To compare the prevalence and factors associated with vertical transmission of human immunodeficiency virus 1 (HIV–1) among pregnant women treated in the periods of 1998–2004 and 2005–2011 in a reference service for the care of HIV-infected patients in southern Brazil.
MethodsThis was a descriptive and analytical study that used the databases of laboratories from the CD4 and STDs/AIDS Viral Load National Laboratory Network of the Brazilian Ministry of Health. HIV-1-infected pregnant women were selected after an active search for clinical information and obstetric and neonatal data from their medical records between the years of 1998 and 2011.
Results102 pregnant women were analyzed between 1998 and 2004 and 251 in the period between 2005 and 2011, totaling 353 children born to pregnant women with HIV-1. It was observed that the vertical transmission rate was 11.8% between 1998 and 2004 and 3.2% between 2005 and 2011 (p<0.001). The increased use of antiretroviral drugs (p=0.02), the decrease in viral load (p<0.001), and time of membrane rupture lower than 4h (p<0.001) were associated with the decrease of vertical transmission factors when comparing the two periods.
ConclusionIt was observed a decrease in the rate of vertical transmission in recent years. According to the studied variables, is suggested that the risk factors for vertical transmission of HIV-1 were absence of antiretroviral therapy, high viral load in the pregnant women, and membrane rupture time >4h.
Comparar a prevalência e os fatores associados à transmissão vertical de HIV-1 entre grávidas tratadas nos períodos de 1998-2004 e 2005-2011 em um serviço de referência de cuidado de pacientes com HIV no sul do Brasil.
MétodosEstudo descritivo e analítico que utilizou as bases de dados de laboratórios da Rede Nacional de Laboratórios de CD4 e Carga Viral de DSTs/AIDS do Ministério da Saúde. As grávidas com HIV-1 foram selecionadas em uma pesquisa ativa de informações clínicas e dados obstétricos e neonatais em seus prontuários médicos entre 1998-2011.
Resultados102 grávidas foram analisadas entre 1998 e 2004 e 251 entre 2005-2011, totalizando 353 crianças nascidas de grávidas com HIV-1. Observou-se que a transmissão vertical foi de 11,8% entre 1998 e 2004 e de 3,2% entre 2005-2011 (p<0,001). O maior uso de medicamentos antirretrovirais (p=0,02), a redução na carga viral (p<0,001) e o tempo de ruptura de membranas menor de 4h (p<0,001) foram associados à redução nos fatores de transmissão vertical quando os dois períodos são comparados.
ConclusãoObservou-se uma redução na taxa de transmissão vertical nos últimos anos. De acordo com as variáveis estudadas, sugere-se que os fatores de risco de transmissão vertical de HIV-1 foram ausência de terapia antirretroviral, alta carga viral das grávidas e tempo de ruptura maior de 4h.
The mother-to-child transmission (MTCT) of human immunodeficiency virus type 1 (HIV-1) can occur in three major periods: in utero, at birth, or during breastfeeding.1 HIV-1 can be transmitted in utero via transplacental cellular transport, through a progressive infection of the placenta's trophoblasts until the virus reaches the fetal circulation or due to ruptures in the placental barrier followed by microtransfusions that occur from mother to child.2 The transmission during delivery occurs via the contact of the fetus with infected maternal secretions while passing through the birth canal, through ascending infection from vagina to fetal membranes and amniotic fluid or through absorption in the neonatal digestive tract. In the postpartum period, the main form of transmission is breastfeeding.3
The vertical transmission route of HIV-1 can be influenced by several factors, such as the delivery mode,4 the use of antiretroviral therapy,5 oral inflammations in the newborn,6 prematurity, and high maternal viral load.7 In addition to these factors, the viral genetic diversity appears to play an important role in vertical transmission.1,8
The epidemic of acquired immunodeficiency syndrome (AIDS) is in process of stabilization; however, it still presents high rates of transmission, especially among women, which characterizes the feminization of the disease.9 Therefore, it is important to understand the epidemiological profile of pregnant women and of MTCT, since the changes in prevalence depend on factors such as the use of antiretrovirals and the adhesion to prenatal care. These and other factors may lead to a reduction in MTCT, thereby facilitating the adoption of more effective preventive measures.9,10 In Brazil, from 1980 to June of 2013, it was estimated that 718,230 people were living with HIV/AIDS.11 According to the Brazilian epidemiological bulletin of 2013, considered information from 2010, the prevalence of HIV infection in pregnant women was 0.38%.11 Vertical transmission has become a major challenge to public health, as epidemiological data show that 80% of HIV cases in children under 13 years had MTCT as the form of transmission.11
