Dexmedetomidine (DEX) is a highly selective alpha-2 adrenergic receptor agonist, which is the main sedative in the intensive care unit. This study aims to investigate the effectiveness and adverse events of DEX in maintaining hemodynamic stability in pediatric cardiac surgery.
SourcesDatabases such as PubMed, Cochrane, Web of Science, WANFANG STATA and China National Knowledge Infrastructure were searched for articles about the application of DEX in maintaining hemodynamic stability during and after pediatric cardiac surgery up to 18th Feb. 2021. Only randomized controlled trials were included and random-effects model meta-analysis was applied to calculate the standardized mean deviation (SMD), odds ratio (OR) and 95% confidence interval (CI).
Summary of the findingsFifteen articles were included for this meta-analysis, and 9 articles for qualitative analysis. The results showed that preoperative prophylaxis and postoperative recovery of DEX in pediatric patients undergoing cardiac surgery were effective in maintaining systolic blood pressure (SBP), mean arterial pressure (MAP), diastolic blood pressure (DBP) and reducing heart rate (HR) (SBP: SMD = -0.35,95% CI: -0.72, 0.01; MAP: SMD = -0.83, 95% CI: -1.87,0.21; DBP: SMD = -0.79,95% CI: -1.66,0.08; HR: SMD = -1.71,95% CI: -2.29, -1.13). In addition, the frequency of Junctional Ectopic Tachycardia in the DEX treatment group was lower than that in the placebo group.
ConclusionsThe application of DEX for preoperative prophylaxis and postoperative recovery in pediatric cardiac surgery patients are effective in maintaining hemodynamic stability, and the clinical dose of DEX is not significantly related to the occurrence of pediatric adverse events which may be related to individual differences.
Dexmedetomidine (DEX) has a high alpha-2/ alpha-1 receptor activity ratio and a selective binding ratio of 1620 alpha-2: alpha-1 receptors. DEX which has sedative, anti-anxiety and analgesic effects, and does not lead to respiratory inhibition is a highly selective and specific alpha-2 adrenergic receptor agonist.1–5 It is reported that DEX which is the main sedative in the intensive care unit and the only sedative in the diagnosis of invasive surgery and magnetic resonance imaging in the intensive care unit,6–10 is part of a balanced anesthetic for the prevention and treatment of sudden mental disorders. The use of DEX in adults has the opposite effect on blood pressure. The increase of systemic vascular resistance leads to an increase in blood pressure through the activation of peripheral alpha-2b adrenergic receptors, and the sympathetic nerve reduces blood pressure through the activation of alpha-2a adrenergic receptors in the central nervous system.11,12 At the clinical dose, the effect of the central sympathetic nerve is superior to that of peripheral vasoconstriction, resulting in an overall decrease in blood pressure. In children, hemodynamic effects such as heart rate (HR) and arterial pressure decrease caused by slow injection of DEX for more than 10 min have been well described.13 However, rapid administration of DEX may lead to transient hypertension. In adults, biphasic hemodynamic responses can be observed within 2 min after injection of DEX. In the beginning, blood pressure increases and reflex bradycardia occurs, and then blood pressure and heart rate stabilize below the baseline.14 Therefore, the safe dose of DEX needs to be further described in detail.
At present, the use of DEX in pediatric intensive care units is increasing due to its good sedation, analgesia and anti-anxiety effects, especially the prophylactic and postoperative medication should be used to maintain the hemodynamic stability of cardiac surgery in children. Hence, the present study's systematic review focused on investigating the effectiveness and adverse events of DEX in maintaining hemodynamic stability in pediatric cardiac surgery, hoping to provide comprehensive and strong evidence that DEX should be used to maintain hemodynamic stability in pediatric cardiac surgery.
MethodsThis systematic review followed methods depicted in Cochrane Handbook for Systematic Reviews of Interventions version 6.0.15 The authors performed the report according to PRISMA-P (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols).16
Search strategyFive electronic databases such as PubMed, Cochrane, Web of Science, WANFANG DATA, and China National Knowledge Infrastructure were selected to systematically search the articles related to the application of DEX in maintaining hemodynamic stability during and after pediatric cardiac surgery up to February 18, 2021. The search formula was (Dexmedetomidine) or (Dexmedetomidine [MeSH Terms]) and (pediatric cardiac surgery) or (pediatric cardiac surgery [MeSH Terms]) and (Hemodynamic) or (Hemodynamic [MeSH Terms]). In addition, the references included in the systematic search were also searched carefully, in order not to omit any related articles, and to make a comprehensive report on the application of DEX in maintaining hemodynamic stability during and after pediatric cardiac surgery.
