To analyze late-onset sepsis and to describe the etiological agents in newborns with gastroschisis.
MethodsA retrospective cohort, including newborns with gastroschisis whose admissions occurred in the period between January 2012 to December 2018 in a tertiary referral center. Maternal and newborn characteristics, surgical procedures and evolution in hospitalization were verified. A bivariate analysis was performed with patients with proven late-onset neonatal sepsis and according to the simple or complex gastroschisis category, the prevalent microorganisms in positive cultures were identified, statistical tests were carried out and the significance level adopted was p < 0,05. Results are presented in proportions, averages and standard deviation or medians. The level of significance adopted was p < 0.05.
Results101 newborns were analyzed, 45 (44.5%) were confirmed late-onset sepsis. The median birth weight was 2285+498 grams, and the gestational age was 35.9 +1.74weeks. The incidence of complex gastroschisis was 17.8%, the hospitalization time was 48.2+29.67 days and mortality was 9.9%. The newborns were divided into 2 groups: Group 1: late-onset sepsis (44.6%), and Group 2: no late-onset sepsis. The presence of complex gastroschisis was a factor associated with infection (p < 0.009). Fasting time (p < 0.001), parenteral nutrition time (p < 0.001), time to achieve full diet (p < 0.001), and hospitalization stay (p < 0.001) were higher in group 2. Gram-positive were the most frequent (51.1%), followed by Gram-negative (20%), and fungi (4.4%).
ConclusionsNewborns with gastroschisis have a higher risk of evolving with late-onset sepsis, despite this study did not calculate the risk of sepsis statistically, and the main germs detected by cultures were gram-positive bacteria, specifically Staphylococcus epidermidis.
Gastroschisis is a congenital malformation characterized by a defect in the abdominal wall, with the externalization of abdominal viscera, particularly the intestine. The umbilical cord usually does not change because the defect is in the paraumbilical region usually on the right and doesn't have membrane coverage on the externalized viscera1.
The prevalence of gastroschisis has progressively increased in all regions of the world. Around the 1960s, when surveillance and data collection programs on congenital malformations began, it was 1:50,000 births and has increased about 10 to 20 times in several populations since then. Currently, it has ratios of 1-2 to 4-5 per 10,000 depending on the study population.2,3 Calderon et al.4 found the gastroschisis prevalence's between 2005 and 2016 in São Paulo - Brazil significantly increased by 2.6% per year.
Gastroschisis can be classified as simple (isolated defect) and complex (presence of intestinal atresia, perforation, necrosis, and/or volvulus). Complex gastroschisis may lead to short bowel syndrome in some cases.5.
The etiology of gastroschisis is unknown, and even its pathogenesis is still little known. Non-genetic risk factors for gastroschisis include sociodemographic factors, with schooling being the most important, maternal therapeutic medication, exposure to non-therapeutic drugs, with reduced maternal age (< 20 years), smoking, illicit drug use being the most replicated factors. On the other hand, there is no consensus regarding the contribution of genetic factors, and familial recurrence was observed in 4.7% of cases. Recent studies have identified interactions between maternal smoking, genetic variants (single nucleotide polymorphism-SNP) in the nitric oxide synthase enzyme gene and the risk of gastroschisis.6 Opitz et al.7 propose that gastroschisis is a primary midline malformation that involves the umbilical canal from amniotic to peritoneal space and its primordial umbilical ring, either through nonclosure or rupture of the membrane covering the area, mostly to the right, between the cord and the edge of the ring.
Raymond et al.8 found that the presence of complex gastroschisis (atresia, perforation, volvulus), preterm delivery, and very low birth weight were associated with worse clinical outcomes including increased sepsis, short bowel syndrome, parenteral nutrition days, and hospital length of stay.
