To investigate the role of Complex Chronic Conditions (CCCs) on the outcomes of pediatric patients with refractory septic shock, as well as the accuracy of PELOD-2 and Vasoactive Inotropic Score (VIS) to predict mortality in this specific population.
MethodsThis is a single-center, retrospective cohort study. All patients diagnosed with septic shock requiring vasoactive drugs admitted to a 13-bed PICU in southern Brazil, between January 2016 and July 2018, were included. Clinical and demographic characteristics, presence of CCCs and VIS, and PELOD-2 scores were accessed by reviewing electronic medical records. The main outcome was considered PICU mortality.
Results218 patients with septic shock requiring vasoactive drugs were identified in the 30-month period and 72% of them had at least one CCC. Overall mortality was 22%. Comparing to patients without previous comorbidities, those with CCCs had a higher mortality (26.7% vs 9.8%; OR = 3.4 [1.3–8.4]) and longer hospital length of stay (29.3 vs 14.8; OR 2.39 [1.1- 5.3]). Among the subgroups of CCCs, “Malignancy” was particularly associated with mortality (OR = 2.3 [1.0–5.1]). VIS and PELOD-2 scores in 24 and 48 hours were associated with mortality and a PELOD-2 in 48 hours > 8 had the best performance in predicting mortality in patients with CCC (AUROC = 0.89).
ConclusionPatients with CCCs accounted for the majority of those admitted to the PICU with septic shock and related to poor outcomes. The high prevalence of hospitalizations, use of resources, and significant mortality determine that patients with CCCs should be considered a priority in the healthcare system.
Septic shock, the most severe presentation of sepsis, is defined as an acute infection that leads to cardiovascular dysfunction and it is a prominent cause of Pediatric Intensive Care Units (PICUs) admissions, as well as morbidity and mortality in the pediatric population.1,2 Mortality due to severe sepsis and septic shock ranges from 7.4%3 to 59%.4 Geographical location and level of economic development appear to have a significant impact on outcomes (e.g: developed countries present mortality close to 21%, whereas, in developing ones, these values range between 35% and 50%).5 Clinical characteristics also have a prominent influence on mortality being observed as higher mortality in patients with previous comorbidities.4,6 Some specific chronic conditions have a higher association with mortality in patients with severe sepsis, such as previous transplantation (48.2%), malignancy (41.3%), and kidney disease (38.2%).7
The Pediatric Logistic Organ Dysfunction Score (PELOD-2) is a helpful tool to assess the patient's severity concerning organ dysfunction by evaluating ten items related to five organ systems (cardiovascular, neurological, renal, respiratory, and hematological). The score ranges from 0 to 338 and it has already shown a solid correlation with mortality in patients with septic shock.3 The level of cardiovascular dysfunction can be measured indirectly by the Vasoactive Inotropic Score (VIS), which is an objective tool to assess the intensity of hemodynamic support in patients with shock. Its use is well established in the definition of outcomes, especially in patients after cardiac surgery.9 The application of VIS has also been studied in the general PICU population10 and in patients with septic shock.11 These studies showed that the VIS measured 48 hours after admission has a better relation to outcomes compared to earlier measurements.10,11
In this study, the authors sought to estimate the prevalence of CCCs in pediatric patients with septic shock, as well as the impact of these conditions on the characteristics and outcomes of this group of patients. The authors also aimed to evaluate if the level of organ dysfunction (PELOD-2) and the need for hemodynamic support (VIS) in the first 48 hours of shock are appropriate predictors of mortality in this specific population.
MethodsThe authors performed a retrospective cohort study enrolling all patients with septic shock who required vasoactive drugs admitted to the 13-bed PICU in southern Brazil. This university hospital is a national and regional reference to receive pediatric patients with onco-hematological, neurological, and genetic diseases, as well as candidates for liver and bone marrow transplantation. An average of 600 patients are admitted annually to the PICU presenting overall mortality of 5%. The local Ethics and Research Committee approved the present study.
All patients admitted to the PICU between January 2016 and July 2018, aged between 1 month and 18 years, were included in the analysis. Those diagnosed with septic shock on admission or during the PICU stay who required the use of vasoactive drugs for at least one hour were selected. The authors defined septic shock according to the International Guideline for the Management of Septic Shock and Associated Organ Dysfunction in Children.2 The exclusion criteria were: patients who received vasoactive drugs before PICU admission, admissions that lasted less than 24 hours, and previous end-of-life care decisions.
