To compare two polyethylene bags in preventing admission hypothermia in preterm infants born at <34 weeks gestation.
MethodQuasi-randomized unblinded clinical trial conducted at a level III neonatal unit between June 2018 to September 2019. The authors assign infants between 240/7 and 336/7 weeks’ gestation to receive NeoHelp™ bag (intervention group) or a usual plastic bag (control group). The primary outcome was admission hypothermia, considering an axillary temperature at admission to the neonatal unit of <36.0 °C. Hyperthermia was considered if the admission temperature reached 37.5 °C or more.
ResultsThe authors evaluated 171 preterm infants (76, intervention group; 95, control group). The rate of admission hypothermia was significantly lower in the intervention group (2.6% vs. 14.7%, p = 0.007), with an 86% reduction in the admission hypothermia rate (OR, 0.14; 95% CI, 0.03–0.64), particularly for infants weighing >1000 g and >28 weeks gestation. The intervention group also had a higher median of temperature at admission – 36.8 °C (interquartile range 36.5–37.1) vs. 36.5 °C (interquartile range 36.1–36.9 °C), p = 0.001, and showed a higher hyperthermia rate (9.2% vs. 1.0%, p = 0.023). Birth weight was also associated to the outcome, and it represented a 30% chance reduction for every 100-g increase (OR, 0.997; 95% CI, 0.996–0.999). The in-hospital mortality rate was similar between groups.
ConclusionThe intervention polyethylene bag was more effective in preventing admission hypothermia. Nonetheless, the risk of hyperthermia is a concern during its use.
Admission hypothermia has been associated with serious neonatal complications, including brain injury, necrotizing enterocolitis, sepsis, and bronchopulmonary dysplasia, particularly in preterm infants.1 Notably, in this population, some studies described a reduction of 15–19% in the in-hospital mortality rate for every 1 °C increase in admission temperature.2,3
The most common and effective preventive auxiliary measure is the use of transparent plastic bags, as it allows the transference of radiant heat to the infant and reduces water evaporation and heat loss.4–10 The same effect is obtained by covering the infant's head using a cap, preferably made of wool.11,12
Most of the studies that supported this practice evaluated the use of polyethylene bags vs. routine care, but only a few compared different plastic devices and their efficacy in preventing admission hypothermia.4,13,14 Moreover, the authors have no standardized type of plastic bag for neonatal assistance.
Thereby, this study compared two polyethylene plastic bags to prevent hypothermia at neonatal unit admission in preterm infants born <34 weeks gestation. The authors hypothesized that the intervention bag would be more effective than the one commonly used (control), especially for infants with birth weight (BW) <1000 g.
MethodsStudy designThis was a prospective, quasi-randomized, unblinded clinical trial.
Patients and settingInborn infants between 240/7 and 336/7 weeks’ gestation, with BW between 500 and 3000 g, were admitted in a level III university neonatal unit.
Infants with major malformation were excluded as well as infants with an estimated birth weight >3000 g since they did not fit in the control bag.
SelectionParticipants were randomized weekly, from Monday to Sunday, with an initial schedule of 60 weeks, generated by the Statistical Analysis Software (SASࣨ, Cary, NC, USA, version 9.4). The software created a list designating each type of bag according to the week of the study. The authors chose this form of randomization to facilitate the use of the correct bag by the clinical team, who assigned each participant according to the week, avoiding allocation errors.
Sample sizeTo estimate the sample size, the authors conducted a 2-year retrospective audit in the neonatal unit. The mean admission hypothermia rate, considering axillary temperature below 36.0 °C, was 18% in premature infants <34 weeks gestation, who were managed at birth using the customary (control) bag. Considering a reduced admission hypothermia rate of 5% in the intervention group as clinically important, the study required a sample of 94 neonates per group at 5% type I error to achieve a power of 80%.