Due to the increasing number of infected pregnant women, actions such as the development of governmental programs and the monitoring of pregnant women have been implemented since 2000 in Brazil; healthcare staff have the obligation to report cases of infected women and exposed children.11 According to data from the National Disease Notification System (Sistema de Informação de Agravos de Notificação [SINAN]),11 77,066 cases of HIV in pregnant women were reported from 2000 to 2013; The South region of Brazil is in second place with 31.3% of cases, behind only the Southeast region (41.7%), and followed by the Northeast (14.9%), North (6.3%), and Midwest regions (5.7%). The detection rate of AIDS in children under 5 years (the indicator used in Brazil to monitor the vertical transmission of HIV) between 2012 and 2003 presented a reduction of 35.8%.11
This study aimed to compare the prevalence and factors associated with vertical transmission of HIV-1 among women treated from 1998 to 2004 and from 2005 to 2011 in a reference service for the care of HIV-infected patients in Southern Brazil, located at the University Hospital of the Federal University of Rio Grande (HU-FURG), in the city of Rio Grande, RS, Brazil.
MethodsThis was a descriptive and analytic study that included 102 newborns from HIV-1 positive pregnant women in the period 1998–2004 and 251 in the period 2005–2011, with a total of 353 births.
Despite the fact that Brazilian governmental programs and monitoring of pregnant women were implemented in 2000, the care for HIV patients in HU-FURG began in 1994, with testing and subsequent observation of the high incidence of cases in the region. Such attention was ruled from normative of the Brazilian Ministry of Health and subsequently every care protocols met as such recommendations. Due to certain changes, such as the higher prevalence of viral subtype C and the difference of the therapeutic and pharmacological model of the patient with HIV referenced in different analyzed periods, it was decided to stratify the data so that analysis would be feasible. Moreover, it could report the effectiveness of care models recommended by Brazilian Ministry of Health.8,12
Since 1998, all pregnant women attended to at HU-FURG were subjected to HIV/AIDS tests as recommended in the guidelines by the Brazilian Ministry of Health. Pregnant women who presented two positive serologic tests and one confirmation test, or two consecutives tests with detectable viral load, were classified as HIV-infected. The mothers signed an informed consent to participate in this research, which was approved by the research ethics committee of the institution (Protocol No. 23116001368/2003-44).
The study outcome was MTCT of HIV-1 in newborns, and the studied variables were: use of highly-active antiretroviral therapy (HAART) – Biovir® (lamivudina + zidovudina, GlaxoSmithKline Brasil, RJ, Brazil) + Kaletra® (Lopinavir e Ritonavir, Abbot, USA) during pregnancy, CD4+ T cells count in the last three months of pregnancy, pregnant women viral load, delivery mode, membrane rupture time, and birthweight (kg). The use of antiretroviral therapy was classified as: a) complete, – when the mother received antiretrovirals during pregnancy and in the moment of delivery as well as the newborn; and b) incomplete, when at least one of the three procedures were conducted or when the mother did not use antiretroviral therapy. Socio-demographic variables were not standardized between these periods. Therefore, it was not possible to describe the demographic profile of the population in this study.
Data was analyzed using Stata version 8.0 (StataCorp – College Station, TX, USA). An analytical descriptive analysis of numerical variables was performed according to the studied periods, which were presented by their frequencies, mean values, standard deviation, and a significant p-value of 0.05 in a two-tailed test.
ResultsThis study assessed 353 children born from HIV-1 positive pregnant women, attended to at HU-FURG.
