Fungal infections (FI) pose a public health concern and significantly increase mortality rates, especially within Neonatal Intensive Care Units (NICU). Thus, this study aimed to investigate epidemiological indicators, risk factors, and lethality predictors associated with FI in a NICU.
MethodsThis study included 1,510 neonates admitted to the NICU of a reference hospital in Brazil between 2015 and 2022. Demographic data, such as sex, birth weight, gestational age, and use of invasive devices were analyzed.
ResultsThirty neonates developed invasive FI, totaling 33 episodes and an incidence of 1.2 per 1,000 patient days. Candida albicans was the most frequent species (52.9 %), the bloodstream was the most affected site (78.9 %), and 72.7 % of infections occurred between 2015 and 2018. The lethality rate associated with FI was 33.3 %, and 90 % of deaths occurred within 30 days of diagnosis of infection. Weight < 750 g, prolonged hospital stay, use of parenteral nutrition, and broad-spectrum antimicrobials were independent risk factors for infection occurrence, especially glycopeptides and 4th generation cephalosporins, having a considerable role in the increase in fungal infections. Weight < 750 g was considered a significant predictor of lethality, and C. albicans had the highest lethality rate (40 %).
ConclusionThese findings highlight the elevated lethality rate associated with these infections, reinforcing the importance of developing strategies to control FI within NICU.
Invasive fungal infections (IFI) constitute a public health concern worldwide due to their impact on prolonged hospitalization, increased morbidity and mortality rates, and substantial escalation of hospital costs.1 This situation is even more alarming when it affects critically ill patients admitted to Neonatal Intensive Care Units (NICU), as their vulnerability significantly reduces their chances of survival.1,2 In recent years, there has been a considerable rise in IFI cases in the NICU, especially among premature infants. This susceptibility can be attributed to inherent factors such as extremely low weight, immune system immaturity, and compromised natural barriers like the skin and gastrointestinal tract.3,4 Additionally, extrinsic factors associated with the hospital environment, including the use of invasive devices and the prophylactic or therapeutic administration of extended-spectrum antimicrobials, contribute to the increase in these infections.1-4 The range of etiological agents causing IFI in NICU is extensive, encompassing rare fungi such as Trichosporon spp.5 However, Candida spp. is the most prevalent and the fourth main cause of healthcare-associated infections (HAIs) in some NICU.2,6
In developed countries like Canada, the incidence of invasive candidiasis in the pediatric population is 5.1 cases per 1,000 admissions to pediatric units.7 In Europe, 36.4 % of invasive candidiasis cases occur in the NICU, with a 30-day mortality rate of 18.2 %.8 In developing countries, the incidence reaches 22 %.9 In Brazil, the incidence and mortality rates of invasive candidiasis in the NICU are 10.97 % and 20.4 %, respectively.10
Although Candida albicans is still the most frequent species in some countries,1,8 recent studies have highlighted a growing incidence of cases caused by non-albicans species, including Candida lusitaniae, Candida guilliermondii and Candida auris.1,9,10 This trend raises concerns, as these species tend to be more resistant to antifungals, posing challenges in managing infections.8,9
Understanding the epidemiology, IFI-associated risk factors, and their predictors of mortality is crucial for guiding treatment, enhancing neonatal care, and reducing morbidity and mortality rates. Therefore, this study aimed to investigate the epidemiological indicators, risk factors, and lethality predictors associated with IFI in a NICU.
Patients and methodsType and place of studyThis analytical retrospective cohort study was conducted in the NICU of a reference university hospital in the southeastern region of Brazil. The neonatal unit comprises 42 beds, 20 beds for intensive care, 16 for semi-intensive care, and six for intermediate care in the “Kangaroo Care” model.
PatientsThis study included neonates admitted to the NICU for more than 24 h between January 1st, 2015, and June 30, 2022. The control group consisted of patients who did not exhibit any type of infection or colonization. Neonates who had at least one laboratory-confirmed IFI episode were included in the study group. Neonates with solely bacterial infection or non-invasive fungal infection were excluded. Data collection occurred daily, from admission to the outcome, using the National Healthcare Safety Network (NHSN) system model.11
Information such as biological sex, birth weight, gestational age (GA), type of delivery, and indication for hospitalization were analyzed. The use of invasive devices, broad-spectrum antimicrobials, prior antifungals, and antifungal therapy for treatment were also evaluated.
Regarding IFI, factors such as the duration of previous hospitalization, prior bacterial infection, isolated fungal species, affected site, and clinical progression of the neonate were considered.