Data such as gender, age, admission source, length of stay among survivors, need for mechanical ventilation in the first 48 hours, and need for hemo or peritoneal dialysis during shock management were collected by reviewing electronic medical records. At the time of this study, pediatric ECMO was not available in the study's unit. The presence of CCCs was determined according to the criteria proposed by Feudtner,12 thus, every patient was classified as having or not having CCCs, and those who had, could be included in one or more CCC categories. The authors did not collect data on the amount of crystalloid administered before the initiation of vasoactive drugs, however, volume resuscitation is performed routinely in the units based on the Pediatric Shock Management Guidelines.2
Daily PELOD-213 was calculated in the first 24 hours after the initiation of vasoactive drugs infusion (PELOD24h) and in the consecutive 24 hours (PELOD48h) using data and exams contained in the medical records. The authors considered the worst value found for each variable in the 24-hour interval. The authors assumed unavailable data and exams to be within normal limits. The number of organ dysfunctions was determined by the same criteria as PELOD-2,8 considering five dysfunctions (neurological, cardiovascular, respiratory, renal, and hematological). The VIS score was calculated with the formula proposed by Gaies8 (VIS = dopamine (μg/kg/min) + dobutamine (μg/kg/min) + [10 x milrinone (μg/kg/min)] + [100 x epinephrine (μg/kg/min)] + [100 x norepinephrine (μg/kg/min)] + [10.000 x vasopressin (U/kg/min)]). The maximum VIS was also calculated in the first 24 hours of shock and vasoactive drugs requirement (VIS24h) and in the subsequent 24 hours (VIS48h). PICU mortality was the main outcome. Other considered outcomes were the need for dialysis and prolonged PICU stay (> 14 days).
The authors expressed continuous variables as mean and standard deviation (SD) when normally distributed, and as the median and interquartile range (IQR) when non-normally distributed. Comparisons between these continuous variables were performed through Student's T and Mann-Whitney U tests, respectively. The authors expressed categorical variables as absolute value and percentage and performed comparisons using the chi-square test. P-value was considered significant when < 0.05. The risk was estimated by Odds Ratio (OR) and 95% confidence interval (CI). The relation between the number of CCCs and mortality was estimated by Spearman's correlation test. The relation between PELOD-2 and VIS scores and PICU mortality was expressed by the area under the ROC curve (AUROC). The authors used the Youden index to determine the best cutoff point for sensitivity and specificity.
ResultsIn the 30-month period, there were 1442 admissions to the PICU, among which 252 (17.5%) fulfilled the criteria for septic shock and received vasoactive drugs. After exclusions and losses, 218 patients remained in the study. The median age was 14 months [3.0; 58.7], with a predominance of males (56.9%). The primary sources of admission were patients referred from other centers (28.4%), the Pediatric Emergency Unit (26.1%), and the pediatric ward of the hospital (25.2%), as can be seen in Table 1.
Characteristics of patients with septic shock requiring vasoactive drugs admitted to the PICU between January 2016 and July 2018.
| n = 218a | |
|---|---|
| Age (months) | 14 [3,0; 58,7] |
| Gender (masc), n (%) | 124 (56.9%) |
| Admission Source | |
| - Another Hospital | 62 (28.4%) |
| - Emergency Department | 57 (26.1%) |
| - Pediatric Ward | 55 (25.2%) |
| - Onchological Ward | 34 (15.6%) |
| - Operating Room | 7 (3.2%) |
| - NICU | 3 (1.4%) |
| Presence of Complex Chronic Condition | 157 (72.0%) |
| VIS (Vasoactive Inotropic Score) | |
| - 24h | 17,9 [10.4; 38.2] |
| - 48h | 18,1 [10.5; 42.9] |
| Pelod-2b | |
| - 24h | 7,6 + 3,4 |
| - 48h | 6,9 + 4,1 |
| Number of organ disfunctionsc | |
| - 24h | 2,9 + 1,1 |
| - 48h | 2,9 + 1,3 |
| Mechanical Ventilation within 48h | 178 (81.7%) |
| Dyalisis | 14 (6.4%) |
| Length of stay among survivors (days) | 9 [6.0; 14.0] |
| Prolonged stay (> 14 days) | 55 (25.2%) |
| Outcome | |
| - PICU discharge | 159 (73.0%) |
| - Transfer to another hospital | 11 (5.0%) |
| - PICU death | 48 (22.0%) |
Numeric variables with normal distribution are expressed as mean ± standard deviation; variables non-normally distributed are expressed as median [interquartile range]; categorical variables are expressed in absolute number and percentage (%).