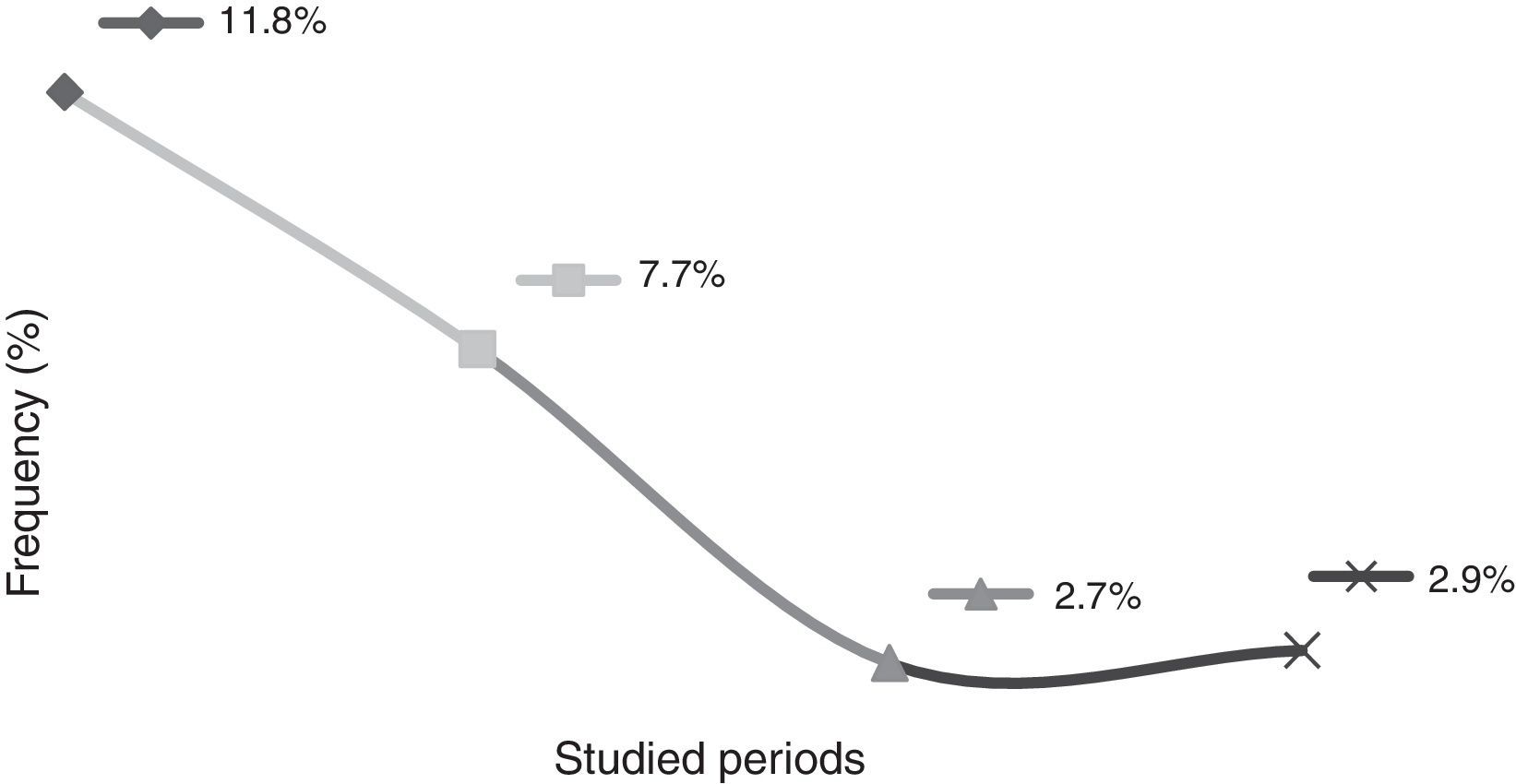
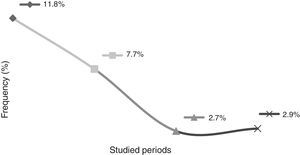
This study showed that the rates of mother-to-child transmission obtained between 1998 and 2004 and between 2005 and 2011 decreased significantly (p<0.001), from 11.8% to 3.2%, respectively. It id interesting to observe the results in different periods: the transmission rate from 1998 to 2000 was 11.8%; from 2001 to 2004, the rate was 7.7%; from 2005 to 2008, 2.7%; and from 2009 to 2011 the transmission rate was 2.9% (Fig. 1).