Inclusion and exclusion criteriaAccording to the inclusion criteria, two researchers assessed the title and abstract of the articles initially searched to determine whether the articles were included in this study. When the tworesearchers disagreed, they consulted a third researcher for a decision. Articles that met the following inclusion criteria according to participants, interventions, comparisons, outcomes, and study design (PICOS) protocol, would be adopted. First, the people studied in the articles were infants and young children. Second, each study included at least one pair of comparisons of hemodynamic parameters before and after cardiac surgery with DEX. Moreover, one group of subjects received DEX treatment, and the other group received control treatment (other treatments without DEX). Third, in the study hemodynamic parameters were measured, including heart rate (HR), mean arterial pressure (MAP), central venous pressure (CVP), diastolic blood pressure (DBP), systolic blood pressure (SBP), and so on. Fourth, only the randomized controlled trials (RCT) were included, to ensure the quality of the combined results. In addition, studies that could not provide effective analytical data were subsequently excluded.
Data extraction and quality assessmentThe two researchers independently extracted the following data provided by the study and entered the data into the extraction table: the study title, the name of the author, the publication time of the study, the sample size, the demographic characteristics of the subjects, the treatment method and dose of DEX, the hemodynamic indicators, the time of measurement, and the specific characteristics of adverse events. Hemodynamic stability was defined as the blood pressure and heart rate change observed no statistically significant. In addition, bradycardia and tachycardia were respectively defined as a 30% decrease and increase from baseline when comparing the lowest and highest HR during dexmedetomidine infusion with the baseline HR. A hypertensive or hypotensive episode was defined as a 30% change from baseline and or if the SBP was below or above the 5th to 95th percentile for age. After data extraction was completed, a third researcher would double-check the consistency of the data extracted by the previous two researchers.
RCT meeting the inclusion criteria was evaluated and then incorporated into the final system review and meta-analysis. Each of the two researchers evaluated the included study according to the RCT quality assessment section of the Cochrane handbook for systematic reviews of interventions 6. 0.15 For each included study, the two researchers assessed several kinds of bias, such as blind bias, selection bias, incomplete outcome data bias, selective reporting bias, and other biases. When they had different opinions, a third researcher would make the final decision on the risk of bias based on the opinions of the previous two researchers. According to the regulations of the Cochrane manual, the authors determined whether the final quality of the study was low-risk, medium-risk, or high-risk based on the conclusions of the overall quality assessment.
Statistical analysisResults were merged across studies by using Stata version 15.1 (Stata Corp MP., College Station, TX, USA).17,18 Given the differences in the demographic characteristics of the subjects, the differences in the use of prophylactic drugs before and after cardiac surgery in children, the differences in dosage and mode of medication, and the inconsistency of measurement time points, a random effect model was applied to combine the results in this study. Considering the high heterogeneity among various studies, it was impractical to calculate the exact amount of effect, but the effect of drug use and the source of high heterogeneity could be discussed, which is of guiding significance for the safe use of drugs in the future and avoiding adverse events in DEX. The authors calculated the standardized mean deviation (SMD) of hemodynamic parameters before and after the administration of DEX in pediatric cardiac surgery and the odds ratio (OR) of the number of adverse events between the DEX group and the control group to evaluate the hemodynamic stability. Besides, the method of calculating and studying the merging standard deviation referred to the Cochrane handbook.15 Q test and I2 statistics were used to estimate the heterogeneity of the study. If the I2 value was 0%, 40%, and 60%, respectively, it was considered to have low, medium, and high heterogeneity, respectively.15 Indicators for evaluating hemodynamic stability will be shown in a forest plot. Egger's test was adopted to weigh the publication bias of the results, and Duval and Tweedie's trim and fill test to assess the sensitivity of the results.19,20 Unless the p value was less than 0.001, the exact p value would be provided. Except that p < 0.10 in Egger's test was considered to be statistically significant, other p < 0.05 could be considered to be statistically significant.
