To describe the current indicators of environmental enteric dysfunction and its association with linear growth deficit and the height-for-age anthropometric indicator.
Data sourcesNarrative review with articles identified in PubMed and Scopus databases using combinations of the following words: environmental, enteric, dysfunction, enteropathy, and growth, as well as the authors’ personal records.
Data synthesisIn the last 15 years, new non-invasive markers have been investigated to characterize environmental enteric dysfunction; however, the best tests to be used have not yet been identified. There is evidence that, in environmental enteric dysfunction, a systemic inflammatory process may also occur as a consequence of increased intestinal permeability, in addition to intestinal mucosa abnormalities. Bacterial overgrowth in the small intestine and changes in fecal microbiota profile have also been identified. There is evidence indicating that environmental enteric dysfunction can impair not only full growth but also the neuropsychomotor development and response to orally administered vaccines. It is important to emphasize that the environmental enteric dysfunction is not a justification for not carrying out vaccination, which must follow the regular schedule. Another aspect to emphasize is the greater risk for those children who had height impairment in early childhood, possibly associated with environmental enteric dysfunction, to present overweight and obesity in adulthood when exposed to a high calorie diet, which has been called “triple burden.”
ConclusionsAccording to the analyzed evidence, the control of environmental enteric dysfunction is very important for the full expression of growth, development, and vaccine response in the pediatric age group.
Descrever os indicadores atuais da disfunção entérica ambiental e sua relação com déficit de crescimento linear e com o indicador antropométrico estatura-idade.
Fontes dos dadosRevisão narrativa com artigos identificados no PubMed e Scopus com o uso de combinações das seguintes palavras: environmental, enteric, dysfunction, enteropathy e growth e dos artigos dos arquivos pessoais dos autores.
Síntese dos dadosNos últimos 15 anos, vem sendo pesquisados novos marcadores não invasivos para caracterizar disfunção entérica ambiental. No entanto, ainda não foram identificados os melhores testes a serem usados. Existem evidências de que na disfunção entérica ambiental, além das anormalidades da mucosa intestinal, pode ocorrer também processo inflamatório sistêmico em consequência da maior permeabilidade intestinal. Sobrecrescimento bacteriano no intestino delgado e mudança no perfil da microbiota fecal também estão sendo identificados. Evidências indicam que a disfunção entérica ambiental pode comprometer não somente o pleno crescimento como também comprometer o desenvolvimento neuropsicomotor e a resposta de vacinas administradas por via oral. É importante destacar que a disfunção entérica ambiental não é justificativa para não fazer a vacinação, que deve seguir o calendário normal. Um outro aspecto a ser ressaltado é o risco maior dessas crianças que tiveram comprometimento da estatura na infância precoce, possivelmente associado à disfunção entérica ambiental, apresentarem na idade adulta excesso de peso e obesidade quando expostas a uma dieta rica em calorias, o que tem sido chamado “triple burden”.
ConclusõesDe acordo com as evidências analisadas, o controle da disfunção entérica ambiental é muito importante para plena expressão do crescimento, desenvolvimento e resposta vacinal na faixa etária pediátrica.
In the first half of the 1960s, it was observed in Thailand that adult individuals without gastrointestinal symptoms presented reduced intestinal absorption of d-xylose and abnormalities in the small intestine mucosa (reduction in villus height and increase in lymphocyte infiltrate in the lamina propria) in comparison with individuals living in developed countries.1 In other tropical countries, the same morphological and functional abnormalities were described, and the condition became known as tropical enteropathy.2 Evidence showed that these abnormalities were acquired, i.e., they were not present in the newborn and appeared after the first semester of life.3–5 Considering that the geographic distribution of tropical enteropathy was not restricted to tropical regions, in the 1980s, authors from South America proposed the term environmental enteropathy.6,7 In this context, it is worth noting that, in some countries in the tropical region, such as Qatar and Singapore, these types of abnormalities have not been identified in the small intestine.8
Another fundamental aspect, known since the 1970s, is the spontaneous reversibility of environmental enteropathy. In Pakistani and Indian patients who moved to New York and did not receive any type of therapeutic intervention, an improvement was observed in intestinal function indicators.9 The opposite phenomenon, that is, the occurrence of small intestine functional alterations, was observed a few months after North-Americans individuals moved from the United States to Pakistan.10 For decades, it has also been observed that the intensity of environmental enteropathy oscillates over time.
