Functional echocardiography is a valuable tool in the neonatal intensive care unit, but training programs are not standardized. The aim was to report an functional echocardiography training program for neonatologists and to describe the agreement of their measurements with the pediatric cardiologist.
MethodsFunctional echocardiography training lasted 32h. After training program, the neonatologists performed functional echocardiography in the neonatal intensive care unit and were required to measure left cardiac chambers dimensions, left ventricle systolic function, right and left ventricular output, ductus arteriosus diameter, and flow pattern. Images were recorded by the equipment and reviewed offline by the pediatric cardiologist. The Bland–Altman test was used for quantitative variables and the kappa test, for qualitative variables.
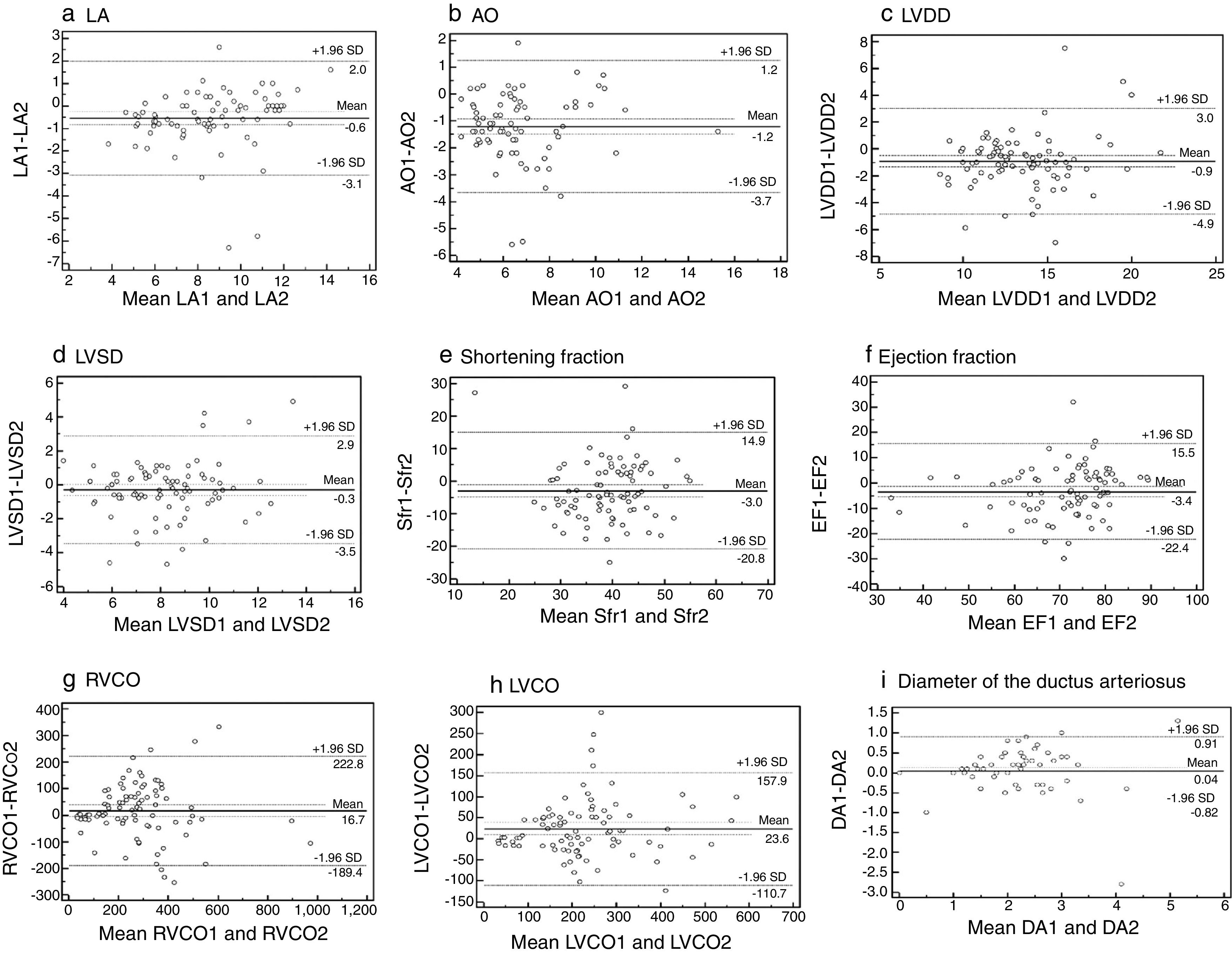
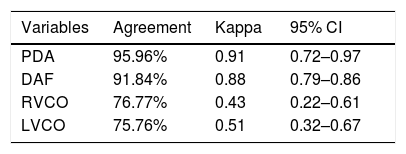
ResultsTwenty-two trained neonatologists performed 100 functional echocardiography exams. Ductus arteriosus identification and flow pattern had substantial agreement (kappa=0.91 and 0.88, respectively), as well as its diameter (mean difference=0.04mm). The mean difference for the aortic root was −1.2mm; left atrium, 0.60mm; left ventricle diastolic diameter, −0.90mm; left ventricle systolic diameter, −0.30mm. Shortening fraction and ejection fraction correlated well with broad limits of agreement, −2.96% (14.88; −20.82%) and −-3.43% (15.54; −22.40%), respectively. Right and left ventricular output had broad limits of agreement, 16.69mL/kg/min (222.76; −189.37) and 23.57mL/kg/min (157.88; −110), respectively. There was good agreement between interpretations of normal or low cardiac output (76.7% for right ventricular output; 75.7% for left ventricular output).
ConclusionThis functional echocardiography training program enabled neonatologists to obtain adequate skills in performing the images, obtaining good agreement with the cardiologist in simple hemodynamic measurements and ductus arteriosus evaluation.
