To analyze the factors associated with leprosy in children who were intradomiciliary contacts of notified adults with the disease in an endemic municipality in Mato Grosso, Brazil.
MethodCase–control study with 204 children under 15 years of age, living in an endemic municipality. Cases (n=40) were considered as the children with leprosy registered at the National Information System of Notifiable Diseases in 2014 and 2015, who were intradomiciliary contacts of at least one adult diagnosed with the disease in the family, and as a control group (n=164) of children living within a radius of up to 100m of the notified cases. Data were obtained through medical file analysis, interviews, and blood samples for anti-PGL-I serological test by the ELISA method. The binary logistic regression technique was used, with p≤0.05.
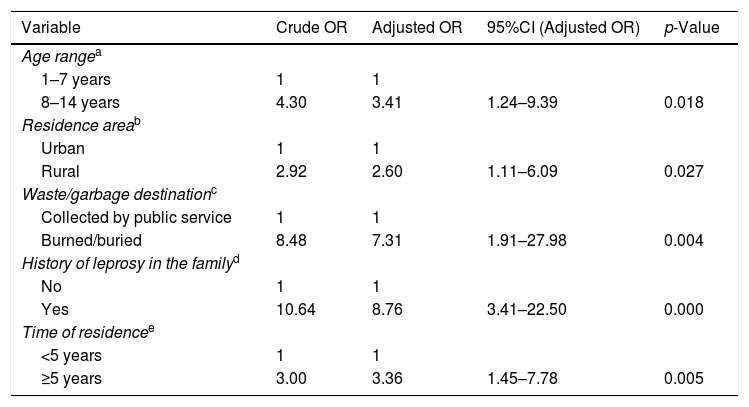
ResultsAfter adjustments, the following were associated with leprosy: age (95% CI: 1.24–9.39, p=0.018), area of residence (95% CI: 1.11–6.09, p=0.027), waste disposal (95% CI: 1.91–27.98, p=0.004), family history of the disease (95% CI: 3.41–22.50, p=0.000), and time of residence (95% CI: 1.45–7.78, p=0.005).
ConclusionFactors associated with the disease indicate greater vulnerability of children aged 8–14 years, associated with living conditions and time of residence, as well as the family history of the disease.
Analisar os fatores associados à hanseníase em crianças contatos intradomiciliares de adultos notificados com a doença em município endêmico, Mato Grosso, Brasil.
MétodoEstudo caso-controle com 204 menores de 15 anos residentes em um município endêmico. Consideraram-se casos (n = 40) crianças registradas com hanseníase no Sistema Nacional de Agravos de Notificação em 2014 e 2015 e que eram contatos intradomiciliares de pelo menos um adulto diagnosticado com a doença na família e como grupo controle (n = 164) crianças residentes a um raio de 100 metros dos casos notificados. Os dados foram obtidos por meio de análise de prontuários, entrevistas e coleta de amostras de sangue para investigação sorológica do anti-PGL-I pelo método Elisa. Usou-se a técnica de regressão logística binária e p ≤ 0,05.
ResultadosMostraram-se associados à hanseníase após ajustes: idade (IC 95%: 1,24–9,39; p = 0,018), zona de residência (IC 95%: 1,11-6,09; p = 0,027), destino de lixo (IC 95%: 1,91-27,98; p = 0,004), história da doença na família (IC 95%: 3,41-22,50; p = 0,000) e tempo de moradia (IC 95%: 1,45-7,78; p = 0,005).
ConclusãoOs fatores associados à doença indicam maior vulnerabilidade em menores de 8 a 14 anos, ligadas as condições e ao tempo de moradia, bem como a história da doença na família.