Only the initial episode of infection was considered to analyze predictors of mortality associated with IFI and calculate mortality rates.
Epidemiological indicators, risk factors and lethality predictorsIFI rates were presented as incidence density (ID-patient-days) and calculated as previously described.6,12 Risk factors for developing fungal infections and mortality were assessed by univariate and multivariate logistic regression, followed by variable selection using the stepwise method. Survival and cumulative probability curves were constructed using the Kaplan-Meier method. P-values ≤ 0.05 were considered statistically significant. The endemic level of infections and the lethality rate associated with IFI were calculated according to Arantes et al. and the Centers for Disease Control and Prevention (CDC), respectively.13,14
Research ethicsThis study was approved by the Ethics Committee for Research with Human Beings of the Federal University of Uberlandia (number 2.173.884; CAAE 68404017.1.0000.5152/2017).
ResultsCharacterization of the populationA total of 1,510 non-repeat neonates were considered eligible for the study, totaling 24,438 patient days. Most neonates were male (55.6 %), premature (40 %), and underweight (40.1 %), with a mean birth weight of 2,090.12 g (range: 345–5,015 g). Issues in the respiratory system constituted the primary indication for NICU admission (52.8 %), and the average length of stay in the unit was 16.18 days (range: 2–130 days). The year with the highest number of admissions was 2019, with 252 neonates (16.7 %). However, it was in 2017 that the authors observed the admission of the most significant number of critically ill neonates to the NICU, accounting for 19.2 % of those with extremely low birth weight and 17.4 % of those with GA < 28 weeks. Furthermore, 22.3 % of deaths occurred in 2017. Supplemental Table 1 presents the characterization of the population included in the study in each year.
Thirty neonates (2 %) had at least one episode of IFI, of which 56.7 % were extremely premature, and 43.3 % had a birth weight < 750 g. Of these patients, 83.3 % were admitted to the NICU due to low birth weight, and 20 % were transferred from other hospitals to the referred unit due to the severity of their health status. Invasive device use, such as umbilical venous catheter (UVC), mechanical ventilation, and parenteral nutrition (PN), was significantly higher among patients with IFI, increasing the risk of infection by up to 24.02 times (PN). Moreover, pre-emptive prescription of antifungals, especially those belonging to the azole class, was more prevalent among newborns with fungal infections (36.7 %) (Supplemental Table 1 and Table 1).
Risk factors associated with invasive fungal infections in the NICU at HC-UFU between January 2015 and June 2022.
Note: CI, confidential intervals; OR, odds ratio; PICC, peripherally inserted central catheter; PN, parenteral nutrition; SD, standard deviation; UVC, umbilical venous catheter; * and ** P statistically significant. There was no information on the gestational age and type of delivery of 73 and 56 neonates in the control group, respectively.
In this study, fluconazole was the first-choice antifungal treatment for 93.3 % (28 patients) of IFI cases. In 7.1 % of cases, fluconazole was prescribed in association with micafungin (one patient) or amphotericin B (one patient). However, in 14.3 % (4 patients) and 3.6 % (1 patient) of cases, fluconazole was replaced by micafungin and amphotericin B, respectively. On average, these replacements occurred after 6.4 days of fluconazole use. Additionally, 66.7 % of amphotericin B or micafungin prescriptions occurred between 2018 and 2022. Among the nine neonates who used these antifungals for IFI treatment, eight (88.9 %) had extremely low birth weight, and six (66.7 %) were discharged.
Epidemiological indicatorsThe study examined several epidemiological indicators, as presented in Table 2. The fungal infection rate during the study period was 1.9 %, with an ID of 1.2 per 1,000 patient days. The highest densities were observed in 2016 and 2017 (4.1 and 2.5 per 1,000 patient days, respectively). There was an upward trend in IFI cases in the second half of 2015, reaching a peak in March 2016, when it exceeded the pre-established upper control limit. In 2017, the IFI density remained above the average for the period but within the pre-established upper alert limit (Supplemental Figure 1). Between 2018 and 2022, infection rates decreased, reaching the lowest value in 2020 (0.2 per 1,000 patient days).
Epidemiological indicators of invasive fungal infections that occurred in the study population of the NICU at HC-UFU between January 2015 and June 2022.
Note: * Trichosporon sp. (2015); C. guilliermondii (2016); C. lusitaniae (2017); C. glabrata (2018); C. tropicalis (2021).