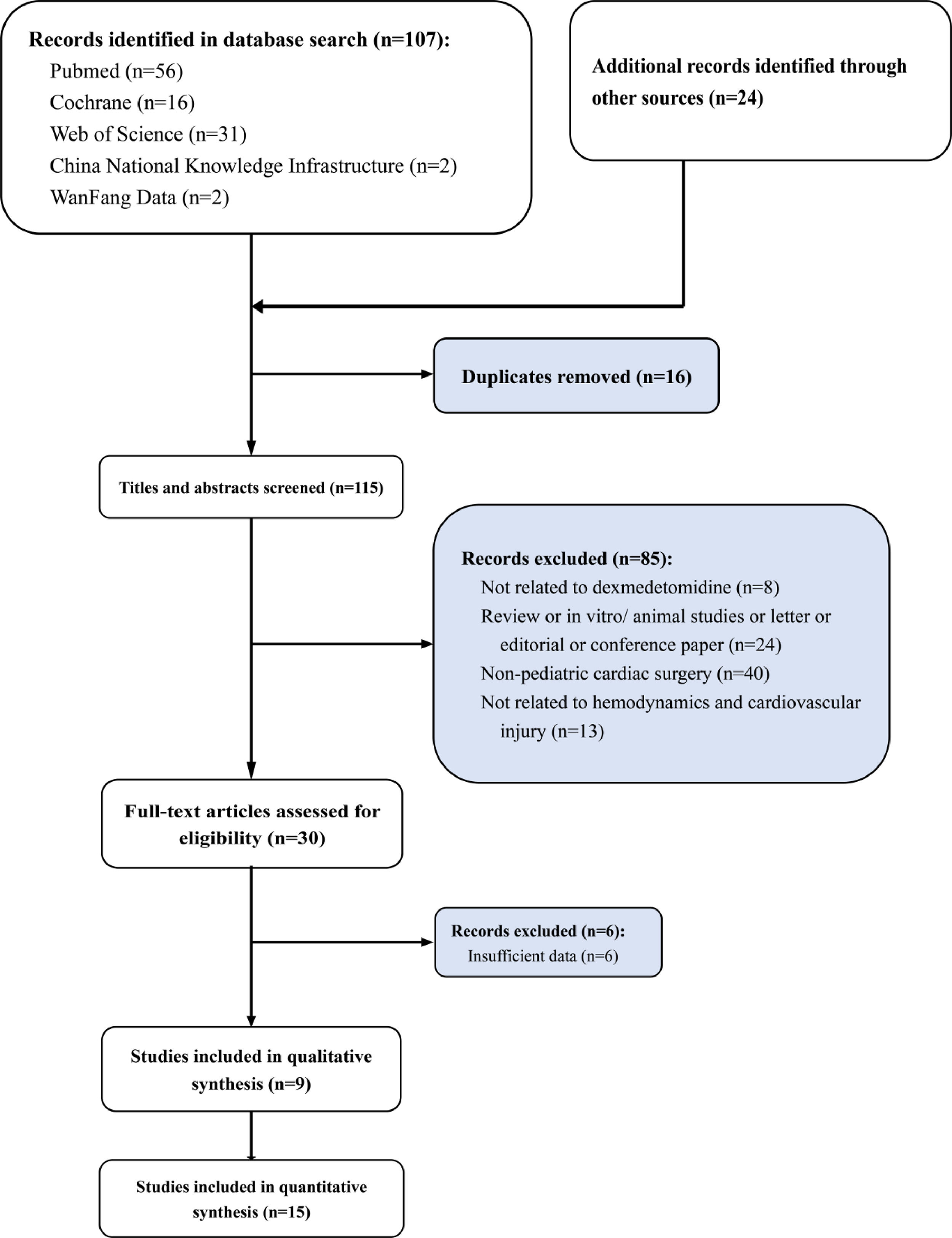
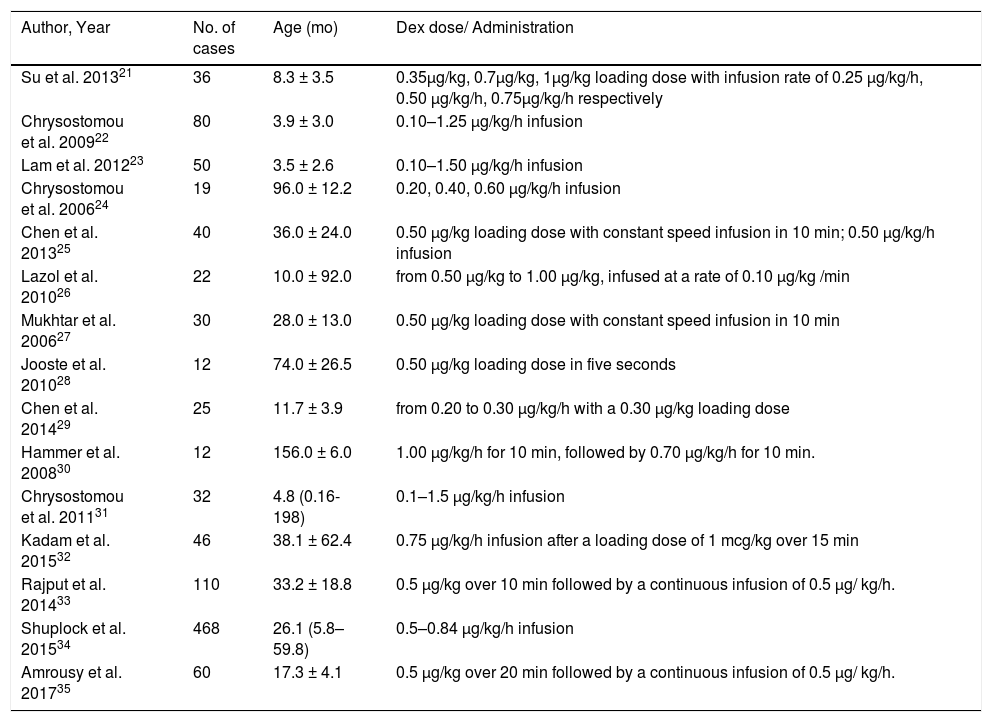
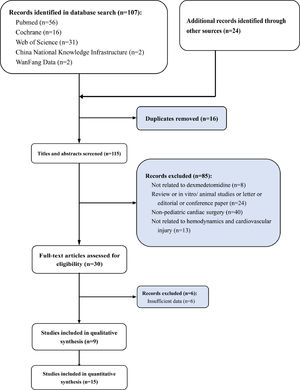
ResultsLiterature search, study characteristics and quality assessment107 articles were retrieved by database system retrieval and 24 by manual retrieval. After 16 repeated articles were removed, 85 articles that did not meet the inclusion criteria were excluded by screening the titles and abstracts of the articles. (not related to dexmedetomidine n = 8; review or in vitro/ animal studies or letter or editorial or conference paper n = 24; Non-pediatric cardiac surgery n = 40; not related to hemodynamics n = 13). Subsequently, 6 of 30 articles screened in the full text were excluded due to a lack of available data. Finally, a total of 9 studies were included in qualitative analysis and 15 in quantitative analysis (Fig. 1). The whole screening process included 1042 pediatric patients who underwent cardiac surgery treated with DEX for meta-analysis and 1332 pediatric patients who underwent cardiac surgery without DEX treatment. The basic features of 15 studies included in the meta-analysis are shown in Table 1.21–35
Baseline characteristics of included studies for meta-analysis.
| Author, Year | No. of cases | Age (mo) | Dex dose/ Administration |
|---|---|---|---|
| Su et al. 201321 | 36 | 8.3 ± 3.5 | 0.35μg/kg, 0.7μg/kg, 1μg/kg loading dose with infusion rate of 0.25 μg/kg/h, 0.50 μg/kg/h, 0.75μg/kg/h respectively |