Leprosy in children and adolescents is a sensitive indicator of the magnitude of the disease in the community, demonstrates the limited efficacy of health programs, and allows the identification of risk factors in this population.1,2 The presence of deformities and physical disabilities in children suggests the persistence of the disease's transmissibility, with a negative impact on self-esteem, school performance, and psychological profile.1–3
In Brazil, although the rate of disease detection in children under 15 years indicates a downward trend in all the regions in the last ten years, some federal units and Brazilian capitals remain hyperendemic and show stationary tendencies, which require additional preventive strategies due to the difficulty in eliminating the disease in the country.4,5 In the state of Mato Grosso, some epidemiological indicators, such as the proportion of multibacillary cases and the presence of grade 2 physical disability, reveal the disease worsening due to a late diagnosis with the persistence of transmission sources.3,6
The transmission of Mycobacterium leprae (M. leprae) in children does not differ from that in adults. However, the conversion of infection into the disease depends on the intrinsic characteristics of the bacillus, of this microorganism's relationship with the host's immune system, and may be influenced by genetic and environmental factors, nutritional status, Bacillus Calmette-Guerin (BCG) vaccine, bacillary load, other mycobacteria, and unfavorable socioeconomic factors.3,7 Contacts of bacilliferous cases, mainly intradomiciliary contacts younger than 15 years, are more likely to contract the infection than an adult, and the risk of developing the disease increases between contacts from 5 to 15 years of age and peaks between 15 and 19 years of age, followed by a decrease in the risk between the ages of 20 and 29 years.3,8,9
Moreover, when the patient is a multibacillary (MB) case, the risk of their contacts developing the disease is greater in relation to the paucibacillary form (PB), and when these cases have from two to five lesions, the risk of developing the disease may be similar to the MB contacts.8
In this context, serological tests capable of identifying specific antibodies against M. leprae, including the anti-glycolipid phenol-1 test (anti-PGL-I), were developed to assist in the early diagnosis, aiming to detect the presence of bacillus infection before the manifestation of signs and symptoms of the disease.10,11 A systematic review study and meta-analysis indicates that the sensitivity of the PGL-I test as a predictor of the clinical development of leprosy among contacts with the disease was less than 50%, and the specificity was higher than 80%.12
In cases of leprosy contacts, the risk of developing the disease is approximately three times higher in those with a positive result for anti-PGL-I than in those with a negative result.12 However, a negative serological result does not necessarily mean absence of infection, but rather could be associated to a low titer of antibodies against M. leprae, as in clinical PB forms,13,14 which reinforces the need for systematic follow-up of these individuals with the purpose of reducing the sources of infection.15,16
The identification of risk factors for leprosy in children under 15 years old and the use of serological tests can be considered a screening strategy for new cases in this more vulnerable population group, in addition to aiding interventions aimed at the early diagnosis and reduction of the risk of the disease. Currently, there are few studies, mainly in the pediatric population, on factors related to the higher risk of developing the disease. Considering this context, the present study aimed to analyze the factors associated with leprosy in children who were intradomiciliary contacts of notified adults with the disease in an endemic municipality in the state of Mato Grosso, Brazil.
MethodsCase–control study of children under 15 years of age living in a municipality endemic for leprosy, Cuiabá, Mato Grosso, Brazil. Cases were considered as all children under 15 years of age diagnosed with leprosy and registered in the National Information System of Notifiable Diseases (Sistema Nacional de Agravos de Notificação [SINAN-MT]) from 2014 to 2015 and who were intradomiciliary contacts of at least one adult diagnosed with the disease in the family, and as a control group all the neighborhood contacts without signs and symptoms of leprosy, with a negative anti-PGL-1 (optical density [OD]<0.150) and residing within a radius of up to 100m of the cases. All children were evaluated by specialists in the referral units for the disease.
Of the total number of participants (n=215), the authors excluded those who were not located (case group: n=5), as well as the children evaluated during screening for the control group with a diagnostic confirmation of leprosy (n=2) and those with a positive anti-PGL-I test (OD: ≥0.150) (n=4). Of these, 40 cases and 164 controls were included in the analysis.
Data sources included: notification files, medical records (case group), and interviews for the collection of sociodemographic, cohabitation, and immunological data and blood samples for the analysis of the anti-PGL-I serological test using the enzyme-linked immunosorbent assay (ELISA) method. Data collection and screening for the identification of the control group were carried out from February to July 2016.
A pilot test was carried out to adjust the research project activities and the collection tool for all information from the present study.