There were 33 episodes of IFI in 30 neonates, totaling 34 isolates. The bloodstream was the most affected site (26–78.8 % - incidence: 1.0 per 1,000 patient days). C. albicans was the predominant species (18–53 % - incidence: 0.6 per 1,000 patient-days), and it was isolated in more than one site in three neonates (2015: bloodstream and central nervous system [cerebrospinal fluid]; 2016: bloodstream and urinary tract; 2021: urinary tract and ascitic fluid). Furthermore, in 2016, one neonate had mixed candidemia caused by C. albicans and C. parapsilosis.
Risk factors for fungal infectionsSeveral factors were considered independent risk conditions for IFI, with emphasis on weight < 750 g [P: 0.0028; OR: 5.12 (1.75 - 14.95)]; previous use of some classes of broad-spectrum antimicrobials, such as aminoglycosides [P: 0.0479; OR: 3.41(1.01 - 11.49)], 4th generation cephalosporins [P: 0.0028; OR: 20.1(2.81 - 144.08)] and glycopeptides [P: 0.0003; OR: 1.21(1.09 - 1.33)]; and PN [P: 0.0047; OR: 27.19(2.76 - 268.22)] (Table 1).
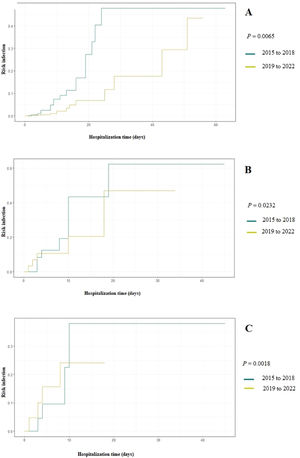
Figure 1 Displays the cumulative probability of developing fungal infection based on the duration of antimicrobial use in two time periods: 2015–2018 and 2019–2022. Between 2015 and 2018 (the period with the highest incidence of fungal infections) antimicrobials were significantly more used and increased the chances of IFI to up to 50 % after 20 days of use. When evaluating the cumulative probability by antimicrobial classes, the prescription of glycopeptides and 4th generation cephalosporins between 2015 and 2018 significantly increased the risk of IFI occurrence to 40 % after ten days of use (P: 0.0232 and P: 0.0018).
Lethality predictors and their indicatorsAll IFI-associated deaths occurred between 2015 and 2018. The overall mortality rate of the study population was 9.2 %, while the mortality associated with IFI in the general population was 0.7 %. Statistical analysis revealed a higher mortality rate in the fungal infection group [P < 0.001; OR 5.24 (2.4 - 11.43)]. Furthermore, the lethality rate of IFI was 33.3 % (10 newborns), reaching 50 % in 2015, 2016, and 2017.
Of the total number of deaths associated with IFI, nine (90 %) occurred within 30 days after diagnosis of infection, with an average time of 15.2 days. Among the newborns affected by IFI and died, 70 % were male, extremely premature, and weighed less than 750 g. Low birth weight was the only significant risk factor for mortality (P: 0.0449; OR: 5.44).
Additionally, five of the ten neonates who died had a prior bacterial infection before the IFI, occurring on average 8.4 days earlier. All neonates who died had a fungal bloodstream infection, with one case being a mixed infection of C. albicans and C. parapsilosis. Another neonate had an episode of invasive candidiasis in the cerebrospinal fluid in addition to the bloodstream infection.
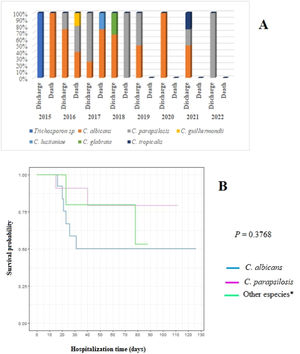
C. albicans had the highest lethality rate in all years of the study (40 %), especially in 2017 when the lethality rate reached 75 % (Table 2, Figure 2). Although no statistical difference was found in the survival of patients according to the species of Candida, newborns affected by C. albicans had the chances of survival reduced by up to 50 % in the first 30 days of hospitalization (Figure 2). Moreover, they had a 2.25 times higher risk of death (data not shown).
Distribution of fungal species in relation to patient evolution (discharge or death) by year of study (A) and survival curve using the Kaplan-Meier method for patients with C. albicans infection compared with those with C. parapsilosis and other Candida species (B). Note: *Other species: Trichosporon sp. (2015); C. guilliermondii (2016); C. lusitaniae (2017); C. glabrata (2018); C. tropicalis (2021).