Of the 218 patients included in the study, 157 (72%) had CCCs. Most patients presented one (43.3%) or two (40.1%) CCCs. Among the categories, the most frequent conditions were “Neurological and Neuromuscular” (29.9%), “Malignancy” (20.4%), and “Genetic and Congenital Defects” (19.1%). In addition, 22.3% of patients were dependent on technology, and 11.5% had already undergone a previous organ transplant (Table 2).
Influence of categories and number of CCC on septic shock mortality. Survivors vs. Non-Survivors.
| n(157) | Survivors115 (73,3 %) | Non-Survivors42 (26,7%) | Statisticsa | |
|---|---|---|---|---|
| CCC categoriesb | ||||
| Neurologic and Neuromuscular | 47 | 39 (82.9%) | 8 (17.1%) | p = 0.07 |
| Malignancy | 32 | 19 (59.4%) | 13 (40.6%) | p < 0.05OR = 2,3 (1,0 – 5,1) |
| Congenital or Genetic Defect | 30 | 24 (80.0%) | 6 (20.0%) | p = 0.35 |
| Cardiovascular | 29 | 22 (75.9%) | 7 (24.1%) | p = 0.72 |
| Gastrointestinal | 29 | 23 (79.3%) | 6 (20.7%) | p = 0.41 |
| Premature and Neonatal | 27 | 21 (77.8%) | 6 (22.2%) | p = 0.55 |
| Hematologic or Immunologic | 18 | 14 (77.8%) | 4 (22.2%) | p = 0.65 |
| Respiratory | 10 | 9 (90,0%) | 1 (10.0%) | p = 0.21 |
| Metabolic | 5 | 3 (60.0%) | 2 (40.0%) | p = 0.49 |
| Renal and Urologic | 2 | 1 (50 %) | 1 (50%) | p = 0.45 |
| Technology Dependence | 35 | 32 (91.4%) | 3 (8.6%) | p < 0.05 |
| Transplantation | 19 | 11 (57.9%) | 8 (42.1%) | p = 0.10 |
| Number of CCCs | ||||
| 1 | 101 | 70 (69.3%) | 31 (30.1%) | (Spearman's correlation)c |
| 2 | 44 | 34 (77.3%) | 10 (22.7%) | r = -0.13 |
| 3 | 8 | 7 (87.5%) | 1 (12.5%%) | p = 0.09 |
| 4 | 4 | 4 (100 %) | 0 |
The “CCC” group presented a higher median age than the group “without CCC”, with a predominance of males in the latter. Whereas external transfer (outpatients) was the most frequent source of patients without CCCs (45.9%), followed by emergency referral (39.3%), patients with CCC were predominantly from the hospital's pediatric and oncology wards (52.2%) (Table 3).
Comparison of characteristics and outcomes between the groups "CCC" and "without CCC".
| “CCC”a | “Without CCC”a | Statisticsb | |
|---|---|---|---|
| n | 157 (72%) | 61 (28%) | |
| Age (months) | 21 [7; 95] | 3 [1; 14] | p < 0.05 |
| Gender (masc), n (%) | 83 (52.9%) | 41 (67.2%) | p = 0.05 |
| Admission Source | |||
| Another Hospital | 34 (21.7%) | 28 (45.9%) | p < 0.05 |
| Pediatric Ward | 48 (30.6%) | 9 (14.7%) | p < 0.05 |
| Emergency Department | 31 (19.7%) | 24 (39.3%) | p < 0.05 |
| Onchological Ward | 34 (21.7%) | 0 | p < 0.05 |
| Operating Room | 7 (4.5%) | 0 | p = 0.09 |
| VIS (Vasoactive Inotropic Score) | |||
| 24h | 19.0 [10.2; 44.4] | 16.6 [10.6; 27.0] | p = 0.51 |
| 48h | 18.0 [10.0; 46.6] | 18.1 [1.1; 30.0] | p = 0.93 |
| Pelod-2c | |||
| 24h | 8.1 + 3.6 | 6.43 + 2.6 | p < 0.05 |
| 48h | 7.6 + 4.3 | 5.36 + 2.9 | p < 0.05 |
| Number of organ disfunctiond | |||
| 24h | 3.1 + 1.1 | 2.5 + 0.8 | p < 0.05 |
| 48h | 3.1 + 1.2 | 2.3 + 1.1 | p < 0.05 |
| Mechanical Ventilation Within 48h | 124 (79.0%) | 54 (88.5 %) | p = 0.11 |
| Dyalisis | 14 (8.9%) | 0 | p < 0.05 |
| Length of Stay (days) among survivors | 9.0 [6.0; 16.0] | 8.0 [5.5;12.0] | p = 0.1 |
| Prolonged Stay (>14d) | 46 (29.3%) | 9 (14.8%) | OR 2.4 (1.1 – 5.3)p < 0.05 |
| Outcome | |||
| PICU discharge | 104 (66.2%) | 55 (90.2%) | |
| Transfer to another hospital | 11 (7%) | 0 | |
| PICU death | 42 (26.7%) | 6 (9.8%) | OR 3.4 (1.3 – 8.4)p < 0.05 |
Numeric variables with normal distribution are expressed as mean ± standard deviation; variables non-normally distributed are expressed as median [interquartile range]; categorical variables are expressed in absolute number and percentage (%).