Mortality rates range from 3 to 10%, and high morbidity in the neonatal period is associated with factors related to slow intestinal adaptation after surgery, use of prolonged parenteral nutrition, long-term central venous catheters, renal aggression, and infections.9
Late-onset neonatal sepsis is caused by microorganisms acquired from the environment after childbirth. Recent advances in the approach to late-onset neonatal sepsis have resulted in a significant increase in survival, even in the face of prolonged hospitalizations, mechanical ventilation, use of invasive procedures and devices, i.e., intravascular catheters and endotracheal cannulas, which are predisposing factors for this condition. In addition, the immaturity of the immune system makes the newborn particularly susceptible.10
According to the American Neonatal Research Network (NICHD), 70% of late infections are associated with gram-positive organisms; coagulase-negative Staphylococcus (48%), Gram-negative bacteria (18%), and fungal infections (12%), where Candida albicans is the most prevalent agent.11
Prolonged hospitalization greatly increases the incidence of neonatal mortality in preterm newborns, reaching 36% in newborns aged between eight and 14 days and 52% aged between 15 and 28 days.11
The present study aims to analyze the rate of late-onset sepsis and to describe the profile of etiological agents in newborns with gastroschisis and according to the classification of the gastroschisis (simple or complex) evaluating the factors associated with infection and the outcome.
MethodsThis is a retrospective cohort, including newborns with simple and complex gastroschisis, admitted to the Neonatal Intensive Care Center 2 (CTIN 2) of the Instituto da Criança (ICr) of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), from January 2012 to December 2018.
CTIN 2 of the ICr of HC-FMUSP is a tertiary reference center for newborns with predominantly surgical congenital malformations and the Fetal Medicine Service of the Obstetric Clinic receives pregnant women with fetuses with gastroschisis and does prenatal follow-up. The delivery occurs in the Obstetric Center of the Central Institute of HC-FMUSP and the newborn is transported to the ICr, directly to the Surgical Center of the ICr located in the same complex but in a building nearby. Most cases were diagnosed with gastroschisis during prenatal care, only 5 cases were outborn and were referred after birth to the ICr. ICr is a reference center for a metropolitan region of São Paulo and receives newborns from several centers.
The following information was collected the clinical records of these newborns:
- ‐
Maternal data: age (in years), parity, route of delivery, and social habits (alcohol, tobacco and recreational drugs).
- ‐
Newborn data: birth weight, gender, gestational age (completed weeks, by date of last menstrual age or first-trimester ultrasound, in this order), presence of other major malformations.
- ‐
Data of the surgical procedure: type of surgery (primary closure or silo), classification of gastroschisis in simple or complex according to the intraoperative finding.
- ‐
Data on the newborn evolution: fasting time; time of parenteral nutrition; time to achieve full enteral nutrition; the presence of confirmed late-onset sepsis, results of cultures (urine, and blood); isolated etiological agent; length of hospital stay, and outcome (death or discharge).
- ‐
Statistical analysis: the sample calculation was not made because it is a convenience sample. The comparison between the two groups (Group 1: simple gastroschisis and confirmed late-onset sepsis and Group 2: complex gastroschisis and confirmed late-onset sepsis) was performed. Results are presented as numbers with proportions (%), mean (SD), or median (IQR). Continuous variables were tested for normality using the Kolmogorov-Smirnov test. Comparisons between groups were performed using the Mann-Whitney U test or T-test for continuous variables, and Pearson Chi-Square for categorical data. All analysis was performed with IBM SPSS Statistics for Macintosh, version 25.0. The level of significance adopted was p < 0.05.