Fungal HAIs occurrence has increased worldwide, especially those caused by Candida spp.15. However, few studies highlighted the epidemiology of IFI in neonatal units.
In this historical series, the incidence of IFI was 1.2 per 1,000 patient days, lower than the rates reported in NICU in countries like Canada,7 the USA,16 and Taiwan,17 where the incidence ranged from 1.97 to 5.1 per 1,000 patient days. This difference may be related to the profile of neonates admitted to each unit, the level of care provided in the NICU, the clinical practices of the healthcare team, treatment protocols, and geographic differences.10 However, the authors observed an oscillation in the IFI rate throughout the study, in which the year 2016 had the highest rate (4.1 per 1,000 patient days) and 2020 had the lowest rate (0.2 per 1,000 patient days).
The higher occurrence of IFI in the initial years of the study (2015 to 2018) may be attributed to the extensive administration of broad-spectrum antimicrobials in newborns admitted to the unit, especially glycopeptides and cephalosporins, in preceding years (2012 to 2014), in order to reduce the occurrence of late-onset sepsis.18 Similarly, from 2015 to 2018, glycopeptides and 4th generation cephalosporins were more used, significantly increasing the chances of IFI occurrence by up to 50 % (Figure 1). The administration of broad-spectrum antimicrobials is the main risk factor for fungal infections in neonates, especially among those with extremely low birth weight.19
The reduction in the occurrence of fungal HAIs in the second period of the study (2018–2022), especially in 2020, could be related to improvements in the management of patients by the multidisciplinary team over time. This improvement was achieved through an interventionist action based on the Plan-Do-Study-Act (PDSA), which aimed to detect the points that needed improvement to enhance cohesion among the team's professionals and in the execution of procedures performed on neonates. This intervention emphasized the importance of training and implementing actions to improve service quality. Additionally, the classes of antimicrobials used as empirical therapy for bacterial infections changed during this period, when the use of aminoglycosides and penicillins was predominant.
A similar study in a pediatric ICU in Canada also observed fluctuations in IFI incidence rates over the 11 years of surveillance.7 Other studies conducted in Greece and the USA reported significant reductions in the incidence rates of IFI, attributing them to improved patient management, rational use of antimicrobials, and implementation of a protocol for prophylactic use of antifungals high-risk neonates.16,20 These justifications align with the actions taken at the NICU of the hospital, which culminated in the reduction in fungal infections in 2020.
Most of the affected neonates were extremely premature (56.7 %) and had very low birth weight (83.3 %). Due to the severity of their conditions, all newborns used at least one invasive device, with a peripherally inserted central catheter (PICC), PN, and mechanical ventilation being the most frequent. The literature supports that these are the main risk factors for IFI.9,10,21 Prematurity and extremely low birth weight are inherent conditions in neonates that increase the likelihood of fungal HAIs by up to 10 times.21 The invasive device use can facilitate the introduction of fungal cells, especially Candida spp., which, coupled with the microorganism's ability to form biofilms, can facilitate their dissemination and establishment of the infectious process.22 This factor likely contributed to the high incidence of IFI associated with using devices such as PICC, UVC, and PN in 2016, the year when the highest rate of IFI.
The use of broad-spectrum antimicrobials, especially 4th generation cephalosporins and glycopeptides, increased the probability of developing fungal infections by up to 20 times. Fu et al. (2016) observed that the prophylactic use of carbapenems was the main risk factor associated with the occurrence of candidemia. Prolonged use of broad-spectrum antimicrobials can exert selective pressure on microorganisms within the hospital environment, contributing to increased infections by non-susceptible and opportunistic fungal species.21
In recent decades, there has been an increase in fungal HAIs incidence, particularly those caused by Candida spp., with a shift in the predominant infectious agents from C. albicans to non-albicans Candida species, including in NICU.23 Some authors suggest that this shift might result from the increased use of azole antifungals, especially fluconazole.24 The present study also observed a considerable increase in IFI by non-albicans species when compared to previous periods.25 However, C. albicans remained the most frequent fungal agent in the unit, responsible for the majority of IFI in six of the eight years of the study, corroborating the findings of Fu et al. (2016).21 On the other hand, Silva et al. (2023)10 and Cook et al.(2023)9 reported a predominance of non-albicans Candida species, particularly C. parapsilosis complex. Regional differences, hospital service complexity, financial resources, and the severity of the population served may explain this variation in the distribution of Candida species.21