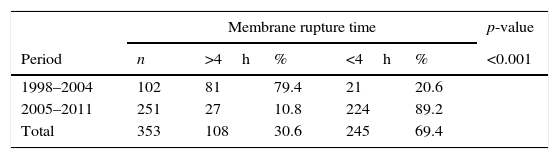
For the analyzed variables in both studied periods (Table 1), it was observed that between the periods from 1998 to 2004, 79.4% of the pregnant women had a membrane rupture time longer than 4h. In contrast, for the period between 2005 and 2011, only 10.8% of pregnant women had a membrane rupture time longer than 4h (p<0.001).
Comparison of factors associated with mother-to-child HIV-1 transmission between the periods 1998 and 2004 and 2005–2011 in a reference service.
| Membrane rupture time | p-value | |||||
|---|---|---|---|---|---|---|
| Period | n | >4h | % | <4h | % | <0.001 |
| 1998–2004 | 102 | 81 | 79.4 | 21 | 20.6 | |
| 2005–2011 | 251 | 27 | 10.8 | 224 | 89.2 | |
| Total | 353 | 108 | 30.6 | 245 | 69.4 | |
| Delivery mode | 0.67 | |||||
|---|---|---|---|---|---|---|
| n | Cesarean | % | Normal | % | ||
| 1998–2004 | 102 | 39 | 38.2 | 63 | 61.7 | |
| 2005–2011 | 251 | 90 | 35.8 | 161 | 64.8 | |
| Total | 353 | 129 | 36.5 | 224 | 63.5 | |
| Mother-to-child transmission | <0.001 | |||||
|---|---|---|---|---|---|---|
| n | HIV+ | % | HIV− | % | ||
| 1998–2004 | 102 | 12 | 11.8 | 90 | 88.2 | |
| 2005–2011 | 251 | 8 | 3.2 | 243 | 96.8 | |
| Total | 353 | 20 | 5.7 | 333 | 94.3 | |
| Antiretroviral therapy during pregnancy | 0.02 | |||||
|---|---|---|---|---|---|---|
| n | Incomplete | % | Complete | % | ||
| 1998–2004 | 102 | 40 | 39.3 | 62 | 60.7 | |
| 2005–2011 | 251 | 67 | 26.7 | 184 | 73.3 | |
| Total | 353 | 107 | 30.3 | 246 | 69.7 | |
| T CD4+ cell count | |||||||
|---|---|---|---|---|---|---|---|
| n | cell 0–199 | % | cell 200–499 | % | Cell>500 | % | |
| 1998–2004 | 95 | 18 | 18.9 | 50 | 52.6 | 27 | 28.4 |
| 2005–2011 | 251 | 13 | 5.2 | 94 | 37.5 | 144 | 57.3 |
| p | <0.001 | <0.001 | 1.00 | ||||
| Maternal viral load – Log10 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 0–2.99 | % | 3.0–3.99 | % | 4.0–4.99 | % | ≥5 | % | |
| 1998–2004 | 95 | 27 | 28.4 | 23 | 24.2 | 35 | 36.8 | 10 | 10.5 |
| 2005–2011 | 251 | 173 | 68.9 | 49 | 19.5 | 26 | 10.3 | 3 | 1.2 |
| p | <0.001 | 0.37 | <0.001 | <0.001 | |||||
| Newborn gender | 0.68 | |||||
|---|---|---|---|---|---|---|
| n | Male | % | Female | % | ||
| 1998–2004 | 102 | 54 | 52.9 | 48 | 47.1 | |
| 2005–2011 | 251 | 127 | 50.5 | 124 | 49.5 | |
| Total | 353 | 181 | 51.3 | 172 | 48.7 | |
There was no significant difference in the mode of delivery between the two studied periods, nor in the average birthweight. The use of antiretroviral therapy throughout the gestational period was observed in 69.7% of the pregnant women. Between 1998 and 2004, 60.7% of pregnant women adhered to HAART and, between the years 2005 and 2011, the adhesion rate was 73.3% (p<0.02), suggesting that adhesion to the antiretroviral therapy by pregnant women is an important factor in the reduction of MTCT. It was considered complete use of antiretroviral therapy when mothers reported having used the drug during the antenatal period, at delivery and her newborn have received prophylaxis with oral suspension of zidovudine (AZT) for six weeks after delivery. The use of injectable AZT and oral AZT was checked with the drug dispensing control spreadsheets during hospitalizations, according to the protocol established by the Brazilian Ministry of Health. Since 1998, PMTCT attention was performed following the recommendation to AZT monotherapy. In 2001, triple therapy was recommended, inserting Biovir® and Nelfinavir. In 2007, following a recommendation of the Brazilian Ministry of Health, Kaletra® was introduced, replacing Nelfinavir.