In the 1980s, it was suggested defining environmental enteropathy as a set of nonspecific small intestinal alterations, both morphological and functional, with or without clinical exteriorization, which potentially show spontaneous reversion after moving to a healthy environment.3 In this context, the environmental enteropathy was considered a societal disease that compromises the health and quality of life of children and adults living under environmental conditions that are below the dignity deserved by all individuals of a civilized society.11
At that time, according to the existing knowledge, it was very difficult to specifically associate the intestinal morphology and function abnormalities with environmental enteropathy, since there was often a coexistence with other common problems of populations living in unfavorable conditions, such as an inappropriate diet regarding macro and micronutrients, energy-protein malnutrition, intestinal parasites, and repeated outbreaks of acute and persistent diarrhea. Although suspected, in the 20th century the negative effect of intestinal malabsorption of environmental enteropathy on linear growth impairment was yet not clear.12
In the 1990s, the idea was established that repeated exposure to environment microbial agents, usually of a bacterial nature, had the potential to harm intestinal health and was the most important factor in the genesis of environmental enteropathy.2,3,5,8 Associated alterations in the microbiota of the proximal intestine leading to bacterial overgrowth in the small intestine could also occur. Considering that, similar to obtaining a small intestine biopsy sample, the collection of enteric fluid for microbiological analysis is an invasive procedure, the hydrogen breath test was used as a non-invasive alternative for the characterization of bacterial overgrowth.13–16
From the 1990s to date, other indicators for the characterization of environmental enteropathy are being investigated, especially the intestinal permeability test of lactulose and mannitol. The d-xylose absorption test, although feasible, has been practically abandoned.4,5,8
In 2013, the term “environmental enteric dysfunction” was proposed as synonymous to the term environmental enteropathy.17 The interest in environmental enteric dysfunction was also increased due to its association with the linear growth deficit (stunting) frequently observed in children living under unfavorable socioenvironmental conditions. Pediatrician started to take into consideration the potential effect of environmental enteric dysfunction on full growth impairment, which occurs faster in the first years of life. However, it must be emphasized that environmental enteric dysfunction can occur at any age group.18–20
The objective of this review was to describe the current environmental enteric dysfunction indicators and its association with linear growth deficit and the height-for-age anthropometric indicator.
MethodsFor this narrative review, articles were retrieved in the PubMed and Scopus databases using combinations of the following words: environmental, enteric, dysfunction, enteropathy, and growth. Not only original articles, but also review articles were considered for the review. References from the selected articles and the authors’ own records were also used.
ResultsEnvironmental enteric dysfunction and other enteropathiesEnvironmental enteric dysfunction is currently defined as an acquired, reversible subclinical disease associated with partial atrophy of intestinal villi, increased crypt depth, and infiltration of T-lymphocytes into the lamina propria and small intestinal epithelium, as a result from repeated bowel exposure to exogenous pathogens.17,20,21 It is also associated with increased intestinal permeability and influx of inflammatory elements through the intestinal epithelium.22
In turn, tropical sprue is characterized by more extensive involvement of the small intestine, including the terminal portion. The most frequent descriptions are from the Caribbean and Southeast Asia. In the past, the disease was mistaken by tropical (environmental) enteropathy, but later it became clear that they were different diseases. Unlike environmental enteric dysfunction, tropical sprue manifests as severe diarrhea associated with abrupt weight loss. In the past, the presence of steatorrhea and vitamin B12 malabsorption were taken into account. There is no specific laboratory indicator for its diagnosis, such as the serological markers of celiac disease.3,5
At present, the most common form of chronic protein-energy malnutrition is the linear growth deficit with height-for-age Z-scores below −2.0 standard deviations (stunting). Environmental enteric dysfunction is considered to be one of the determining factors of stunting. In turn, acute protein-energy malnutrition with significant weight deficit can lead to a diarrheal process, secondary to the atrophy of intestinal villi and systemic inflammation. These lesions are also found in kwashiorkor. In these children, malnutrition reversal is difficult, and the risk of death is high.5 In addition to protein-energy enteropathy and tropical sprue malnutrition, other enteropathies must be differentiated from environmental enteric dysfunction, such as celiac disease, autoimmune enteropathy, enteropathy induced by non-steroidal anti-inflammatory drugs (NSAIDs), among others.5
Role of biomarkers in environmental enteric dysfunctionTo date, the criteria used for the diagnosis of enteric environmental dysfunction are yet to be established.