A ecocardiografia funcional é uma ferramenta valiosa na unidade de terapia intensiva neonatal, mas os programas de treinamento não são padronizados. Nosso objetivo foi relatar um programa de treinamento em ecocardiografia funcional para neonatologistas e descrever a concordância de suas medidas com o cardiologista pediátrico.
MétodosO treinamento em ecocardiografia funcional durou 32 horas. Após o programa de treinamento, os neonatologistas faziam ecocardiografia funcional na unidade de terapia intensiva neonatal e mediam as dimensões das câmaras cardíacas esquerdas, função sistólica do ventrículo esquerdo, débito cardíaco do ventrículo direito e débito cardíaco do ventrículo esquerdo, diâmetro do canal arterial e o padrão de fluxo. As imagens foram registradas no equipamento e revisadas offline pelo cardiologista pediátrico. O teste de Bland–Altman foi usado para variáveis quantitativas e o teste Kappa para variáveis qualitativas.
ResultadosForam feitas por 22 neonatologistas treinados 100 ecocardiografias funcionais. A identificação do canal arterial e o padrão de fluxo apresentaram concordância substancial (Kappa=0,91 e 0,88, respectivamente), bem como seu diâmetro (diferença média=0,04mm). A diferença média foi de -1,2mm para a raiz da aorta, 0,60mm para o átrio esquerdo, -0,90mm para o diâmetro diastólico do ventrículo esquerdo e de -0,30mm para o diâmetro sistólico do ventrículo esquerdo. A fração de encurtamento e a fração de ejeção apresentaram boas correlações, com amplos limites de concordância, respectivamente -2,96% (14,88; -20,82%) e -3,43% (15,54; -22,40%). Os débitos cardíacos do ventrículo direito e do ventrículo esquerdo apresentaram amplos limites de concordância, 16,69mL/kg/min (222,76; -189,37) e 23,57mL/kg/min (157,88; -110), respectivamente. Houve boa concordância entre a interpretação de débito cardíaco normal ou baixo (76,7% de débito ventricular direito; 75,7% de débito ventricular esquerdo).
ConclusãoEsse programa de treinamento em ecocardiografia funcional permitiu aos neonatologistas obter habilidades adequadas na realização das imagens, com boa concordância com o cardiologista em medidas hemodinâmicas simples e avaliação do canal arterial.