The detection of anti-PGL-I IgM antibodies was performed by Instituto Lauro de Souza Lima laboratory, Bauru, São Paulo, Brazil, using the ELISA immunoenzymatic method and was conducted according to the methodology described by Brett17 and Silva.18 To perform the technique, 96-well polystyrene plates were used, coated by natural disaccharide octyl radical-linked and coupled to bovine serum albumin (ND-O-BSA) semisynthetic antigen was used. Spectrophotometric analysis at 490nm was used to perform the enzymatic activity evaluation. The reading of the results was performed through OD, subtracting the mean OD values from the wells with BSA and with ND-O-BSA. An OD≥0.150 was considered the cut-off point for test positivity.
The SPSS (IBM SPSS Statistics for Windows, version 20.0, NY, USA) and STATA (Stata Statistical Software: Release 8, College Station, TX, USA) were used to manage and analyze the data. Data consistency was evaluated through the VALIDATE App (Microsoft®, Power Apps, Validate, WA, USA). For the exploratory analysis of quantitative data such as age, mean and standard deviation were considered. Absolute and relative frequency measures were considered for independent categorical variables. The odds ratio (OR) was used to determine the association between the predictor variables and the outcome, with a 95% confidence interval. The variables with p<0.20 were inserted using the stepwise method in the binary logistic regression model, and those with statistical significance, with a p ≤0.05, were maintained in the final model.
The present research was approved by the Research Ethics Committee (REC) of the State Secretariat of Mato Grosso/School of Public Health, process No. 443830.
ResultsOf the 204 study participants, 19.6% corresponded to the case group, and 80.4% corresponded to the control group. Notified cases of children and adolescents with leprosy showed a mean age of 10.85 years, with a minimum age of 4 years and a maximum of 14 years (SD=±2.74). Regarding the clinical characteristics of the cases, 40% were classified as PB and 60% as MB. The mean age of the control group was 7.35 years, with a minimum age of 1 and a maximum of 14 years (SD=±3.95). The proportion of contacts in the control group evaluated during the screening with a positive result for anti-PGL-1 serological test was 2.4%.