The fact that the signs and symptoms are shared by several diseases makes the search for pathogenicity biomarkers a challenge. The same alterations observed in environmental enteric dysfunction (increased intestinal permeability, enterocyte lesion, mucosal inflammation) are also present in relatively common diseases in the same age group: inflammatory bowel disease, celiac disease, food allergy, and even in some cases of irritable bowel syndrome, where mucosal inflammation predominates.23,24
To facilitate understanding and guide the interpretation of the biomarkers associated with environmental enteric dysfunction, different approaches have been proposed with the objective of evaluating: (a) damage and repair of the intestinal mucosa; (b) intestinal permeability; (c) absorption; (d) bacterial translocation; and (e) systemic and intestinal mucosa inflammation.
An ideal biomarker would have high specificity, which would allow diagnostic refinement. Due to the complexity of the interaction of involved factors, this biomarker is not yet available. However, if it is possible to identify the pathophysiological alteration that underlies the process, it is possible to identify intestinal dysfunction in its initial, subclinical phase, and at a time when interventions can be initiated with greater chance of effectiveness. From the theoretical standpoint, the histopathological study would be the gold standard; however, the performance of an invasive procedure (intestinal biopsy) is a limiting factor.23,24
Considering that tissue changes correlate with increased intestinal permeability, the performance of a diagnostic test capable of measuring it is useful in clinical practice. In this context, the lactulose/mannitol test performs well, although it has some interpretation difficulties, especially in children and when there is an association with bacterial overgrowth in the small intestine and/or dysbiosis. The lack of standardization and validation studies in relation to the technique, the absence of a reference standard are difficulties that still need to be overcome.25,26 The difficulties include the need for fasting, complete urine collection for a prolonged period, and the measurement of lactulose and mannitol, which require the availability of complex laboratory procedures.8
Under normal circumstances, mannitol is absorbed in the small intestine, whereas lactulose is minimally absorbed by humans and, therefore, remains in the intestinal lumen, where it undergoes bacterial fermentation. Thus, if there is any intestinal area impairment, there is a reduction in the monosaccharide absorption and, consequently, reduction in its urinary excretion. In turn, if there is an increase in intestinal permeability, lactulose can also reach the bloodstream and also be excreted by the kidney.23,24
A systematic review of studies from several regions has shown that when the probability of environmental enteric dysfunction occurrence is high, the modified lactulose/mannitol test shows good association with the presence of stunting. Thus, it has been used not only as a marker of the disease but also as a parameter to evaluate intervention effectiveness.26Table 1 presents the main biomarkers that are being investigated in environmental enteric dysfunction.20,23,24,27
Methods proposed for environmental enteric dysfunction evaluation.
| Method | |
|---|---|
| Small intestine biopsy | Evaluation by optical microscopy (reference standard). Abnormalities can also be observed with electron microscopy and confocal endomicroscopy. |
| Biomarker of intestinal mucosa lesion and repair | |
| Citrulline | A non-essential amino acid produced by young enterocytes. It decreases when there is atrophy of intestinal villi and decrease of absorptive surface. |
| GLP-2 (glucagon-like peptide 2) | Produced by ileal endocrine cells. It has a trophic effect on the intestine and strengthens the intestinal barrier. |
| REG (regenerating family proteins) | These proteins increase in the stool when there is intestinal lesion. |
| Intestinal absorption and permeability | |
| d-Xylose | Absorption decreases when there is intestinal villi atrophy and decrease of the absorptive surface. It can be metabolized by bacteria that are found in excess in the small intestine. |
| Lactulose: mannitol or lactulose: rhamnose | The association between the disaccharide and the monosaccharide increases when there are lesions associated with increased permeability and intestinal villi atrophy and decrease of the absorptive surface. |
| Zonulin | Regulation of the junction between enterocytes. It increases when there is a lesion at the junction between enterocytes, indicating increased intestinal permeability. |
| Claudin 2, 4 and 15 | Regulation of the junction between enterocytes. Increase in claudin 2 and 15 indicates decreased intestinal absorption. Increase in claudin 4 indicates increased function of the intestinal mucosal barrier. |
| Bacterial translocation | |
