed are markers of airway disease (AWT, AWT%, AT%), we believe patients with pectus excavatum were an adequate alterative for the control group. Although we could not obtain pulmonary function data for the control group, we believe this limitation was mild as we excluded those with any previous history of airway or lung parenchyma diseases. CT attenuation values are also affected by variation in inspiratory and expiratory lung volumes and acquisition techniques. Also, due to the retrospective design of our study, we could not include some additional clinical parameters to be correlated. Further studies should also focus on using spirometer-controlled or controlled-ventilation CT scanning to try to reduce this variable by standardizing degrees of inspiratory and expiratory efforts during the scan. However, strong correlations identified in previous studies suggest that the variation due to these technical factors is minimal.3
Second, the bronchial airway attenuation, which is already considered a method of good accuracy in the differentiation of obstruction between asthmatic patients and non-asthmatic patients,29 was not evaluated in our study. Third, air trapping at CT was assessed using only AT%, AWT and AWT%, thus, we could not include other tools such as the expiratory/inspiratory ratio of MLD for the analysis, being necessary new studies using different measures of quantification of air trapping to validate the results herein presented. Finally, MLD measurement is influenced by radiation dose and body habitus. The control population was slightly older and therefore received more radiation dose than the cases, as the difference in body habitus could lead to lower values of MLD for a fixed dose of radiation.30
In conclusion, we have found that children with severe asthma present differences in chest MDCT scans compared to healthy controls with higher values of AT%, AWT, and AWT%, and lower MLD. In addition, we found that patients with previous hospitalizations had significant higher percentages of air trapping and worse lung function with lower values of FEV1, FVC and FEF 25–75.
Conflicts of interestThe authors declare that they have no conflict of interest.
Financial ResourcesNone.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Please cite this article as: Silva TK, Zanon M, Altmayer S, Pacini GS, Watte G, Stein R, et al. High-resolution CT pulmonary findings in children with severe asthma. J Pediatr (Rio J). 2021;97:37–43.