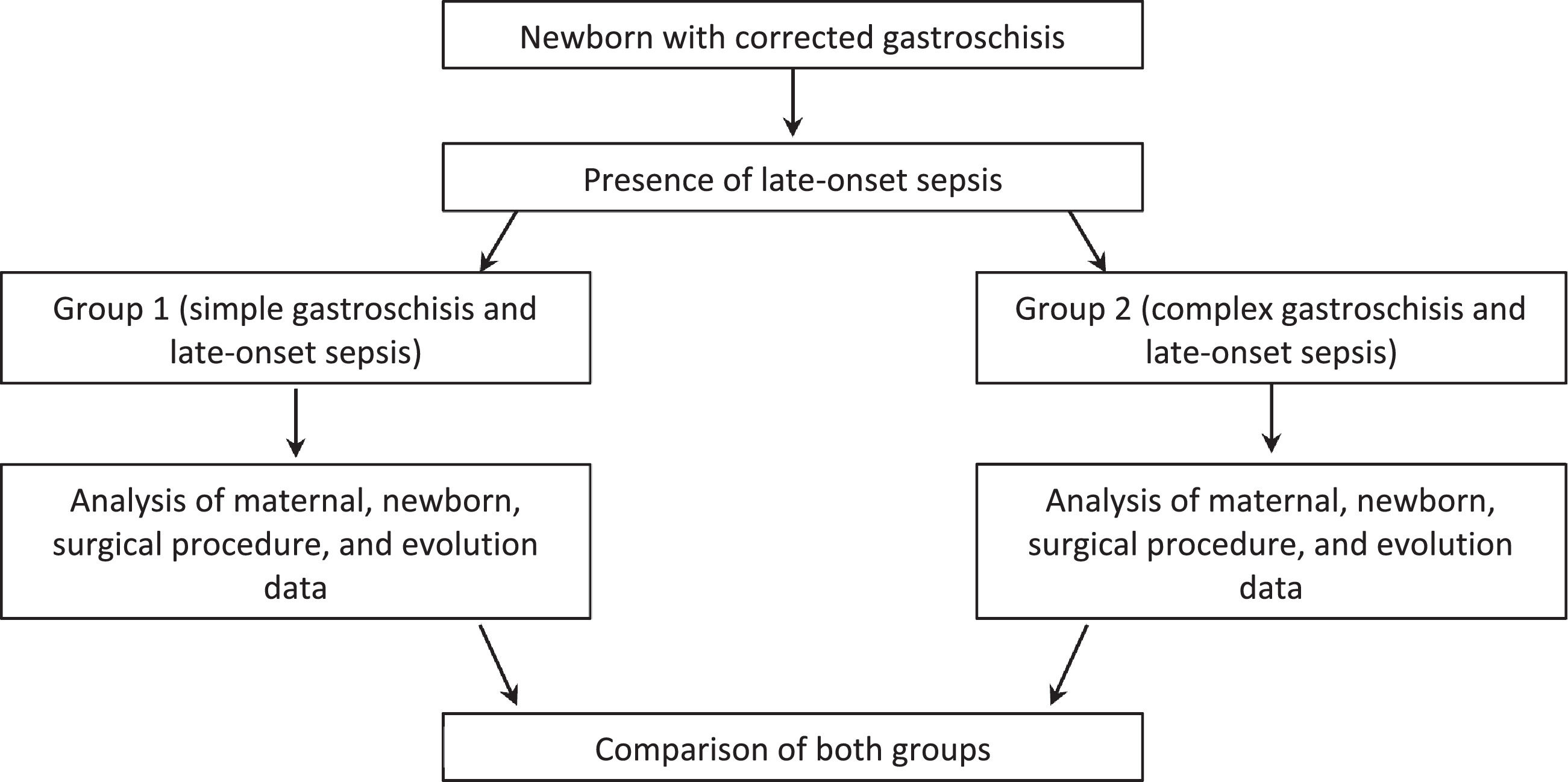
Initially, an analysis was made of newborns with sepsis or not, and then those with infection were divided into simple and complex gastroschisis and analysis was performed (Fig. 1).
The study was approved by the Ethics Committee of the Department of Pediatrics and the Ethics Committee for the Analysis of Research Projects (CAPPesq) of HC-FMUSP, protocol: 2,476,188; there was no need to obtain the consent form because it was a collection of data from medical records.
ResultsIn the study period between January 2012 and December 2018, 101 newborns with gastroschisis were admitted in the service, i.e., 12.6 admissions per year.