In this series, IFI lethality rate of over 30 % is consistent with previous studies in the same hospital.26 Notably, 90 % of neonates with fungal HAI who died, did so within 30 days after the infection was diagnosed, highlighting the severity of these infections and the importance of preventive interventions. Similar studies in neonatal or pediatric units in Canada,7 the USA,16 Europe,8 India27 and Turkey28 reported fungal-associated lethality rates ranging between 13.7 % and 33.9 %. However, the 30-day lethality was lower than that found in this series. Differences between the populations of each study, and in the clinical management protocols among the evaluated hospital centers may explain this wide variation in mortality rates.16
Seventy percent of newborns with IFI who died had a birth weight below 750 g, which increased the probability of death by 5.44 times. However, the limited sample size in this study may have restricted the observation of other risk factors associated with mortality from fungal infections, a limitation also noted by Silva et al. (2023).10 This observation is consistent with a multicenter study that identified a strong association between mortality and birth weight below 1,000 g.9 However, the authors found no relationship between death and the species responsible for the infection. Although no species had a significant relationship with death in this series, C. albicans had a higher lethality rate, especially among newborns weighing less than 1,000 g, since 41.2 % of newborns in this weight category who died were affected by this species. Other reports have also indicated that C. albicans was associated with higher mortality rates compared to non-albicans Candida species, even within the same hospital,26,29 contrary to the findings of Liu et al. (2023),7 and Warris et al. (2020)8, who reported a higher lethality for non-albicans Candida species.
Antifungal prophylaxis with fluconazole is indicated for neonates with a birth weight below 1,000 g admitted to the NICU with a fungal infection rate of 10 %.30 However, the rise of azole-resistant isolates, especially in developing countries, poses a challenge to implementing prophylactic protocols.9 Treatment options for IFI in the NICU include the use of amphotericin B deoxycholate or fluconazole as the first choice, with echinocandins reserved for cases involving resistant isolates or patients with contraindications or limitations related to amphotericin B toxicity.30 In this study, fluconazole was the antifungal of choice for treating most IFI due to its good bioavailability and affordable cost. However, it is noteworthy that between 2018 and 2022, the prescription of micafungin or amphotericin B was higher compared to the period from 2015 to 2017, especially among neonates with extremely low birth weight. This increase in the use of broad-spectrum antifungals among more severe patients aligns with the reduction in mortality associated with IFI during the same period.
This study has limitations: data were obtained from a single health reference center, and the population affected by fungal infections was relatively small. However, studies that evaluate the temporal trends and epidemiological indicators of HAIs by fungal species in NICU over a prolonged period remain scarce, especially in Brazil. Therefore, the findings presented here contribute to expanding knowledge about the epidemiology of IFI in neonatal units worldwide, including Brazil, and can guide healthcare professionals in implementing preventive measures, particularly concerning device management and the use of broad-spectrum antimicrobials. These measures are crucial in controlling and reducing the incidence of these infections, leading to decreased neonatal morbidity and mortality rates.
ConclusionRisk factors inherent to the neonates, such as extreme prematurity and low weight, use of invasive devices, and administration of broad-spectrum antimicrobials, were independent risk conditions for IFI in the NICU. C. albicans was the most prevalent fungal species in the unit and was associated with higher lethality. The mortality rate associated with IFI was high, and most deaths occurred within 30 days of diagnosis of the fungal infection.
Continuous epidemiological surveillance is crucial to effectively combat and reduce the incidence of fungal HAIs in the NICU. Regular review of patient management protocols, including the appropriate use of antimicrobials, is essential to understand the profile of affected patients and the evolving microbial epidemiological patterns over time. This knowledge enables the development of strategies to control and mitigate infections, lower mortality rates associated with IFI, and promote the rational use of antimicrobials. By implementing these measures, healthcare facilities can enhance patient outcomes and minimize the impact of fungal HAIs in the NICU setting.
CRediT authorship contribution statementRalciane de Paula Menezes: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. Isadora Caixeta da Silveira Ferreira: Conceptualization, Writing – original draft, Writing – review & editing. Mallu Santos Mendonça Lopes: Data curation, Investigation, Methodology. Thiago Alves de Jesus: Data curation, Investigation, Methodology. Lúcio Borges de Araújo: Formal analysis, Methodology, Software, Supervision, Validation. Reginaldo dos Santos Pedroso: Conceptualization, Writing – original draft, Writing – review & editing. Denise Von Dolinger de Brito Röder: Data curation, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
This study was financed by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) - APQ00965-18. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) provided journals and scientific fellowships. Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) provided undergraduate research scholarships.