| Lipopolysaccharide (LPS) and anti-LPS | Both substances (LPS and flagellin) are part of the structure of the bacteria found in the intestinal lumen. Passage through the mucosa indicates increased permeability and bacterial translocation. It generates immune response with antibody formation. |
| Flagellin and Anti-flagellin | |
| EndoCAb (endotoxin core antibody) | It increases when there is endotoxemia secondary to inflammation triggered by bacterial translocation. |
| Mucosal inflammation | |
| Myeloperoxidase (MPO) | Produced by neutrophils for bacterial destruction. It increases in the stool when there is inflammation. |
| Neopterin | Produced by macrophages and dendritic cells. It increases in the stool when there is inflammation. |
| Calprotectin | Produced by neutrophils. It increases in the stool when there is inflammation. |
| Alpha-1-antitrypsin | Cell protection through the action of enzymes produced by neutrophils. It increases in the stool when there is inflammation. |
| Systemic inflammation | |
| C-reactive protein (CRP) | It increases in systemic inflammatory processes. |
| Ferritin | |
| Interferon gamma | |
| Tumor necrosis factor | |
| Interleukin 6 and 10 | |
| Alpha-1-acid glycoprotein | |
| Bacterial overgrowth in the small intestine | |
| Enteral fluid culture | Ideally aerobic and anaerobic culture. |
| Hydrogen and methane breath test in expired air | Excessive fermentation of lactulose or glucose administered under fasting conditions increases the concentration of hydrogen and/or methane in the expired air. |
| Fecal microbiota/microbioma | Analysis with molecular biology techniques. |
Based on Refs. 15, 20, 23, 24, 27.
The function of alpha-1-antitrypsin (AAT) is to protect cells from the deleterious effects of proteolytic enzymes released by stimulated neutrophils. This protein is not synthesized in the intestine and its presence in stool reflects increased intestinal permeability and protein loss, secondary to mucosal inflammation. Fecal calprotectin is a protein present in the neutrophil cytoplasm and is detected in feces when there is an active inflammatory process. The presence of high concentrations of zonulin in feces also suggests an increase in intestinal permeability, since its function is to modulate the junctions between enterocytes.23,24,27
Citrulline is an amino acid produced by enterocytes, and its levels are low in the presence of flattening of the villi and consequent reduction of the intestinal area. GLP-2 (glucagon-like peptide 2) is an important trophic factor in intestinal cell repair, stimulating the growth of intestinal villi, the integrity of the mucosal barrier, absorptive functions and has an anti-inflammatory role, reducing the intestinal mucosa inflammation.23,24,27
As a biomarker of bacterial translocation, two structural bacterial components have been studied, the lipopolysaccharides (LPS) and flagellin. As one explanation for the presence of inflammation is systemic exposure to LPS, Endocab measures this exposure and is increased when there is increased intestinal permeability and endotoxemia secondary to inflammation. Other inflammation markers of interest are myeloperoxidase (MPO) – an enzyme present in the cytoplasm of neutrophils – and neopterin (NEO), which is produced by macrophages and dendritic cells.23,24,27
The Bangladesh Environmental Enteric Dysfunction (BEED) project24 aims to compare the performance of different biomarkers often used in the investigation of environmental enteric dysfunction in relation to the histopathological examination of the small intestine mucosa, which is considered the gold standard. The project is still underway and has recruited 400 children with stunting and 400 children at risk of linear growth retardation. One of the expectations is the creation of a score that will allow diagnostic refinement. One of the difficulties is that a typical pattern of histological alterations of the small intestinal biopsy has not yet been identified to allow the definition of the gold standard for the diagnosis of environmental enteric dysfunction.24
In another study carried out in the same geographic region with a high risk of environmental enteric dysfunction, involving approximately 180 infants in the second year of life, the concordance between the lactulose/mannitol test and a set of biomarkers indicative of intestinal mucosal structure impairment and systemic inflammation was assessed. The results were disappointing, and it was concluded that the presence of other factors that can impair intestinal permeability might explain the difficulty in interpreting the tests. Considering the complexity of the pathogenic mechanisms involved in the genesis of environmental enteric dysfunction, it is necessary to search for markers with greater specificity.28
Therefore, a biomarker or set of biomarkers shown to be accurate in refining the diagnostic probability in a patient with clinical suspicion of environmental enteric dysfunction has yet to be found. As the pathophysiological mechanisms and the pathophysiology are common to several other diseases, it can be speculated that this problem will not be solved in the short term.