The groups “CCC” and “without CCC” did not show significant differences regarding the median VIS at 24 and 48 hours, as well as the need for mechanical ventilation support in the first 48 hours of shock management (79 vs 88%, p = 0.11). The median length of PICU stay among survivors also did not significantly differ between the two groups. However, prolonged PICU stays (> 14 days) were more frequent in the “CCC” group, with OR 2.4 [1.1- 5.3]. PELOD-2 and the mean number of organ dysfunctions were higher in the “CCC” group, both at 24 and 48 hours. The need for dialysis throughout shock management was significantly higher in the “CCC” group (8.9% vs 0, p < 0.05).
The overall mortality was 22% (n = 48/218). As expected, mortality in patients with CCC was substantially greater than in those without CCC (26.7% vs 9.8%, OR 3.4[1.3-8.4], p < 0,05). Thus, the majority of deaths in the cohort occurred in patients with CCC (87%, n = 42/48) (Table 3). The authors did not find a significant correlation between the number of CCCs and mortality (r = -0.13; p = 0.1) (Table 2). When comparing the categories of CCCs with each other, the authors observed significantly higher mortality in the “Malignancy” group (40.6%), OR 2.3 [1.0 – 5.1]. The authors also found high mortality rates in the Transplantation (42.1%) and Metabolic (40%) groups, but without a statistically significant difference (p = 0.1 and p = 0.49) (Table 2).
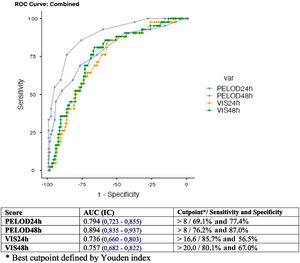
Among patients with CCCs, the authors observed an association of both the VIS and the PELOD-2 scores with mortality (Fig. 1). The VIS24h and VIS48h scores presented AUROC of 0.736 and 0.757. PELOD24h and PELOD48h had AUROC of 0.794 and 0.894, respectively (Fig. 1). A cutoff point higher than 8 in the PELOD-2 at 48 hours score showed a better correlation with mortality (AUROC > 0.8). Patients with CCC and a PELOD-2 higher than 8 on the second day of shock management (PELOD48h) had a mortality rate of 68.1%. On the other hand, patients with PELOD-2 of 8 or less at the same period presented a mortality rate of 9.1%.
DiscussionIn this study, which involved a cohort of 218 patients (Supplementary Fig. 1) with septic shock requiring vasoactive drugs, the authors observed that mortality was strongly associated with the presence of Complex Chronic Conditions (CCCs), especially malignancy. A PELOD-2 greater than 8 after 48 hours of shock management was also associated with higher mortality in patients with CCCs.
Patients with CCCs are a growing population in pediatrics.14 Studies in PICUs have shown that approximately half of the patients admitted to these units have at least one CCC.8,15 When considering studies with patients with septic shock who need vasoactive drugs, such as the present study, the prevalence of previous comorbidities ranged from 51% to 71%.15,16 One study4 has also associated prior comorbidities with double the mortality in patients with volume-refractory septic shock.
In the present study's sample, the authors found a high prevalence of CCCs (72%). These patients seemed to have greater severity of illness at admission, reflected by the elevated PELOD-2 and the higher mean number of organ dysfunctions. They also evolved with a greater need for renal replacement therapy during shock management and had three times more chance of dying in the PICU. Despite the greater severity and worse outcomes, the authors observed that some aspects of the initial management of the shock in these patients were not significantly different from patients without previous comorbidities, such as hemodynamic support (VIS score) and the need for invasive ventilation.