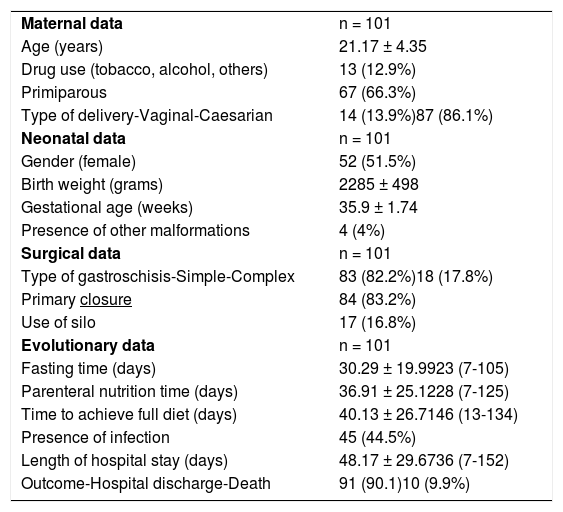
Table 1 shows maternal, newborn, surgical procedure, and neonatal evolution data.
Maternal, neonatal, surgical, and evolutionary data of newborns with gastroschisis.
Of these 101 newborns with gastroschisis, 45 (44.6%) were confirmed late-onset sepsis by blood cultures. The isolated agents were gram-positive: Staphylococcus aureus, Staphylococcus capitis, Staphylococcus epidermidis, Staphylococcus haemolyticus; gram-negative: Acinetobacter baumannii, Enterobacter cloacae, Enterococcus faecalis, Klebsiella oxytoca, Klebsiella pneumoniae, Stenotrophomonas maltophilia, and fungus: Candida albicans. Gram positive were the most common agents (51.1%) followed by gram-negative (20%) and fungi (4.4%) and the others had more than one agent.
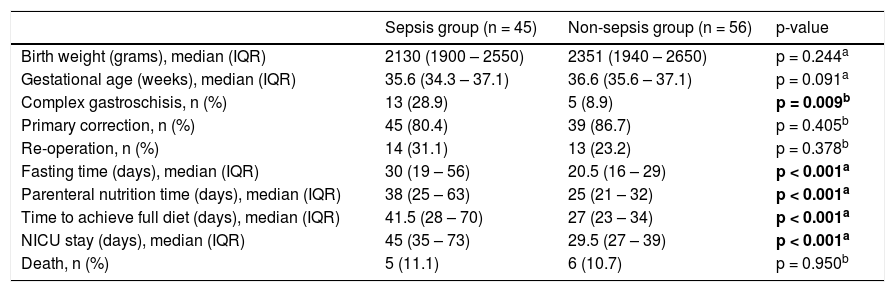
The factors related to sepsis were the presence of complex gastroschisis, fasting days, days of parenteral nutrition, time to reach a full diet, and days in the ICU (Table 2).
Factors related to sepsis in newborns with gastroschisis.
| Sepsis group (n = 45) | Non-sepsis group (n = 56) | p-value | |
|---|---|---|---|
| Birth weight (grams), median (IQR) | 2130 (1900 – 2550) | 2351 (1940 – 2650) | p = 0.244a |
| Gestational age (weeks), median (IQR) | 35.6 (34.3 – 37.1) | 36.6 (35.6 – 37.1) | p = 0.091a |
| Complex gastroschisis, n (%) | 13 (28.9) | 5 (8.9) | p = 0.009b |
| Primary correction, n (%) | 45 (80.4) | 39 (86.7) | p = 0.405b |
| Re-operation, n (%) | 14 (31.1) | 13 (23.2) | p = 0.378b |
| Fasting time (days), median (IQR) | 30 (19 – 56) | 20.5 (16 – 29) | p < 0.001a |
| Parenteral nutrition time (days), median (IQR) | 38 (25 – 63) | 25 (21 – 32) | p < 0.001a |
| Time to achieve full diet (days), median (IQR) | 41.5 (28 – 70) | 27 (23 – 34) | p < 0.001a |
| NICU stay (days), median (IQR) | 45 (35 – 73) | 29.5 (27 – 39) | p < 0.001a |
| Death, n (%) | 5 (11.1) | 6 (10.7) | p = 0.950b |
IQR, interval quartil range; NICU, Neonatal Intensive Care Unit.