| Chrysostomou et al. 200922 | 80 | 3.9 ± 3.0 | 0.10–1.25 μg/kg/h infusion |
| Lam et al. 201223 | 50 | 3.5 ± 2.6 | 0.10–1.50 μg/kg/h infusion |
| Chrysostomou et al. 200624 | 19 | 96.0 ± 12.2 | 0.20, 0.40, 0.60 µg/kg/h infusion |
| Chen et al. 201325 | 40 | 36.0 ± 24.0 | 0.50 μg/kg loading dose with constant speed infusion in 10 min; 0.50 μg/kg/h infusion |
| Lazol et al. 201026 | 22 | 10.0 ± 92.0 | from 0.50 μg/kg to 1.00 μg/kg, infused at a rate of 0.10 μg/kg /min |
| Mukhtar et al. 200627 | 30 | 28.0 ± 13.0 | 0.50 μg/kg loading dose with constant speed infusion in 10 min |
| Jooste et al. 201028 | 12 | 74.0 ± 26.5 | 0.50 μg/kg loading dose in five seconds |
| Chen et al. 201429 | 25 | 11.7 ± 3.9 | from 0.20 to 0.30 μg/kg/h with a 0.30 μg/kg loading dose |
| Hammer et al. 200830 | 12 | 156.0 ± 6.0 | 1.00 μg/kg/h for 10 min, followed by 0.70 μg/kg/h for 10 min. |
| Chrysostomou et al. 201131 | 32 | 4.8 (0.16-198) | 0.1–1.5 μg/kg/h infusion |
| Kadam et al. 201532 | 46 | 38.1 ± 62.4 | 0.75 μg/kg/h infusion after a loading dose of 1 mcg/kg over 15 min |
| Rajput et al. 201433 | 110 | 33.2 ± 18.8 | 0.5 μg/kg over 10 min followed by a continuous infusion of 0.5 μg/ kg/h. |
| Shuplock et al. 201534 | 468 | 26.1 (5.8–59.8) | 0.5–0.84 μg/kg/h infusion |
| Amrousy et al. 201735 | 60 | 17.3 ± 4.1 | 0.5 μg/kg over 20 min followed by a continuous infusion of 0.5 μg/ kg/h. |
Dex, dexmedetomidine; mo, month.