Echocardiography in the neonatal intensive care unit (NICU) has been used for many years only by echocardiographers to diagnose structural congenital heart diseases. However, in recent years it has also been used by neonatologists for hemodynamic evaluation in various clinical situations.1–3 This use of cardiac ultrasound by neonatologists is termed functional echocardiography (FEcho). FEcho allows assessment of cardiac function, cardiac output, and pulmonary pressure on a continuous basis, if necessary, and detects intracardiac and interarterial shunts. Information obtained with this procedure in association with clinical findings and conventional cardiac monitoring techniques can help guide therapeutic decisions.4 In addition, FEcho is also important because many centers do not have specialists in pediatric echocardiography easily available for the potentially wide-spread and everyday application for diagnosis and follow up in NICU.5
However, FEcho is currently available in very few centers. One of the reasons is lack of an efficient and continuous training program for neonatologists.6,7 For hemodynamic evaluations of neonates by echocardiography, some measures are necessary which require proper training to minimize error.8 International societies have suggested completion of basic FEcho training mainly in an echocardiography laboratory, with a minimum of 150 exams in the pediatric age group over a period of four to six months.9 This basic training proposal does not include echocardiography training focused on hemodynamic studies and specific aspects of newborns in NICU, and therefore, may not reflect the main scenario of the neonatologists’ routine.10
Although recent studies have reported the need to standardize neonatologist training for FEcho, few have analyzed the learning level, particularly regarding quantitative parameters. Thus, this study aimed to describe the measurement agreement between the neonatologist and pediatric cardiologist after a theoretical and practical training program.
MethodsThis was an analytical-prospective study composed of an educational intervention followed by the evaluation of the agreement of the echocardiography measurements performed by the neonatologist and the cardiologist.
Neonatologists from neonatal division in Federal University of São Paulo (UNIFESP) pediatric department, a reference center for undergraduate and graduate studies in the medical area, were invited to participate in FEcho training. They had no prior training in ultrasound technology. Participation was voluntary and did not involve any expense or monetary compensation. There was no previous selection in order to participate in this study. The course took place from January 2011 to December 2012. The study was approved by University's Research Ethics Committee.
The study's aim was to assess concordance of basic echocardiographic measurements between neonatologists and an experienced pediatric cardiologist (MZ) after an educational intervention. This outcome was assessed several months after course completion by reviewing exams performed by neonatologists in the NICU.
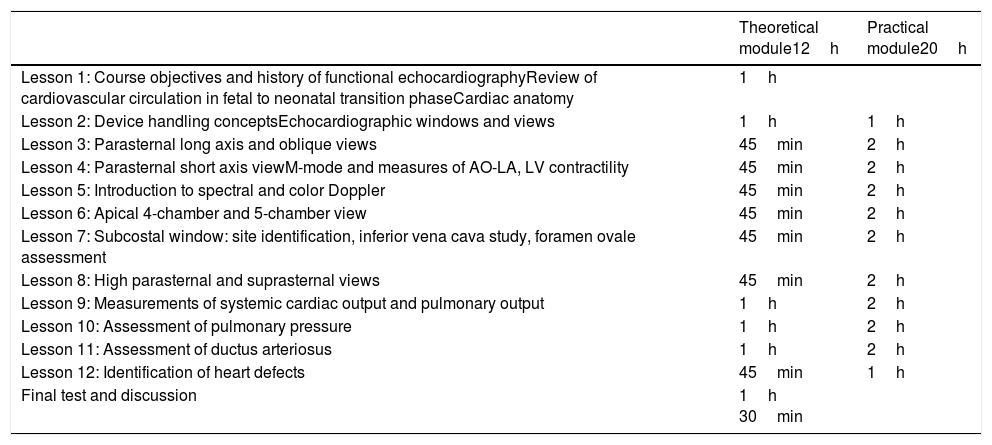
The training program had a 12-lesson theoretical module and 20 practical training lessons performed under the supervision of an experienced pediatric cardiologist certified by the national society of cardiology and echocardiography. A neonatologist with two years experience in FEcho assisted in practical training as well. The echocardiographic system used for practical training and exam performance was a Siemens X300 pre-edition (Siemens Medical Solutions, Korea) with a 4–9MHz transducer and predefined settings for optimal image resolution.