Then these newborns with confirmed late-onset sepsis (n = 45) were divided into 2 groups, according to the classification of gastroschisis (simple or complex), as follow:
- ‐
Group 1: late-onset sepsis and simple gastroschisis (n = 32).
- ‐
Group 2: late-onset sepsis and complex gastroschisis (n = 13).
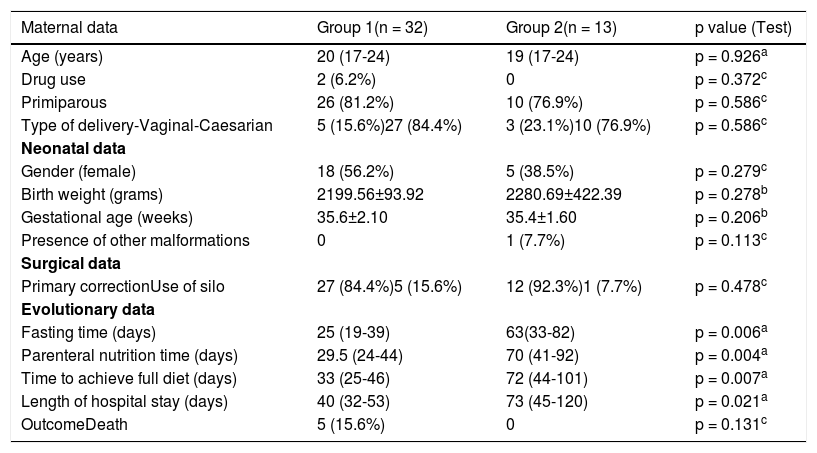
Table 2 shows maternal, newborn, surgical procedure, and neonatal evolution data in Group 1 and Group 2.
DiscussionDespite advances in perinatal, surgical, and technological care, infant newborns with gastroschisis continue to be a challenge for obstetricians, neonatologists, pediatric surgeons and, also for multidisciplinary teams. Care standardization for newborns with gastroschisis enables practice transformation, cost-effective outcome improvement, and supports an organizational culture dedicated to continuous improvement.12
Of the 101 newborns studied, there was no predominance between genders in the incidence of gastroschisis (female 51.5% and male 48.5%), a fact that is comparable to the literature, according to Mastroiacovo et al.5
The incidence of gastroschisis was higher among primiparous women (66.3%), which was also compatible with the literature (60.8%).13 The prematurity rate (gestational age less than 37 weeks) was 59.4%, compatible with the literature (56%).13
The rate of cesarean deliveries found (86.1%) was considerably higher than in the literature (50%), a fact probably related to cultural factors of each country and to protocols of the Obstetrics Service.8 In Brazil, Calcagnotto et al.2 studying the risk factors associated with mortality in newborns with gastroschisis, found a cesarean section rate of 92.2%. Rates of cesarean births in Brazil have increased in the last decades, the rate increased to 30% in the early 1980s, achieving 40% in the early 1990s. The stability observed in the period 1990–2000 was followed by an additional increase, which took the cesarean rates above 50% in 2012. There is a variation in rates according to the region of the country and if it occurs in the public or private service.14
Several authors have argued in favor of routine cesarean section as a means of protecting the exposed intestines from the contracting uterus and avoiding trauma.15 Most studies looking at routes of delivery, including a recent metanalysis,16 do not demonstrate any outcome benefit to elective cesarean versus vaginal delivery. With regards to gestational age, it has been observed that the average gestational age of spontaneous delivery of gastroschisis is less than 37 weeks, leading some investigators to suggest that even earlier, planned preterm delivery results in attenuated bowel injury and improved outcomes.
The incidence of complex gastroschisis was 17.8% slightly higher than that of Raymond et al.8 (14%) and the presence of other associated malformations was 4%.