InterventionThe control group received the plastic bag commonly used in the hospital (Figure 1), made of a non-sterile single layer of polyethylene, measuring 30 × 45 cm, with one open side – that can be closed by a resealable closure zipper – and a closed opposite side, where a collar is made using scissors to allow the newborn's head and neck pass through. Soon after birth, the fontanelle area was dried; then, a 15 × 15 cm plastic cap was placed directly on the newborn's head and a wool cap was on top.
The intervention group received the NeoHelp™ device (Vygon®, France) (Figure 1), consisting of a sterile double-layer polyethylene bag, available in three sizes (small, medium, and large), with an internal pre-shaped foam cushion, and a self-adjusting double plastic hood attached. According to the manufacturer's recommendations, the infant was placed into the bag, and it was closed by a hermetic Velcro seal. The infant's head was covered by a plastic cap included in the kit, and a wool cap was placed on top.
Both the radiant warmers (target temperature of 36–37 °C) and the transport incubator (target temperature of 35–37 °C) remained ready for use 24 h a day. The servo control probe of the heated warmer was continuously attached to the mattress sheet by microporous tape (Micropore™, 3M™, Brazil), and the temperature was adjusted during stabilization as needed. For promoting heating of the plastic bag, they were extended on the mattress of the radiant warmers, previously the birth.
Infants were attended to in a stabilization room 10 m away from the delivery room (DR), reachable in about two to three minutes. The environmental temperatures of the DR and stabilization room were maintained between 24 °C and 26 °C. According to the local routine, briefly after birth, the preterm infants were placed in a conventional bassinet without drying, wrapped in a heated surgical drape, and transported to the stabilization room. Following the Brazilian Pediatrics Society guidelines applicable at the time of the study,6 preterm infants were placed inside the plastic bag, under a radiant warmer, and resuscitated or stabilized as needed. The bag was continuously closed, being removed only in the neonatal unit when the body temperature reached >36.5 °C. The right upper limb was left out, where the pulse oximeter sensor was placed. Heated gases for ventilation were unavailable.
The infant's temperature was measured in the axillary region using the BD® thermometer (Sparks, Maryland, USA) with a minimum recorded temperature of 32 °C and a 0.1 °C resolution, in two-time points: immediately before the newborn left the stabilization room and at admission to the neonatal unit, still inside the transport incubator. The maternal temperature was measured just before delivery (15 min, on average), using the same thermometer. Temperatures of the delivery and stabilization rooms were registered by Herweg® thermometers (model 1610; Timbo, Brazil).
Discontinuation criteriaOutcomeThe primary outcome was admission hypothermia, defined as an axillary temperature at admission to the neonatal unit of <36.0 °C. The secondary outcome was the intra-hospital mortality rate.
Hyperthermia was considered an adverse effect, defined as an axillary temperature of >37.5 °C at admission.
Statistical analysisContinuous variables were presented as the median and interquartile range (IQR) due to non-parametric distribution and compared by Mann–Whitney test. Categorical variables were expressed by percentage (%) and compared by χ2 tests, or Fisher's exact test.
To assess the factors associated with admission hypothermia, the authors performed a univariable logistic regression analysis. Then, the authors conducted a multivariable logistic regression analysis using the forward stepwise regression. This method can be used in settings where the number of variables under consideration is larger than the sample size, as in the present study, and it minimizes correlated variables (such as GA and BW). The results were expressed as odds ratios (OR) and 95% confidence interval (95% CI).
To verify the different effects of the interaction between the type of bag and the BW and GA, the authors adjusted the logistic regression model considering the independent interaction between these two variables vs. the bag.
To evaluate the effect size, the authors calculated 95% CI for frequency, risk ratio, the relative and absolute reduction of risk rates, and the number needed to treat.
Data analysis was performed using SAS version 9.4 (Cary, NC, USA), Level of significance was set at 5%.