There was an increase in the CD4+ T cells count (CD4+ T cells>500) when comparing both studied periods. In the period from 1998 to 2004, 28.4% of pregnant women presented CD4+ T cells count higher than 500. In the period from 2005 to 2011, the percentage of pregnant women with CD4+ T cells count higher than 500 increased to 57.3% (p<0.001).
When analyzing the maternal viral load, it was observed that the percentage of pregnant women with viral load between undetectable and log of 2.9 was 68.0% in the period from 2005 to 2011 and 28.4% in the period from 1998 to 2004 (p<0.001).
DiscussionBrazil aims to eliminate HIV-1 vertical transmission (less than 1% of transmission) until 2015.11 Studies have shown that it is possible to prevent new HIV infection in children when HIV-positive pregnant women have timely and appropriate access to prenatal care and to HAART.13 In the present study, a 5.7% rate of vertical transmission was observed from a total of 353 children born from seropositive mothers for HIV-1 between the years 1998 and 2011. However, it is interesting to analyze the rate of MCTC in different periods. In this study, between 1998 and 2000, the MTCT rate was 11.8%; from 2001 to 2004, 7.7%; from 2005 to 2008, 2.7%; and from 2009 to 2011, 2.9%. This demonstrates that the MTCT rates are relatively lower when analyzed at different times. There was a small increase in MTCT rates between the years 2005 and 2008 (2.7%) and 2009–2011(2.9%), which may be justified due to the fact that a portion of HIV-positive pregnant women still do not make use of chemoprophylaxis during the pregnancy, especially drug users and currently the use of crack cocaine. Data showed that pharmacy records can help identify less-than-optimal adherence to treatment.14 Given the stabilization of MTCT rates observed not only in this study, but throughout in Brazil, the Brazilian Ministry of Health implemented in 2012 the use of nevirapine (NVP; Technical Note n. 388/2012). In Brazil, the use of AZT associated with NPV has been advocated for the prevention of MTCT, since a recently published study demonstrated that the oral treatment with a solution containing AZT during six months associated to an oral suspension with NPV (three doses in the first week of life) significantly reduces the rate of MTCT from pregnant women who did not use chemoprophylaxis during pregnancy.11
Taking into account the aim of this study, observing the rates obtained between 1998 and 2004 and 2005–2011, a significant drop in the transmission rates (from 11.8% to 3.2%, respectively) was observed, clearly demonstrating a reduction in MTCT rates. Comparing these results with studies conducted in the same Brazilian region, a studied published in 2006 observed a MTCT rate of 11.8% in infants born between 1998 and 2003.12 In another study published in 2010 from the same region, a MTCT rate of 4.8% was observed between the years 2003 and 2007.8 In the present study, the MTCT rate was only 2.9% when considering only the period from 2007 to 2011. These results clearly demonstrate that there has been a decrease in the MTCT rates of HIV-1, which highlights the effectiveness of the national policy for the control of MTCT.