The authors believe that some characteristics associated with CCCs, such as immunosuppression, have a key role in determining the higher mortality in these patients. In a previous study of pediatric sepsis, the presence of comorbidities by itself did not determine higher mortality, but specific conditions such as malignancy, kidney disease, and transplantation did.7 In the present study's sample, the authors found significantly higher mortality in patients with malignancy when compared to other CCCs, which the authors attribute to immunosuppression, related not only to the disease itself but also to the specific treatments. The authors also observed a high mortality rate in patients with a history of transplantation and metabolic diseases. However, a larger sample would be necessary to validate these findings.
In the present study, the authors observed a PICU mortality related to septic shock of 22%, a value slightly higher than the mortality reported in severe sepsis and septic shock in developed countries.5 Studies with populations similar to ours, that aimed to evaluate patients with septic shock and the need for vasoactive drugs, have also been performed in other centers. For this specific group, a mortality of 59% was observed in a developing country,4 while developed countries presented mortality rates between 9 and 18%.10,17 It is clear that the burden of sepsis is higher in low- and medium-income countries, not only for its incidence (85% of sepsis cases occur in these countries)18 but also for its higher mortality.
Markers of worse prognosis have been widely studied in pediatric patients with sepsis. A significant discriminatory power for mortality of PELOD-2 in children with sepsis has already been observed.3 The VIS score has recently been analyzed in patients with septic shock4,11 and in the general population of PICUs10 to predict the length of stay, mechanical ventilation,11 and mortality.4,10 Haque et al.4 showed a good association with mortality and a VIS score >20. Musick et al.10 suggest the better performance of the VIS when measured 48 hours after initiation of vasoactive treatment (AUC = 0.736). However, it does not present an optimal discriminatory power for mortality since other scores have better performances in PICUs for this purpose. Although studies in patients with septic shock and the need for vasoactive drugs report a high prevalence of CCCs in their samples, the present study appears to be the first one validating VIS and PELOD-2 specifically in the CCC population.
The authors found that PELOD2 at 48h (AUROC = 0.894) had a better correlation with mortality than PELOD24h (AUROC = 0.794). The authors figure that the persistence of organ dysfunction 48 hours after shock management may have a stronger association with worse outcomes than earlier measurements. PELOD48h >8 was a good marker of worse prognosis, with a sensitivity of 76% and specificity of 87%. As for the VIS score, the authors also found better performance when measured after 48 hours (AUROC = 0.757) and better sensitivity and specificity when using a cutoff point of 20. Nevertheless, the authors considered the specificity of this marker, both after 24 and 48 hours of treatment (56% and 67%), too low to be used as an individual prognostic marker. The PELOD-2, when measured at the same time, was a better predictor of mortality in the present study. The authors believe this score to be a better tool to predict negative outcomes as it encompasses several organ dysfunctions and not just hemodynamics, such as the VIS score.
The present study has some limitations. Firstly, it is a single-center, retrospective study that presents disadvantages inherent to such a format. For example, the authors did not have data on the amount of crystalloid administered prior to or after the initiation of vasoactive drugs, a factor that is known to have an impact on outcomes.19 Additionally, although the present sample was satisfactory to evaluate and compare the two groups (with and without CCC), it does not provide us with the possibility of making comparisons between the subgroups due to the small sample size in each CCC category. Despite being a great result, the low mortality in the “without CCC” group (6 deaths) does not allow the predictive power for mortality of the VIS and PELOD-2 scores to be compared with due accuracy between the groups “CCC” and “without CCC”. Nevertheless, the present study's results are similar to and compatible with other related studies. In addition, they describe the reality of severe infections in complex patients living in developing countries such as Brazil.
In conclusion, patients with sepsis and CCCs represent an increasing population demanding PICU admission. Defining prevalence, prognostic factors, and outcomes is extremely important for a better understanding and management of this group. The authors believe that these patients should be a priority in the healthcare system due to the high number of hospitalizations, great morbidity, and significant mortality, especially in low-income countries, where the burden of sepsis is significantly higher. The main contribution of the present study is to demonstrate the high prevalence of CCCs and their impact on the need for support, length of stay, and mortality of patients with septic shock. Further studies are needed to assess the role of each category of CCC in the evolution and outcomes of septic shock.
Supplementary Fig. 1 - Flowchart of patients is included in the study.