Environmental enteric dysfunction and growthThe final height of humans depends primarily on the genotype and environmental factors that influence growth.18,19 Even after reducing its prevalence, stunting continues to be an important public health problem present in practically all regions of the world.29 In Brazil, there was a significant reduction in the prevalence of stunting, which decreased from 37% in 1974 to 7% in 2007 in children under 5 years of age.30 However, worldwide, it is estimated that approximately 25% of children exhibit stunting in the first five years of life, characterized by a Z-score <−2.0 standard deviations in relation to the World Health Organization growth curve, mainly in Africa and Southeast Asia.18,19
The mechanism for the development of height deficit is complex and not fully understood. It is considered that nutrient deficits in intrauterine and postnatal life may contribute to its development. In recent years, the role of enteric environmental dysfunction in the onset of linear growth deficit has also been evaluated.18,19
In the past, subclinical intestinal malabsorption observed in environmental enteric dysfunction was suspected of being the most important mechanism of nutritional status and growth impairment. More recently, not only the involvement of the small intestine but also the occurrence of systemic inflammation has been taken into consideration.
Systemic inflammation may be secondary to increased intestinal permeability, which allows bacterial translocation and/or the passage of substances with the potential to generate an inflammatory response. Another aspect is the presence of alterations in the intestinal microbiota. Thus, the following mechanisms have been recently presented to explain the interference of enteric environmental dysfunction in growth: (1) increased intestinal permeability; (2) bacterial translocation; (3) intestinal inflammation; (4) systemic inflammation; (5) dysbiosis; (6) nutrient malabsorption.18
A recent systematic review of the literature analyzed the publications between 2010 and 2018 that associated environmental enteric dysfunction indicators with growth or height deficit.20 Of the five published articles that analyzed indicators of injury and/or repair of the small intestine (citrulline, intestinal fatty acid binding protein, regenerating protein family [REG], and glucagon-like peptide 2 [GLP-2]), only two identified a positive association between environmental enteric dysfunction and growth deficit.20 Regarding the analysis of the ten articles that evaluated intestinal permeability (lactulose/mannitol or rhamnose ratio; percentage of lactulose, zonulin, claudulin-4 and 15 absorption), an association was found between increased intestinal permeability and growth deficit in half of the publications.20 In turn, of the eleven studies that assessed bacterial translocation indicators (anti-lipopolysaccharide [LPS] antibody and anti-flagellin antibody), only four showed an association with linear growth impairment. Similar results were found in association with intestinal inflammation indicators (myeloperoxidase, neopterin, and alpha-1-antitrypsin), i.e., no association was observed between linear growth and inflammation in four of the five publications.20 Regarding the indicators of systemic inflammation (cytokines, C-reactive protein, and ferritin, among others) conflicting information was also retrieved. The attempt to associate the different environmental enteric dysfunction indicators did not show any relevant association.20 Thus, up to date, it is not possible to choose a noninvasive marker that will allow the diagnosis of environmental enteric dysfunction to be established with accuracy and to define its association with the occurrence of linear growth deficit.20
Regarding the changes in the intestinal microbiota in environmental enteric dysfunction, most of the studies evaluated small intestine bacterial overgrowth and fecal microbiota composition. Currently, the microbiological study of duodenal secretion is the most accurate method to evaluate small intestine bacterial overgrowth. However, considering the complexity of specimen collection and processing, the Exhaled Breath Test has been used in the investigation of small intestine bacterial overgrowth, because it is a noninvasive and low-cost test.14,15 A study carried out in South Asia evaluated 430 children under 5 years of age and showed small intestine bacterial overgrowth in 12.5% of children in the first year of life and approximately 30% in the subsequent years. The distribution of bacterial overgrowth in relation to age was similar to that observed in the number of episodes of acute diarrhea.13