These 20 practical sessions were conducted on low- and medium-risk neonatal units during 15 days after theoretical classes on the respective topic. Two meetings were scheduled, for two hours each, in pairs so they could examine a total of 20 neonates and observe another 20 exams from their partner.
These hands-on sessions were performed in a tertiary university hospital, mainly in normal neonates (Level 1).
Neonatologists were trained to acquire all echocardiographic windows as described in Table 1, and to perform echocardiographic measurements on left cardiac chambers dimensions and left ventricle systolic function using the M-mode technique. The main measures were aortic root diameter in diastole (AO), left atrium diameter in systole (LA), and left ventricular systolic and diastolic diameters (LVSD, LVDD). The left ventricular shortening fraction (Sfr) and ejection fraction (EF), obtained by the Teichhoz formula, were used as left ventricular systolic function parameters. To calculate left and right ventricle cardiac outputs (LVCO, RVCO), respective LV and RV outflow tract systolic diameter, and velocity time integral by pulsed wave Doppler were measured, using several echocardiographic windows (parasternal long axis view and apical five chamber for LVCO and parasternal long axis oblique for pulmonary artery and parasternal short axis view for RVCO). They were also trained to identify and measure ductus arteriosus diameter and to classify its flow pattern as left–right, right–left, or bidirectional.
Functional echocardiography training (FEcho) for neonatologists: theoretical and practical content and workload.
| Theoretical module12h | Practical module20h | |
|---|---|---|
| Lesson 1: Course objectives and history of functional echocardiographyReview of cardiovascular circulation in fetal to neonatal transition phaseCardiac anatomy | 1h | |
| Lesson 2: Device handling conceptsEchocardiographic windows and views | 1h | 1h |
| Lesson 3: Parasternal long axis and oblique views | 45min | 2h |
| Lesson 4: Parasternal short axis viewM-mode and measures of AO-LA, LV contractility | 45min | 2h |
| Lesson 5: Introduction to spectral and color Doppler | 45min | 2h |
| Lesson 6: Apical 4-chamber and 5-chamber view | 45min | 2h |
| Lesson 7: Subcostal window: site identification, inferior vena cava study, foramen ovale assessment | 45min | 2h |
| Lesson 8: High parasternal and suprasternal views | 45min | 2h |
| Lesson 9: Measurements of systemic cardiac output and pulmonary output | 1h | 2h |
| Lesson 10: Assessment of pulmonary pressure | 1h | 2h |
| Lesson 11: Assessment of ductus arteriosus | 1h | 2h |
| Lesson 12: Identification of heart defects | 45min | 1h |
| Final test and discussion | 1h 30min |
After finishing the training program, neonatologists were encouraged to perform FEcho at bedside. They have full echocardiography equipment availability in the NICU. Neonates with congenital heart disease were excluded.
For each exam, the neonatologist completed a paper form with the following data: newborn identification, anthropometric data (weight and height), use of mechanical ventilation or vasoactive drugs, and description of echocardiographic measurements. Finally, they were required to report the time taken to perform the FEcho. These FEcho were not used for making recommendations to the NICU medical team. When there was a clinical indication for echocardiographic evaluation, the hospital pediatric cardiology team, regardless of this study's purposes, performed echocardiography.
Neonatologists recorded and saved all clips and still images from each exam in the ultrasound equipment with proper identification; therefore, measurements could be repeated offline. Measures conducted by the neonatologists were not visible in the equipment. Stored images and clips of each exam were reviewed by the pediatric cardiologist responsible for the study when 100 exams were completed, thus each frame and cardiac cycle that was considered most appropriate to carry out measures directly in the equipment could be chosen. This analysis was recorded in a table with proper identification. After completing all exam measurements, the neonatologists’ analyses were compared with the cardiologist.
FEcho exam quality and accuracy by neonatologists were evaluated by comparing their findings (images, measurements) with those from the cardiologist (gold standard).
Student's t-test was used to compare independent numerical samples. Agreement between the measures by the cardiologist and neonatologists was assessed by Bland–Altman method.11 Agreement for categorical variables was analyzed using the kappa test. Stata/SE 9.0 for Windows (Stata Corporation – College Station, TX, United States) was used for statistical analysis. For all tests, p<0.05 was considered statistically significant.