Mortality in this study was 9.9%. World mortality varies widely according to the region and socio-economic condition. While Raymond et al.8 found a 5% mortality in an American multicenter study, mortality in the northern region of Brazil was 51.2% according to Bilibio et al.17 and 11.4% in the southeast region of Brazil.18 In Europe, in a meta-analysis published in 2016,19 mortality in the Netherlands was 8.8%. These differences can be also be explained by lower adequate prenatal rates of pregnant women, the presence of more cases of complex gastroschisis, and infections.
Sepsis is one of the main causes of morbidity and mortality in the neonatal period, despite advances in the care provided to this population. The clinical evolution of late-onset sepsis is due to several factors such as prematurity and deficiencies in immune response (innate and adaptive).10
Table 1 shows that of the 101 patients with gastroschisis, 44.5% evolved with infectious conditions, which was extremely relevant and far above the literature.19,20 The justification for this fact could be the higher number of complex gastroschisis; rates of prematurity, as well as longer, fasting time, and prolonged parenteral nutrition. However, when comparing the incidence of late sepsis in this study with data published in 2016 with 50 newborns with gastroschisis from the same service, a decrease was observed from 58% to 44.5%.21
Despite the greater susceptibility of the newborn to infectious conditions, it is observed that in gastroschisis there are predisposing factors, such as exposure of the viscera to the external environment, with consequent serositis, surgical approach, prolonged fasting with an increased chance of bacterial translocation, prolonged venous access for parenteral nutrition, and a long stay in the neonatal intensive care unit.8 In this study, the authors found associated factors for sepsis: the presence of complex gastroschisis, longer time of parenteral nutrition fasting, days to reach full diet, and length of hospital stay however without correlation with mortality (Table 2).
Patients with complex gastroschisis start enteral feeding later and take longer to achieve full enteral feeding, with a subsequent longer duration of parenteral nutrition and hospitalization. Thus, the risk of late-onset neonatal sepsis, short bowel syndrome and necrotizing enterocolitis is higher.20
The analysis of Table 3 found that groups 1 and 2 (Group 1: confirmed late-onset sepsis and simple gastroschisis; Group 2: confirmed late-onset sepsis and complex gastroschisis) did not differ in relation to maternal variables - age, parity, and type of delivery. Fasting time was 25 days vs 63 days (p = 0,006), parenteral nutrition time was 29 days vs 70 days (p = 0,004), time to achieve full diet 33 days vs 72 days (p = 0,007), and hospitalization stay 40 days vs 73 days (p = 0,021) were higher in group 2, showing that this group is composed of more severe children with greater difficulty to introduce enteral nutrition. Such results are comparable to those of Bergholz et al.20 that they found in a systematic review and meta-analysis started on enteral feedings later and they take longer to full enteral feedings with a subsequent longer duration of parenteral nutrition and their risk of sepsis, short bowel syndrome and necrotizing enterocolitis is higher.19
Maternal, neonatal, surgical, and evolutionary data in both groups.
| Maternal data | Group 1(n = 32) | Group 2(n = 13) | p value (Test) |
|---|---|---|---|
| Age (years) | 20 (17-24) | 19 (17-24) | p = 0.926a |
| Drug use | 2 (6.2%) | 0 | p = 0.372c |
| Primiparous | 26 (81.2%) | 10 (76.9%) | p = 0.586c |
| Type of delivery-Vaginal-Caesarian | 5 (15.6%)27 (84.4%) | 3 (23.1%)10 (76.9%) | p = 0.586c |
| Neonatal data | |||
| Gender (female) | 18 (56.2%) | 5 (38.5%) | p = 0.279c |
| Birth weight (grams) | 2199.56±93.92 | 2280.69±422.39 | p = 0.278b |
| Gestational age (weeks) | 35.6±2.10 | 35.4±1.60 | p = 0.206b |
| Presence of other malformations | 0 | 1 (7.7%) | p = 0.113c |
| Surgical data | |||
| Primary correctionUse of silo | 27 (84.4%)5 (15.6%) | 12 (92.3%)1 (7.7%) | p = 0.478c |
| Evolutionary data | |||
| Fasting time (days) | 25 (19-39) | 63(33-82) | p = 0.006a |
| Parenteral nutrition time (days) | 29.5 (24-44) | 70 (41-92) | p = 0.004a |
| Time to achieve full diet (days) | 33 (25-46) | 72 (44-101) | p = 0.007a |
| Length of hospital stay (days) | 40 (32-53) | 73 (45-120) | p = 0.021a |
| OutcomeDeath | 5 (15.6%) | 0 | p = 0.131c |
- Group 1: late-onset sepsis and simple gastroschisis.