In Brazil, the breath test was used to investigate bacterial overgrowth in school-aged children living under unfavorable environmental conditions. A study published in 2007 evaluated 50 children aged 5–11 years who lived in a slum in the countryside of the state of São Paulo, Brazil.31 The results were compared with those obtained from the control group, which comprised children from a private school in the same municipality. The breath test was carried out utilizing two substrates: glucose and lactulose. Glucose did not present a significant hydrogen production in children living in a slum when compared with the controls. In turn, small intestine bacterial overgrowth was more prevalent in the children living in a slum (37.5%) than in the controls (2.1%; p<0.001).31 The mean height-for-age Z-score of the control children (+0.19±0.84) was higher (p<0.05) than in children living in a slum, with (−0.63±0.91) or without (−0.81±1.19) small intestine bacterial overgrowth. In those living in slums, small intestine bacterial overgrowth was not associated with greater height impairment. Two other studies were carried out at different times in a slum in the metropolitan region of the city of São Paulo, Brazil.32,33 The data were compared with those of children of good socioeconomic status recruited from a private school in the same region. The first showed a higher prevalence of small intestine bacterial overgrowth in the children living in the slum (30.9%) than in the ones from the private school (2.4%; p=0.0007). Among the children living in a slum, the anthropometric indicators were similar in children with and without bacterial overgrowth. Conversely, they were lower than those observed in the children from the private school, including height.32 The second study showed small intestine bacterial overgrowth in 61.0% of 100 children who lived in a slum.33 A lower mean height-for-age (−0.48±0.90 vs. −0.11±0.97) and capillary hemoglobin (12.6±1.0 vs. 13.4±1.2g/dL) Z-score were verified in the group of children with small intestine bacterial overgrowth when compared to those who did not have it (p<0.05).33 Unfortunately, that study did not evaluate systemic inflammation indicators.
The development of small intestine bacterial overgrowth in residents of areas with unsatisfactory environmental conditions was attributed to the inhibition of the migrating motor complex (MMC), secondary to repeated exposure of the digestive tract to lipopolysaccharides (LPS) through ingestion of water or soil contaminated with bacteria.14 A decrease in peristalsis increases intestinal transit time and facilitates microbial proliferation. This hypothesis is based on animal models, in which a decrease in the frequency and vigor of peristaltic contractions was shown in the small intestine induced by LPS produced by Escherichia coli. Additionally, in germ-free mice, Lactobacillus acidophilus and Bifidobacterium bifidus cause an increase in the MMC, while Micrococcus luteus and E. coli cause a decrease. There is little evidence to demonstrate effective nutritional status impairment due to small intestine bacterial overgrowth. In Brazil, it was verified that, in children aged 5–11 years who lived in a slum, those with bacterial overgrowth had lower values of height-for-age Z-score.33 These data can be explained by intestinal malabsorption, as characterized in the 1990s in a community in Burma/Myanmar.34,35 Using the hydrogen breath test, the authors found lower absorption of a test meal with rice in children with small intestine bacterial overgrowth. They observed longitudinally that the growth rate in height of children with malabsorption of rice was lower.34,35 This is the only longitudinal study showing that bacterial overgrowth can impair growth.
Another possible mechanism to explain the effect of small intestine bacterial overgrowth is the increased intestinal permeability.14 Thus, functional impairment of the tight junction between enterocytes and consequent endotoxemia and systemic inflammation may occur. The role of the intestinal microbiota in the intestinal homeostasis must be remembered. It is well known that the microbiota regulates not only the intestinal barrier but also the intestinal immune function, with a complex interaction that results in the maintenance of a minimum basal level of inflammation and controls the occurrence of a full inflammatory response.14
From the microbiological standpoint, the fecal microbiota of children and adults living in slums in Bangladesh, where environmental enteropathy is prevalent, shows marked differences when compared with those of residents of the United States.5 Samples from a small number of children and adults (four to six) were evaluated monthly for approximately six months. It was observed that the microbiota of the individuals living in Bangladesh showed greater diversity and lower stability than those observed in the North-American individuals. In contrast to the Americans, the feces of the Bangladesh sample were richer in Prevotella, Butyrivibrio, and Oscillospira and had a greater abundance of Bacteroides.36 Differences in the microbiota may be related to the type of diet in communities with a high prevalence of environmental enteric dysfunction, which are usually rich in starch and dietary fiber and low in protein and fat from animal sources.5 One example is the study comparing the microbiota of 11 African children from rural communities in Burkina Faso with 13 children from urban areas of the same country and 13 Italian children from Florence.37,38 It was observed that, when food of animal origin and simple carbohydrates are included in the diet in urban areas of the African country, the microbiota starts to show an increased abundance of bacteria with a higher capacity to metabolize these foods, as observed in European children. This process is associated with a reduced production of short-chain fatty acids by the intestinal microbiota. Children who live in rural communities persist with a microbiota richer in Prevotella, Treponema, and Succinivibrio, which have greater specificity for foods rich in dietary fiber and other complex carbohydrates found in vegetables.37,38