Ethical aspectsThe Research Ethics Committee approved this study with authorization number CAAE 88760918.8.0000.5404. Before they signed the written consent form, all parents were informed about the study data and intervention to be performed throughout the study.
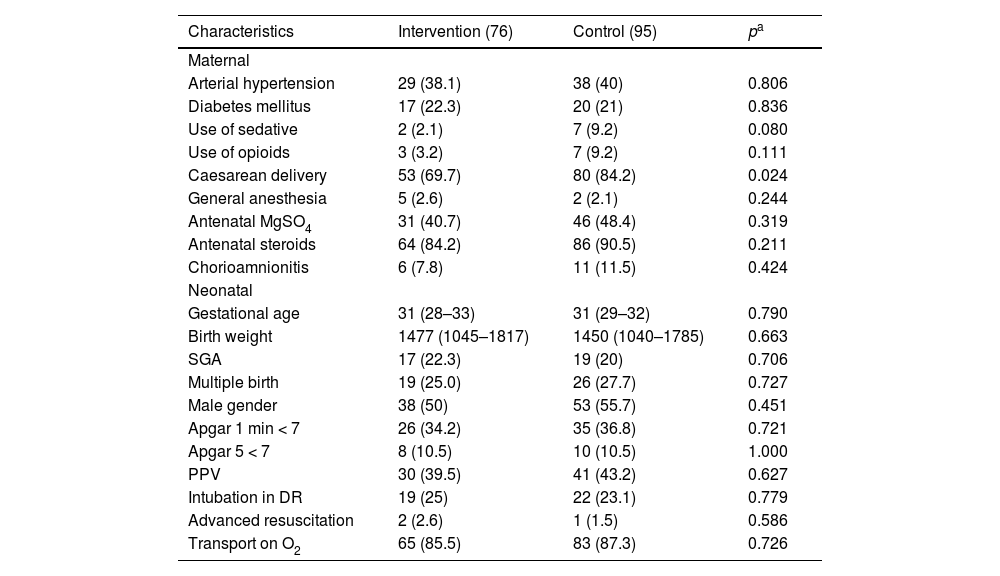
ResultsSelection of patientsFrom June 2018 to September 2019, this level III neonatal unit registered 187 births at <34 weeks gestation, and 185 were eligible (two died in the DR). Of these, the authors excluded 14 infants: seven with major malformations, two with BW >3000 g, and five born outside the DR. The final sample, thus, comprised 171 infants randomized into two groups: 76 in the intervention group and 95 in the control group (Figure 2). There was no allocation bias between the groups.
Clinical characteristicsBoth groups showed similar baseline demographic characteristics, including BW and GA, but the control group had a significantly higher frequency of cesarean delivery (Table 1). Infants <1000 g corresponded to 19.3% of the total sample and those <28 weeks gestation to 17.5%.
Maternal and neonatal characteristics according to group (intervention and control).
| Characteristics | Intervention (76) | Control (95) | pa |
|---|---|---|---|
| Maternal | |||
| Arterial hypertension | 29 (38.1) | 38 (40) | 0.806 |
| Diabetes mellitus | 17 (22.3) | 20 (21) | 0.836 |
| Use of sedative | 2 (2.1) | 7 (9.2) | 0.080 |
| Use of opioids | 3 (3.2) | 7 (9.2) | 0.111 |
| Caesarean delivery | 53 (69.7) | 80 (84.2) | 0.024 |
| General anesthesia | 5 (2.6) | 2 (2.1) | 0.244 |
| Antenatal MgSO4 | 31 (40.7) | 46 (48.4) | 0.319 |
| Antenatal steroids | 64 (84.2) | 86 (90.5) | 0.211 |
| Chorioamnionitis | 6 (7.8) | 11 (11.5) | 0.424 |
| Neonatal | |||
| Gestational age | 31 (28–33) | 31 (29–32) | 0.790 |
| Birth weight | 1477 (1045–1817) | 1450 (1040–1785) | 0.663 |
| SGA | 17 (22.3) | 19 (20) | 0.706 |
| Multiple birth | 19 (25.0) | 26 (27.7) | 0.727 |
| Male gender | 38 (50) | 53 (55.7) | 0.451 |
| Apgar 1 min < 7 | 26 (34.2) | 35 (36.8) | 0.721 |
| Apgar 5 < 7 | 8 (10.5) | 10 (10.5) | 1.000 |
| PPV | 30 (39.5) | 41 (43.2) | 0.627 |
| Intubation in DR | 19 (25) | 22 (23.1) | 0.779 |
| Advanced resuscitation | 2 (2.6) | 1 (1.5) | 0.586 |
| Transport on O2 | 65 (85.5) | 83 (87.3) | 0.726 |
Categorical variables are presented as frequency (N) and percentage (%); continuous variables are presented as median and interquartile range. MgSO4: magnesium sulphate; SGA: small for gestational age; PPV: positive pressure ventilation; DR: delivery room; O2: oxygen.