- Group 2: late-onset sepsis and complex gastroschisis.
Regarding the outcome, 84.4% were discharged from the hospital in group 1 and 100% in group 2. In relation to mortality, 15.6% were obtained in group 1 and zero in group 2 (p = 0,131). It is interesting to note that, although late-onset sepsis is pointed out as a major cause of mortality in children with gastroschisis, 10 deaths were noted in the general analysis (101 cases), in group1 5 cases and in group 2 zero, i.e., 50% of deaths did not occur due to infectious causes. Raymond et al.8 showed a mortality rate of 13% in complex gastroschisis and 4% in simple gastroschisis.
Regarding the isolated agents in the cultures, Gram-positive microorganisms were the most frequent (51.1%), followed by Gram-negative microorganisms (20%). In this series, positivity for fungi was quite low (4.4%). This finding is compatible with Baird et al.22 who showed in a Canadian study with 395 newborns with gastroschisis a predominance of coagulase-negative Staphylococcus.
In a study in three tertiary Brazilian pediatric surgery centers, where one of the centers is the same as in the present study, published in 2011, 163 medical records of newborns with gastroschisis were analyzed between January 2003 and June 2009. Late-onset sepsis was the most frequent cause of death, with 69.5% of all deaths. In this study, the etiological agents of sepsis were not described.23
Another study by the same service, published in 2016, showed a frequency of 58% of late-onset sepsis in newborns with simple and complex gastroschisis, with 37.9% of infections associated with the use of a central venous catheter.21 Patients who presented late-onset sepsis did not differ in gestational age, gender, birth weight, or time for the surgical approach when compared to patients who did not present an infection. On the other hand, the time of mechanical ventilation and hospitalization was longer in those patients with complex gastroschisis and infection and who required more than one surgical approach. The only mention of etiological agents that 72.7% of these infections were related to Staphylococcus epidermidis.22
The study of Calcagnotto et al. (2013)2 showed that mortality was significantly higher in cases of more surgeries are needed and sepsis and this pathology accounted for 66.7% of deaths.
In a Brazilian retrospective study, including 89 newborns with gastroschisis, all neonates received at least one initial antibiotic regimen during hospitalization, and 66.7% received more than one course of antibiotics. Infection rates, as well as etiological agents, have not been described. The use of two or more cycles of antibiotics during hospitalization influenced the time of hospitalization.24
In an older Brazilian study, published in 2001, the authors report that sepsis is a factor of poor prognosis in gastroschisis, but they did not include this variable in the regression model to determine the prognosis of newborns with gastroschisis.25
Infectious complications remain an important consideration in the management of neonates with simple and complex gastroschisis. Measures to prevent infection in this population should be considered given the high incidence.22
The present study's data has some limitations because it is a single-center, retrospective sample and in a period of long analysis when the authors had important technological and care advances. In contrast, during this period the authors developed a multidisciplinary managed protocol aimed at improving care for the newborn with gastroschisis. This protocol covers prenatal care with a focus on nutritional therapy and infection prevention.
In conclusion, this is the first study that lists the etiological agents of late-onset sepsis in the immediate and mediated postoperative period of gastroschisis. This report is important to direct the prescription of antibiotics in the face of the suspicion of sepsis in these children. It is also important to know the profile of etiological agents of the unit that the newborn is hospitalized to rationalize the prescription of antibiotic therapy in the suspicion of sepsis in this population. The authors did not find any statistical difference between the groups regarding mortality. In addition, it is important that every unit that cares for newborns with gastroschisis is aware of the propensity of these children to evolve with late-onset sepsis, to act effectively in its prevention.