A project carried out in the metropolitan region of São Paulo compared the microbiota of 100 school-aged children living in a slum with 30 children living under good environmental conditions recruited from private schools. Real-time PCR was used to quantify the selected phyla, genus and species. Children living in unfavorable conditions had a higher number of bacteria, organisms from the Firmicutes and Bacteroidetes phyla, the Escherichia and Lactobacillus genus, and lower counts of Salmonella. A lower prevalence and counts of Clostridium difficile were also observed. Comparatively, it was speculated that a greater prevalence of Salmonella and C. difficile could indicate negative aspects in the microbiota of children living in good environmental conditions.39 It is interesting to mention that a larger amount of Archaea Methanobrevibacter smithii was observed in the children living in a slum, accompanied by a larger production of methane in the exhaled air, indicating a different pattern of bacterial metabolism.40 In children living in a slum with small intestine bacterial overgrowth, according to the respiratory test with lactulose, it was found that the fecal microbiota had a lower count of bacteria and Firmicutes and a higher count of bacteria of the Salmonella genus.
However, there are still many questions about the role of the microbiota on the development of environmental enteric dysfunction, such as the stability of the microbiota profile of children at risk of stunting, whether there are particularities in the microbiota of children without stunting that live in non-industrialized countries, and whether interventions to correct any eventual intestinal microbiota deviations are possible.41 Another question is about the validity of considering fecal microbiota as an indicator of abnormalities that occur in the small intestine, including bacterial overgrowth. Studies using molecular biology techniques can contribute to a better understanding of the microbiota in the proximal small intestine. However, it should be remembered that collecting samples from this portion of the intestine is a difficult and invasive procedure, such as the collection of tissue fragments for histological studies.
Environmental enteric dysfunction and response to oral immunizationThere is evidence that the local and systemic inflammatory state described in environmental enteric dysfunction may also decrease the response to oral vaccines, as well as being the main cause of stunting.42,43
In this context, decreased response to the rotavirus vaccine, oral polio vaccine, and attenuated cholera vaccine observed primarily in Africa and Asia have been associated with environmental enteric dysfunction. Several hypotheses have been raised to explain the phenomenon, from the heterogeneity of strains, which would make it difficult to develop a vaccine that had the same universal performance, to factors associated with host and environment.42,43 A recent editorial44 discussed the hypothesis that the characteristics of the intestinal microbiota, changes in the intestinal mucosa caused by the inflammatory process that affects mainly the innate immune response, and the systemic proinflammatory state could be involved in the impaired response to oral vaccines documented in children living in regions with poor environmental conditions.
Case–control studies nested in clinical trials carried out in Ghana and India showed that the intestinal microbiota analyzed before the children received the rotavirus vaccine was qualitatively different among the children who responded and those who did not respond to the vaccine challenge.45,46 These studies also compared the intestinal microbiota of children from Ghana and India who responded to the vaccine challenge with a group of German children, and found that the microbiota profile of the two groups was similar. Conversely, the microbiota of the non-responder children showed differences in comparison to the German children.45,46 These findings cannot be extrapolated to the entire child population living in contaminated environments, as this hypothesis has not been corroborated in other contexts where the prevalence of environmental enteric dysfunction is high.47
A recent review article48 analyzed eight studies carried out in Africa, Asia, and South America with the aim of evaluating the response to oral vaccines in regions where the frequency of enteric environmental dysfunction is high. Several difficulties were found when carrying out the integrated interpretation of these studies, including: different diagnostic criteria for enteric environmental dysfunction, use of biomarkers that can be altered by other diseases, and other methodology limitations of these articles. Therefore, it was not possible to reach robust conclusions. Of the eight articles, four showed evidence that the vaccine response was lower in children with high probability of environmental enteric dysfunction, while two found an opposite result, i.e., higher vaccine immunogenicity, and two studies did not find a statistically significant association. They concluded that, although biologically plausible, there is still insufficient empirical data to allow a definitive conclusion about the possibility that environmental enteric dysfunction reduces oral vaccine responsiveness in underdeveloped regions.