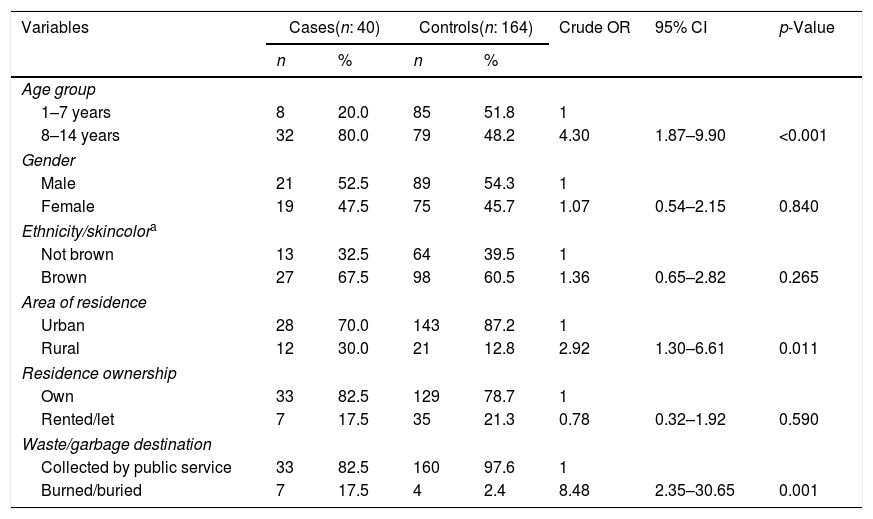
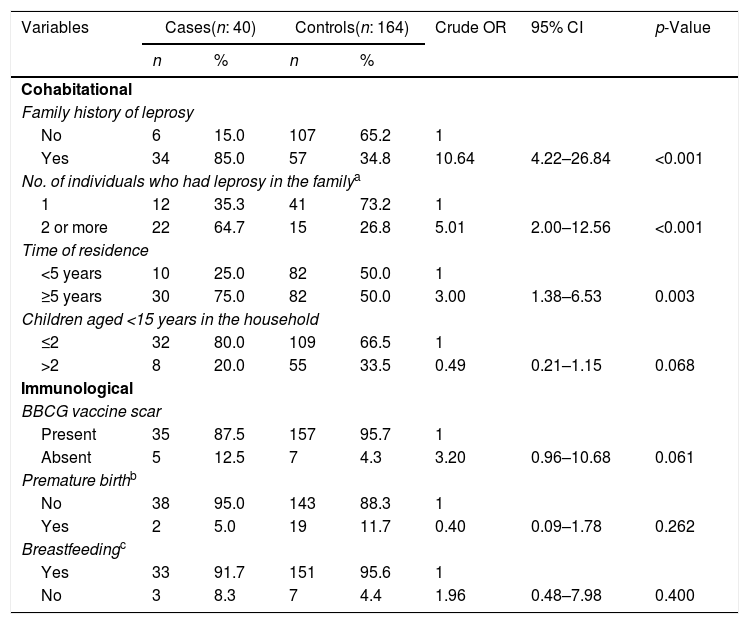
The results of the bivariate and adjusted analyses are shown in Tables 1–3. It was found after adjustments that the chance of occurrence of leprosy in individuals aged 8–14 years was 3.4 times higher than that in individuals aged 1–7 years (95% CI: 1.24–9.39). For those living in rural areas, the chance of the disease was 2.6 times higher when compared to those living in urban areas (95% CI: 1.11–6.09). It was observed that when garbage was burned or buried, the chance of leprosy was 7.3 times higher in comparison to those with access to garbage collection (95% CI: 1.91–27.98). Children with a family history of leprosy had an 8.7-fold higher chance of having the disease in comparison to those who did not have individuals with the disease in the family (95% CI: 3.41–22.5). The probability of leprosy occurrence was about 3.3-fold higher among those with a time of residence ≥5 years compared to those with a shorter time of residence (95% CI: 1.45–7.78).
Bivariate analysis of leprosy cases younger than 15 years according to sociodemographic variables, Cuiabá, Mato Grosso, Brazil.
| Variables | Cases(n: 40) | Controls(n: 164) | Crude OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Age group | |||||||
| 1–7 years | 8 | 20.0 | 85 | 51.8 | 1 | ||
| 8–14 years | 32 | 80.0 | 79 | 48.2 | 4.30 | 1.87–9.90 | <0.001 |
| Gender | |||||||
| Male | 21 | 52.5 | 89 | 54.3 | 1 | ||
| Female | 19 | 47.5 | 75 | 45.7 | 1.07 | 0.54–2.15 | 0.840 |
| Ethnicity/skincolora | |||||||
| Not brown | 13 | 32.5 | 64 | 39.5 | 1 | ||
| Brown | 27 | 67.5 | 98 | 60.5 | 1.36 | 0.65–2.82 | 0.265 |
| Area of residence | |||||||
| Urban | 28 | 70.0 | 143 | 87.2 | 1 | ||
| Rural | 12 | 30.0 | 21 | 12.8 | 2.92 | 1.30–6.61 | 0.011 |
| Residence ownership | |||||||
| Own | 33 | 82.5 | 129 | 78.7 | 1 | ||
| Rented/let | 7 | 17.5 | 35 | 21.3 | 0.78 | 0.32–1.92 | 0.590 |
| Waste/garbage destination | |||||||
| Collected by public service | 33 | 82.5 | 160 | 97.6 | 1 | ||
| Burned/buried | 7 | 17.5 | 4 | 2.4 | 8.48 | 2.35–30.65 | 0.001 |
OR, odds ratio; 95% CI, 95% confidence intervals; p, p-value.