The overall percentage of admission hypothermia was 9.3%, the median temperature at admission was 36.6 °C (IQR, 36.2–37.0 °C), and the hyperthermia rate was 4.7%.
Admission hypothermia (AH) was significantly less frequent in the intervention group (2.6% vs. 14.7%, P = 0.007), which also presented a higher median admission temperature (36.8 °C IQR 36.5–37.1 °C vs. 36.5 °C IQR 36.1–36.9 °C, P < 0.001) (Supplementary appendix – Table 1). A subgroup analysis considering the endpoint temperature of 36.5 °C showed that the prevalence of admission hypothermia was also significantly different between the intervention and control group (23.6% vs. 47.3%, P = 0.001).
When the study reached the expected number of participants in the control group (95), only 76 participants were enrolled in the intervention group. As a result of the proposed randomization schedule, the following weeks would be dedicated to the control bag. Due to the unpredictable weekly birth rate and having reached the pre-specified study enrollment, the authors analyzed the present data, and given the statistical difference between the AH rates between the groups (2.6% vs. 14.7%, p = 0.007), we calculated the size effect indices: 95% CI for intervention bag was 0.0–6.2% vs. 7.6–21.8% for control bag; the risk ratio for the intervention group was 0.18 (95% CI 0.04–0.79); the relative risk reduction was 82%, and the absolute risk reduction was 12.1% in favor of the intervention bag; and an NNT of 8. Thus, the authors decided to interrupt the allocation of patients.
Regarding environmental variables associated with admission hypothermia, both the maternal temperature (36.1 °C vs. 35.7 °C, p = 0.011) and newborn temperature at the stabilization room (36.7 °C vs. 36.5 °C, p = 0.044) were higher in the intervention group. Other environmental temperatures were adequate and similar in both groups. Transport duration and age of admission at the neonatal unit were also similar in both groups (see the supplementary appendix – Table 1).
HyperthermiaThe intervention group showed a higher rate of hyperthermia (9.2% vs. 1%, p = 0.023) (supplementary appendix – Table 1). When compared with the non-hyperthermic group, the hyperthermic infants showed no statistical difference in BW – 1677 g (IQR 1308–1950) vs. 1420 g (IQR 1040–1795), p = 0.195 – nor in GA – 31.5 weeks (IQR 28.5–32.5) vs. 31.0 weeks (IQR 28.0–32.0), p = 0.862. Other maternal and neonatal characteristics were also similar. Variables related to temperature, however, were different: the hypothermic group had higher maternal temperatures 36.7 °C (IQR 36.2–37.2 °C) vs. 35.8 °C (IQR 35.3–36.2), p < 0.001; higher DR temperature 26.1 °C (24.7–26.4 °C) vs. 23.4 °C (22.3–26.0 °C), p = 0.039; and a higher temperature at stabilization room 37.3 °C (IQR 36.9–37.8 °C) vs. 36.5 °C (IQR 36.1–36.9 °C), p < 0.001. The temperature returned to a normal range around two hours of life. The authors observed no significant clinical event, except self-limited tachycardia.