ResultsTwenty-two neonatologists participated in theoretical and practical training. Neonatologist participants had 1–7 years of practice in neonatology (median 2.8 years). After finishing training, 100 FEcho exams were performed by neonatologists in the NICU during an 18-month period. The median number of FEcho exams done by each neonatologist was 2 (1.25–5.25 interquartile range). Newborns had a mean (±standard deviation) gestational age of 31 weeks (±4), birth weight of 1361g (±835), and a chronological age of 12 days (±11.8); 65% of newborns were on mechanical ventilation and 43% were under treatment with vasoactive drugs. The mean time required by each neonatologist to perform the FEcho was 29.6 (±9.5)min.
Bland–Altman analyses (Fig. 1) show mean difference and measurements’ limits of agreement between neonatologists and the pediatric cardiologist. Measurements of LA, AO, LVDD, and LVSD were adequate, with narrow limits of agreement. Measures of Sfr and EF had good agreement, but large limits of agreement. RVCO and LVCO measurements presented broad agreement limits. When cardiac output was categorized as normal or low, diagnostic concordance was 76.7% for RVCO and 75.7% for LVCO (Table 2). Patent ductus arteriosus was identified by the cardiologist in 57 FEcho exams, and in 55 by neonatologists. For patent ductus arteriosus identification and flow pattern, kappa values were 0.91 and 0.88, with a substantial agreement between neonatologists and the cardiologist (Table 2). For ductus arteriosus diameter measurement, the difference between neonatologists and the cardiologist was very low, with narrow limits of agreement (0.04mm mean difference; 95% limits of agreement: 0.91–0.82mm; Fig. 1).
Bland–Altman plot analysis: agreement between neonatologists (1) and cardiologist (2) measurements: a. left atrium (LA), b. aorta (AO), c. left ventricle diastolic diameter (LVDD), d. left ventricle systolic diameter (LVSD), e. shortening fraction (Sfr), f. ejection fraction (EF), g. right ventricular cardiac output (RVCO), h. left ventricular cardiac output (LVCO), i. ductus arteriosus diameter (DA).
Agreement between the examinations done by neonatologists and the cardiologist for qualitative analysis of the diagnosis of a patent ductus arteriosus (PDA), type of ductus arteriosus flow (DAF), and classification of normal or abnormal right ventricular cardiac output (RVCO) and left ventricular cardiac output (LVCO).
| Variables | Agreement | Kappa | 95% CI |
|---|---|---|---|
| PDA | 95.96% | 0.91 | 0.72–0.97 |
| DAF | 91.84% | 0.88 | 0.79–0.86 |
| RVCO | 76.77% | 0.43 | 0.22–0.61 |
| LVCO | 75.76% | 0.51 | 0.32–0.67 |
The results of this study showed that this FEcho training program enabled neonatologists to obtain conventional echocardiographic images and perform simple measurements for hemodynamic evaluation. The best results were obtained in the detection and measurement of ductus arteriosus. This is an important finding, since the search for persistent ductus arteriosus is not only a frequent exam indication but may also have therapeutic implications in daily care of critically ill preterm infants.12–14
Cardiac examination with ultrasound performed by non-specialists has received great attention, but there are different types of training for neonatologists. In 2008, in Australia, a training and certification program in FEcho was developed.15 This program comprises a basic two-day classroom course, followed by a recommendation to perform 50 cardiac studies in neonates over a maximum period of two years. After this period, an advanced two-day course should be taken, followed by completion of another 25 cardiac exams in neonates. In this program, all exams had to be recorded in a logbook and reviewed by a neonatologist experienced in FEcho. In Canada, FEcho was introduced in 2006 and the duration of training courses ranges from less than eight weeks (14%) to more than 16 weeks (29%).6 The actual results of such trainings in learning level have not been published. Guidelines and training recommendations in FEcho have been published in consensus between the American Society of Echocardiography and the European Associations of Pediatric Cardiology and Ecocardiography.9 This consensus recommends a basic course lasting four to six months, after which every student should perform 150 exams and interpret another 150 exams. This consensus also recommends an advanced training with the same duration and number of exams as the basic course, aiming to enable students to perform and interpret FEcho exams independently. However, the mentioned consensus recommends training in an echocardiography laboratory with patients and diseases that do not necessarily represent the reality of neonatologists.10
The design of this training program included the basic topics of image acquisition and hemodynamic evaluation in FEcho, with each theoretical class followed by corresponding practical training, which may have contributed to satisfactory learning level observed in this relatively short training model. Other factors that may have contributed to this better performance included full availability of and full access to the echocardiographic equipment. These points were identified as limiting factor to FEcho learning maintenance in other studies.6,7
In post-training activities, average exam duration by the neonatologists was approximately 30min, which was satisfactory considering that recommended duration for a study with an echocardiography specialist is 45–60min.16 The group of neonates submitted to FEcho was preterm: over half were on mechanical ventilation and many were receiving vasoactive drugs. This type of patient is more difficult for image acquisition and performance measures, and even in this group, the neonatologists were able to perform basic images and measures in a satisfactory time.