Bivariate analysis of leprosy cases under 15 years of age according to cohabitational and immunological variables, Cuiabá, Mato Grosso, Brazil.
| Variables | Cases(n: 40) | Controls(n: 164) | Crude OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Cohabitational | |||||||
| Family history of leprosy | |||||||
| No | 6 | 15.0 | 107 | 65.2 | 1 | ||
| Yes | 34 | 85.0 | 57 | 34.8 | 10.64 | 4.22–26.84 | <0.001 |
| No. of individuals who had leprosy in the familya | |||||||
| 1 | 12 | 35.3 | 41 | 73.2 | 1 | ||
| 2 or more | 22 | 64.7 | 15 | 26.8 | 5.01 | 2.00–12.56 | <0.001 |
| Time of residence | |||||||
| <5 years | 10 | 25.0 | 82 | 50.0 | 1 | ||
| ≥5 years | 30 | 75.0 | 82 | 50.0 | 3.00 | 1.38–6.53 | 0.003 |
| Children aged <15 years in the household | |||||||
| ≤2 | 32 | 80.0 | 109 | 66.5 | 1 | ||
| >2 | 8 | 20.0 | 55 | 33.5 | 0.49 | 0.21–1.15 | 0.068 |
| Immunological | |||||||
| BBCG vaccine scar | |||||||
| Present | 35 | 87.5 | 157 | 95.7 | 1 | ||
| Absent | 5 | 12.5 | 7 | 4.3 | 3.20 | 0.96–10.68 | 0.061 |
| Premature birthb | |||||||
| No | 38 | 95.0 | 143 | 88.3 | 1 | ||
| Yes | 2 | 5.0 | 19 | 11.7 | 0.40 | 0.09–1.78 | 0.262 |
| Breastfeedingc | |||||||
| Yes | 33 | 91.7 | 151 | 95.6 | 1 | ||
| No | 3 | 8.3 | 7 | 4.4 | 1.96 | 0.48–7.98 | 0.400 |
Logistic regression of factors associated with the occurrence of leprosy in children under 15 years of age, Cuiabá, Mato Grosso, Brazil.
| Variable | Crude OR | Adjusted OR | 95%CI (Adjusted OR) | p-Value |
|---|---|---|---|---|
| Age rangea | ||||
| 1–7 years | 1 | 1 | ||
| 8–14 years | 4.30 | 3.41 | 1.24–9.39 | 0.018 |
| Residence areab | ||||
| Urban | 1 | 1 | ||
| Rural | 2.92 | 2.60 | 1.11–6.09 | 0.027 |
| Waste/garbage destinationc | ||||
| Collected by public service | 1 | 1 | ||
| Burned/buried | 8.48 | 7.31 | 1.91–27.98 | 0.004 |
| History of leprosy in the familyd | ||||
| No | 1 | 1 | ||
| Yes | 10.64 | 8.76 | 3.41–22.50 | 0.000 |
| Time of residencee | ||||
| <5 years | 1 | 1 | ||
| ≥5 years | 3.00 | 3.36 | 1.45–7.78 | 0.005 |
The present study is a pioneer in the screening of neighborhood contacts through the anti-PGL-I serological test in children under 15 years old, aiming to investigate factors associated with the occurrence of leprosy in children. The findings indicated a greater chance of developing the disease in children aged 8 to 14 years, living in rural areas, with burned/buried garbage, time of residence longer than five years, and history of the disease in the family.
Although the study excluded the neighborhood contacts with a positive result in anti-PGL-I test in children under 15 years of age (control group), as it resulted in selection bias, a seropositivity of 2.4% was found in this group. Other studies have indicated a prevalence of seropositivity between 14% and 18%, albeit among intradomiciliary contacts in the general population by age group.19,20 The risk of contracting leprosy is not restricted to the group of close relatives living under the same roof, but also includes neighborhood and social contacts (school, work, etc.).8
Regarding disease surveillance, the contacts of leprosy, especially the pediatric population, should be the priority groups for disease control. The health teams are advised to carry out an annual clinical evaluation and follow-up of the cases with the highest risk of contracting the disease for a period of at least five years, immunization with the BCG vaccine for contacts with absence of signs and symptoms of the disease, and recommendations to the family regarding the disease.3
A positive serological result among children under 15 years of age may be indicative of early exposure to M. leprae and the persistence of disease transmissibility in the community.19 Exposures and re-exposures to the bacillus during intradomiciliary or extradomiciliary relationships with cases of the disease may lead to infection. Moreover, the immunological immaturity of children favors a greater susceptibility to infections.10
Studies indicate that children living in an endemic area may develop specific antibodies against M. leprae in response to early exposure to the bacillus, and levels of anti-PGL-I antibodies in the body correlate with the present bacillary index.16,19
It was observed that children and adolescents aged 8–14 years have a greater chance of developing the disease. Other studies indicated that the most affected age group among children under 15 years of age was between 10 and 14 years, which could be related to the individuals’ immunopathological response, genetic factors, and the long disease incubation period.21
The higher risk of occurrence of the disease for those living in rural areas may be justified by local and sociodemographic characteristics. Rural areas present difficulties for access to health services, which consequently contributes to late diagnosis, persistence of active transmissibility of the infection, and development of new cases of the disease.4 The lack of awareness and non-suspicion of the disease by the individuals and family and the scarcity of skilled health professionals, also cooperate to bring about the delay in the disease detection and its dissemination.14 Thus, it is important to develop health education actions aimed at the population and to train the health teams, enabling them to suspect the disease, allowing an early diagnosis and interruption of the transmission chain by implementing treatment of the disease.