Associated factors with hypothermia and hyperthermiaSupplementary appendix – Table 2 presents a stratified assessment by BW and GA vs. the bag used. Regarding admission hypothermia, the intervention bag showed to be more effective in newborns with BW ≥1000 g (0 vs. 7, p = 0.015) and gestational age ≥28 weeks’ gestation (0 vs. 11, p = 0.003). Hyperthermia was more prevalent in the intervention group for BW ≥1000 g (7 vs. 1, p = 0.025). The authors found no differences related to GA subgroups (see the supplementary appendix – Table 2).
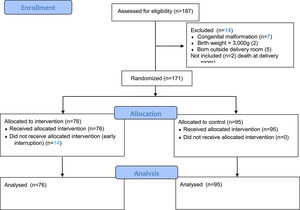
Univariate logistic regression analysis for admission hypothermia, considering axillary temperatures of <36.0 °C.
Categorical variables are presented as frequency (N) and percentage (%); continuous variables are presented as median and interquartile range. OR: odds ratio; 95% CI: 95% confidence interval; MgSO4: magnesium sulphate; SGA: small for gestational age; g: gram; O2: oxygen; min: minute; DR: delivery room; SR: stabilization room; TI: transport incubator.
The univariate logistic regression analysis showed that the intervention group (OR, 0.16; 95% CI, 0.03–0.71), GA (OR, 0.80; 95% CI, 0.67–0.97), BW (OR, 0.99; 95% CI, 0.99–0.99), SGA (OR, 3.38; 95% CI, 1.16–9.83), and need for tracheal intubation in the DR (OR, 3.70; 95% CI, 1.29–10.6) were positively associated with AH (Table 2).
In the multivariable logistic regression, the intervention bag remained independently associated with admission hypothermia, showing an 86% risk reduction of the condition (OR, 0.14; 95% CI, 0.03–0.64), and with BW, presenting a 30% chance reduction for every 100-g increase in BW (OR, 0.997; 95% CI, 0.996–0.999).
After adjusting the multiple models, considering the independent interaction between BW and GA and polyethylene bag, the authors found that the interaction with BW showed an approximately 81% reduced risk of admission hypothermia. Conversely, the interaction between GA and the device resulted in a 71% reduced chance of admission hypothermia (see the supplementary appendix – Table 3).
Mortality rateThe global mortality rate was 4.7% (8/171), and the death rate was similar in both groups (p = 0.464). None of the hyperthermic infants died.
DiscussionThe present study showed that the intervention bag was more effective in reducing admission hypothermia in infants born at <34 weeks’ gestation when compared with the conventional (control) bag, with a significantly reduced AH rate. This finding probably results from its physical characteristics: two layers of polyethylene that reduce evaporative and convective heat losses; a pre-shaped foam cushion that reduces conductive heat loss; and a hermetic Velcro that provides better sealing and fewer chances of inadvertent opening. Another important aspect of the intervention bag is its adjustable hood, which limits heat dispersion by the head. The regular bag, in turn, is made of a single layer of plastic, has no foam, and its closing zipper often opens during DR care.