With the approval of Law No. 9313, on November 13, 1996, Brazil began to rely in its legal system with a legislation that ensures the access to antiretroviral medication for individuals living with HIV/AIDS. Thus, Brazil became the first emerging country to provide antiretroviral therapy. In 2009, the Secretary of Substitute Health Surveillance started to use fast HIV tests in pregnant women, according to the authority conferred by the Article 45 of the Decree No. 6860 of May 27, 20099,11 Therefore, it can be suggested that these control measures had an influence in the decline of the HIV infection rates in infants between the studied periods, demonstrating the importance of these control measures in public healthcare services. Similar decreases in MTCT rates were observed in several countries that adopted control measures, especially the use of antiretroviral therapy by HIV-positive pregnant women.15 In the present study, it was observed that 69.7% of mothers made use of antiretroviral medication throughout the gestational period, suggesting a decline in the vertical transmission rate. In a study published in 2011, it was observed that, from 25 seropositive children, 9% were born from mothers who received inadequate antiretroviral therapy during pregnancy, a fact that occasioned a rate of only 1.7% of vertical transmission.16
When analyzing the different periods of this study, it was observed that, between 1998 and 2004, 60.7% of pregnant women adhered to antiretroviral therapy and, between 2005 and 2011, there was an increase in this adherence: 73.3%. This suggests a low viral load in pregnant women and a decrease in vertical transmission rates between the periods from 2005 to 2011, only 3.2%. These results corroborate those from a previous study, which demonstrated that the main risk factors for HIV transmission were failure of antiretroviral therapy, late maternal diagnosis and, consequently, high viral load of pregnant women at delivery.8 The use of antiretroviral therapy during pregnancy is extremely important in order to prevent vertical transmission; it can be used during any period, regardless of the clinical condition of the mother.17 Studies have reported that a high viral load and a low CD4+ T cells count during pregnancy are significant factors in MTCT.8,18
It is noteworthy that a membrane rupture time lower than 4h is extremely important to reduce MTCT.19 In the present study, observed a significant decrease (p<0.001) in rupture time was observed when analyzing the studied periods, since 79.4% of pregnant women had a rupture time higher than 4h in the period from 1998 to 2004. In contrast, from 2005 to 2011, this rate was 10.8%. The significant difference between membrane rupture time in both studied periods is the result of the update of care protocols for HIV-infected pregnant women. In 2004, HIV testing during prenatal care and proper implementation of prevention actions of vertical transmission of HIV were initiated, and the first protocol was published in 2007. According to the Brazilian guideline for prophylaxis of HIV transmission and antiretroviral therapy in pregnant women, the active management of labor should occur to prevent prolonged membrane rupture time, since a reduced time decreases the risk of vertical transmission.9
Despite of the effort to reduce MTCT, the residual risk of that transmission is still relatively high in comparison of what it is observed in countries who adopt HAART.20 The fact that Southern Brazil is characterized by having a highere prevalence of HIV-1 subtype C, which is more transmissible in utero,8 may explain the rate of MTCT found in the present study. Additionally, late entrance and lack of adherence to prenatal care, especially in drug users, favor MTCT. A study demonstrated that low prenatal screening of maternal HIV infection, impairing maternal treatment or prophylaxis, and the incorrect use of the rapid screening test at admission for delivery are impediments to the effective reduction of MTCT.21 Aiming to increase the care for pregnant women with low adherence to prenatal, especially those drug users, the referral service of HU-FURG conducts active search of women in favor of the effectiveness of compliance with care protocols to prenatal care as recommended by the Brazilian Ministry of Health.
Therefore, the results of the present study suggest that the increase of antiretroviral therapy during pregnancy, time for membrane rupture lower than 4h, and low viral load contributed to the decline MTCT in both studied periods. These results are in agreement with the data obtained in the literature.2,7,22 However, more studies should be conducted to establish which factors are involved in MTCT.
Conflicts of interestThe authors declare no conflicts of interest.
To the Laboratory of AIDS Support, the infectious diseases physicians and obstetricians from the HIV/AIDS Service of UnHU-FURG, and the Brazilian Ministry of Health.
Please cite this article as: da Rosa MC, Lobato RC, Gonçalves CV, da Silva NM, Barral MF, de Martinez AM, et al. Evaluation of factors associated with vertical HIV-1 transmission. J Pediatr (Rio J). 2015;91:523–8.