Digital image acquisition and offline measurements were critical to the relatively short exam duration. The authors believe that it is possible to further reduce exam duration by increasing the number of exams per neonatologist and improving their skills in image acquisition and equipment handling.
The quantitative measurement analysis performed by neonatologists and the experienced pediatric cardiologist in this study presented good agreement, especially regarding left ventricle systolic function. FEcho is an important tool in a longitudinal patient follow-up beyond the subjective analyses and may contribute to therapeutic modification, especially considering cardiac systolic function.
Right and left ventricular cardiac output measurements demonstrate broad limits of agreement, 16.69mL/kg/min (222.76–189.37mL/kg/min) and 23.57mL/kg/min (157.88–110.0mL/kg/min), respectively. However, it must be observed that normal values are also broad and considered normal in this age group when between 150 and 450mL/kg/min. When considering normal interpretation or low cardiac output, concordance of 76.7% for RVCO and 75.7% for LVCO was found. Low accuracy of non-invasive cardiac output assessment is reported in literature.17–19 The main source of error in the estimate of cardiac output is the measurement of the ventricular outflow tract radius, which is squared: small measurement differences may cause large variations in calculation and, consequently, in cardiac output estimation.
In order to improve this hemodynamic measurement's reproducibility, particularly in preterm newborns, it has been suggested to use diameter percentiles adjusted for weight, as proposed by de Waal et al.20; using this proposal, radius measurement is predetermined. Currently, in the NICU where the study was conducted, neonatologists are instructed to perform ventricle outflow tract measurement in the first exam and to adopt it as a measure in subsequent exams in the same patient to improve reproducibility. Thus, only velocity time integral measurement in the respective outflow becomes necessary. Other methods have been proposed to evaluate LVCO in neonates, such as magnetic resonance (MRI), with limits of agreement considered acceptable between −79.6 and +60.0mL/kg/min,21 which are lower than the values found in this study (−110.7 to +157.9mL/kg/min), but this technique is not feasible in most centers, nor for everyday application.
LimitationsThe number of exams performed by each neonatologist after training was variable and many of them did few exams. It is possible that neonatologists who performed more exams had better performance, but authors chose not to analyze this subgroup due to the small sample size of this study. Even after well-established training programs, only 27% of trained neonatologists reported having incorporated FEcho into their clinical practice.6
Implications for practice and researchUpon completion of this study and presentation of results to the hospital board, this theoretical and practical educational intervention was incorporated into the training of all pediatricians specializing in neonatology at this institution.
ConclusionThis FEcho training program enabled neonatologists to obtain adequate skills in performing images with good agreement with the specialist in simple hemodynamic measurements and ductus arteriosus evaluation. These point-of-care hemodynamic evaluations may improve the delicate management of critically ill preterm infants. Advanced hemodynamic measurements, such as cardiac output, may require a longer training and enhancement in measurements in order to be more effective.
FundingThis study was financed in part by the Coordenaçao de Aperfeiçoamento de Pessoal de Nível Superior - Brasil ( CAPES) - Finance code 001.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Zamith MM, Figueira SA, Oliveira AC, Metolina C, Castro JS, Santos CN, et al. Functional echocardiography training in the neonatal intensive care unit: comparing measurements and results with the pediatric cardiologist. J Pediatr (Rio J). 2020;96:614–20.