To our knowledge, this is the first study to evaluate two polyethylene bags. A clinical trial conducted in Portugal comparing NeoHelp™ to another single-layer polyethylene bag in <28 weeks’ gestation infants has no published data yet.16
The study by Çaglar et al. compared the use of a polyethylene plastic sheet vs. a vinyl bag in 59 preterm infants ≤32 weeks gestation, with no mention of admission temperature, and found higher temperature 60 min after birth in the vinyl group (36.0 °C vs. 35.7 °C; p = 0.041).14 Using a mannequin, the study by Lahana et al. tested three polyethylene bags. The authors found a significant reduction in heat loss prevention, when comparing no bag vs. one of the three plastic bags under radiant warmer and observed that hyperthermia developed briefly. The inanimate mannequin used, however, did not correspond to a live newborn, lacking behavioral and vasomotor responses when submitted to heating.17
Besides the plastic bag type, the present study identified a decrease in the chance of admission hypothermia by approximately 30% for every 100-g increase in BW, consistent with other studies.2,3 This association is related to body surface and mass, favoring heat loss mainly through conduction and radiation.18,19
Compared with the literature, the present results for the global admission hypothermia rate were relatively low. This may be due to the intervention bag used. However, the other thermoregulatory interventions during delivery room stabilization and transport are part of the thermal care bundle that has been developed and implemented since 2014 in the unit. Continuing education and reinforcement of the guidelines allowed us to sustain acceptable low admission hypothermia rates.20,21
In addition to the primary outcome, the study also assessed hyperthermia, finding an overall rate of 4.7%. This result was similar to McCall et al.’s review, which analyzed 12 studies with preterm infants <37 weeks’ gestation comparing interventions designed to prevent hypothermia vs. routine care and found a hyperthermia rate (>37.5 °C) of 5.4% vs. 3.9%, respectively.4
Hyperthermia becomes a concern when many procedures for thermal care are used concomitantly in the delivery room. As premature infants have a reduced ability to dissipate heat due to a decreased response by sweat glands that develop around 30–32 weeks,22 and higher temperatures may cause vasodilation, tachycardia, lethargy, and apnea,23 measuring and monitoring the newborn's temperature in the delivery/stabilization rooms is essential. Using a skin temperature servo-control probe during the infant's permanence on the radiant warmer and in the transport incubator is useful to monitor thermal care.5,24 Although the clinical team received instructions to manipulate radiant warmer temperatures if hyperthermia occurred, the authors were unable to measure this event. Each three to four months the authors evaluated the hyperthermia rate and established a 15% rate limit to interrupt the present study, based on the highest hyperthermia rate found in McCall et al.’s review (near 14%).4,15
While the hyperthermic infants in this study showed no consequences of high temperature, except auto-limited tachycardia, overheating is always a concern, and it has been associated with brain damage and death.25 However, it is important to emphasize that the early termination of patient allocation could underestimate the real rate of hyperthermia in the intervention group.
To avoid allocation error, this study adopted weekly randomization, thus helping the clinical team (who were not blinded) to follow the sequence. Evaluating the results, the authors found no evidence of team bias favoring the use of one of the devices. Also, the authors only released the preliminary results to the clinical team after completing the recruitment.
Another limiting and unverified factor was the cord clamping time, as its later completion could lead to a longer exposure time to the DR's colder environment. At the time of the study, the unit's protocol followed the Brazilian pediatric Society guidelines of late cord clamping,6 but the timing register was not routine.
Another limiting factor was the low number of infants enrolled as <28 weeks and <1000 g, the main group that would be benefited from the intervention. The ongoing clinical trial whose main objective is to evaluate the effect of the plastic bag intervention in extremely preterm newborns can answer this question.16
Importantly, the intervention bag is more expensive than the common polyethylene bag used (US $13.20 vs. US $0.27), and this fact may be a limiting fact for its use in current care. As a limitation, this study did not compare the occurrence of major morbidities between groups. Thus, no actual analysis of costs vs. life/morbidity savings was performed.
In conclusion, the intervention bag was more effective in preventing hypothermia compared with the control device. Its use was less effective at BW <1000 g and <28 weeks’ gestation, which may be due to sampling limitations. Also, the intervention bag carries a higher risk of hyperthermia.
This study was supported by the Fund for Support to Teaching, Research and Outreach Activities – FAEPEX – State University of Campinas (grant number 0014/18 to J.P.S.C.).
Institution: This research was developed at the Women's Hospital of the State University of Campinas.