However, it is important to note that these results should not discourage the use of these vaccines in populations at risk for enteric environmental dysfunction, albeit less effective, immunization is able to protect a significant number of children and contributes to the reduction of hospitalizations and deaths.42 That is, these data should be considered as an indication of the urgent need to control enteric environmental dysfunction.
Environmental enteric dysfunction and neurocognitive developmentExperimental studies have shown that an inflammatory state can induce neurocognitive alterations. It is theorized that, in addition to the inflammatory state, metabolites associated with intestinal dysbiosis stimulate the production of neurotransmitters that interfere with the brain development process. In humans, it is more difficult to evaluate this process, because inflammatory processes can be associated with other factors, such as micronutrient deficiency (iron), lack of environmental stimuli, poverty, and intestinal dysbiosis.8
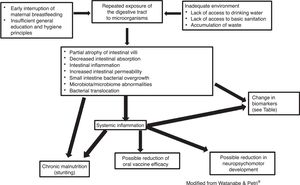
A multicenter study entitled MAL-ED (The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development) is following-up a cohort of 1600 children recruited at birth in eight countries (South Africa, Bangladesh, Brazil, India, Nepal, Pakistan, Peru, and Tanzania). Data on environmental exposures, intestinal inflammation, intestinal permeability assessment, exposure to enteropathogens, periodic measurements of food intake, including micronutrients, response to oral vaccines, cognitive development, and growth monitoring are being analyzed. This project allows the study of the consequences of environmental enteric dysfunction in different geographic and epidemiological contexts.27Fig. 1 outlines the etiology and consequences of environmental enteric dysfunction.
Final considerationsTo date, there is no fully effective preventive or therapeutic measure for enteric environmental dysfunction. Since the 1970s, there has been evidence that environmental enteric dysfunction abnormalities may regress with the improvement of environmental conditions.9 Considering that repeated exposures of the intestine to infectious agents are one of the most valued mechanisms in the genesis of enteric environmental dysfunction,21 preventive measures and acute diarrhea treatment may have a significant impact on the reduction of the problem.21,49,50
In this context, not only treated water supply and sanitation, but also the optimization of oral rehydration therapy, zinc, and vitamin A supplementation should be considered.21,49,50
The use of non-absorbable antimicrobials was not accompanied by intestinal permeability normalization, as measured by the lactulose/mannitol test.21 In turn, normalization of the hydrogen test in the expired air with lactulose was verified after the two-week administration of antimicrobials to schoolchildren with small intestine bacterial overgrowth that lived in a slum located in a metropolitan region of Brazil.51 The treatment of small intestine bacterial overgrowth should be considered, mainly when the patient has gastrointestinal clinical manifestations.
Finally, it should be emphasized that gastrointestinal tract disorders traditionally described as associated with severe primary malnutrition have been described in children with milder malnutrition and are included in the diagnosis of environmental enteric dysfunction. This finding is interesting because it helps to understand why interventions focused only on food supply fail to impact nutritional recovery in many cases, mainly in linear growth recovery. The overlap of the two disorders makes it difficult to recover the children's nutritional status.49
Another aspect to be emphasized is the greater risk for those children who had height impairment in early childhood to develop overweight and obesity in adulthood, when exposed to an energy-rich diet, which has been called the “triple burden.” Early in life, children living in an environment with a high degree of environmental contamination have greater exposure to intestinal infections and malnutrition (double burden) and in adulthood, to overweight/obesity and the associated comorbidities (triple burden).49
From what is known so far, robust epidemiological studies show that stunting is mostly observed in poor regions, with high environmental contamination, where a high prevalence of enteric environmental dysfunction is most frequently reported. However, overlapping with other factors that may compromise linear growth makes it difficult to reach a definitive conclusion. Several cohort studies are ongoing and, in the future, it is possible that more reliable data will be available and allow a causal inference to be safely made.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Morais MB, Silva GA. Environmental enteric dysfunction and growth. J Pediatr (Rio J). 2019;95:S85–S94.