Another factor that remained associated with the occurrence of leprosy in this population was the treatment of garbage and waste. It is known that the collection of household waste through public collection is an effective measure aiming to control factors that might be harmful to health.18 However, part of the population – such as those living in rural areas and those in the urban outskirts – do not have access to this type of service, using other forms of waste disposal. In this study, it was observed that those who burned or buried the household waste showed higher chances of having leprosy when compared to those with access to public collection of waste/garbage. Therefore, it is emphasized that environmental and health conditions influence the quality of life of a population and the risk of disease development.
Children and adolescents with a family history of leprosy were more likely to be infected, and the risk was higher among those who had two or more cases of leprosy in the family, which remained strongly associated in the multivariate analysis. The genetic and physical distance between the susceptible individual and the case and the intensity of exposure may favor incidence of the disease.8,19 Other studies have reported that the risk of a healthy person having leprosy increases nine-fold when a family member is affected.3 Thus, the higher the number of patients in the family, the greater the exposure to the bacilli and the risk of infection among the exposed individuals.8,19
This study identified that there is a greater chance of occurrence of the disease among the children who lived in the residence for a period of five years or longer. This may be related to the fact that they inhabit a spatial clustering of endemic disease and share situations of poverty, in which transmissibility is facilitated, since unfavorable socioeconomic conditions and precarious housing conditions influence the risk of acquiring the disease.18,22 In endemic regions, such findings are determinant factors for the persistence of the disease and increased infectivity in the pediatric population.
The importance of systematically monitoring the populations at risk is observed, aimed at attaining an early diagnosis, timely treatment, and interruption of the transmission chain, an essential triad for disease control.21,23 However, there are evident difficulties in the routine implementation of these action by the health services, which are impaired by the shortage of human and material resources, work overload, organizational maladministration, and lack of knowledge about the disease, among other reasons.
Serological test associated with clinical evaluation may be a useful strategy for the identification of infected individuals at higher risk of developing the disease, helping in the early detection of new cases.19,23 The use of these technologies has become relevant to aid health services when planning and implementing a more effective methodological follow-up of high-risk contacts.19
Moreover, knowing the factors associated with leprosy in contacts under 15 years of age contributes to the expansion of knowledge, redirection of public policies and health service practices, as well as improving the surveillance of the disease in this specific population. It is recommended that health professionals develop educational activities between individuals and families, aiming to contribute to the reinforcement of knowledge about the disease, which is an important condition for the exercise of self-care in this more vulnerable population.
The present study has as limitations those related to the type of study used and the data related to selective memory, which may influence some findings. However, to ensure comparable groups and minimize possible biases, such as selection bias, participants were included from the same risk area, who belonged to the same age group.
It was concluded that the factors determining the occurrence of leprosy in the studied population indicate greater vulnerability in individuals aged 8–14 years, associated to the living conditions and time of residence, as well as to history of the disease in the family.
FundingThis study was supported by the Ministry of Health, Applied Research in Health Surveillance, Public Call No. 20_2013.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Rodrigues TS, Gomes LC, Cortela DC, Silva EA, Silva CA, Ferreira SM. Factors associated with leprosy in children contacts of notified adults in an endemic region of Midwest Brazil. J Pediatr (Rio J). 2020;96:593–9.