Age show in mean ± SD or median (interquartile range).
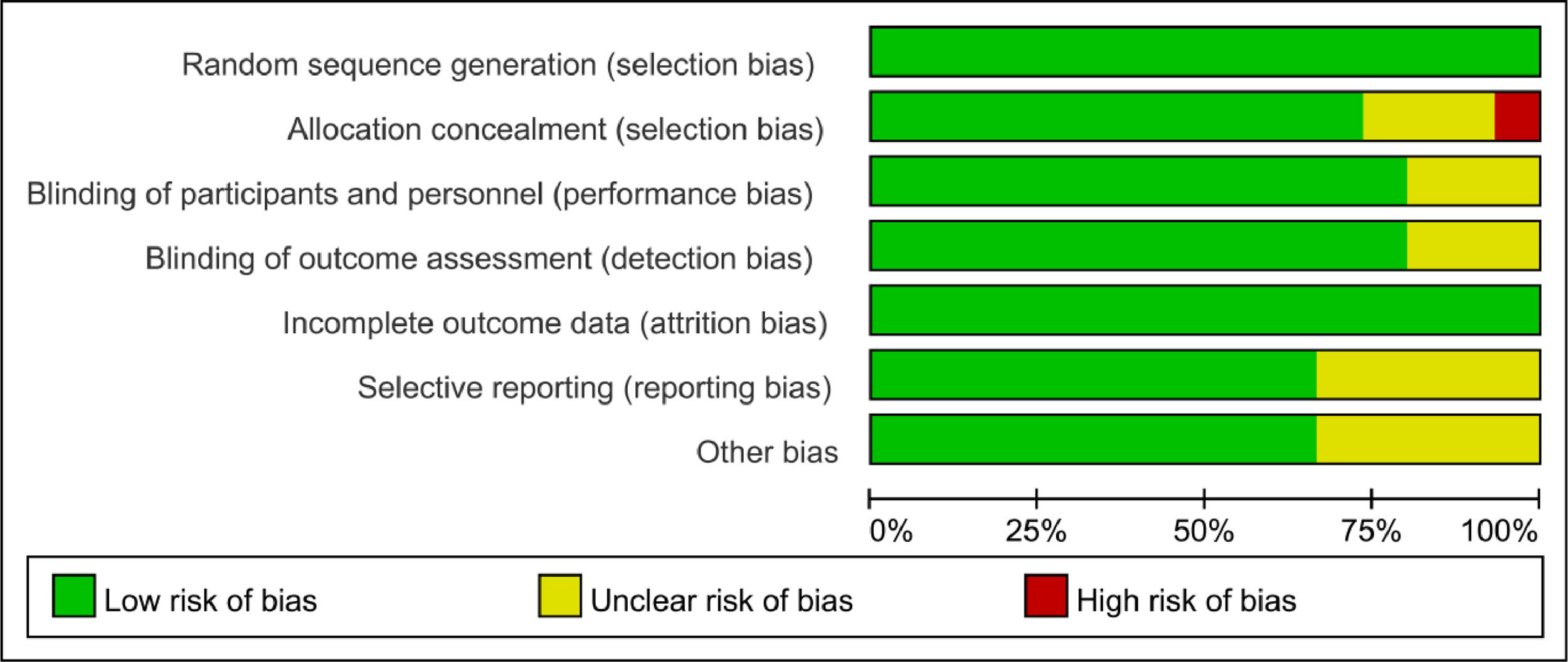
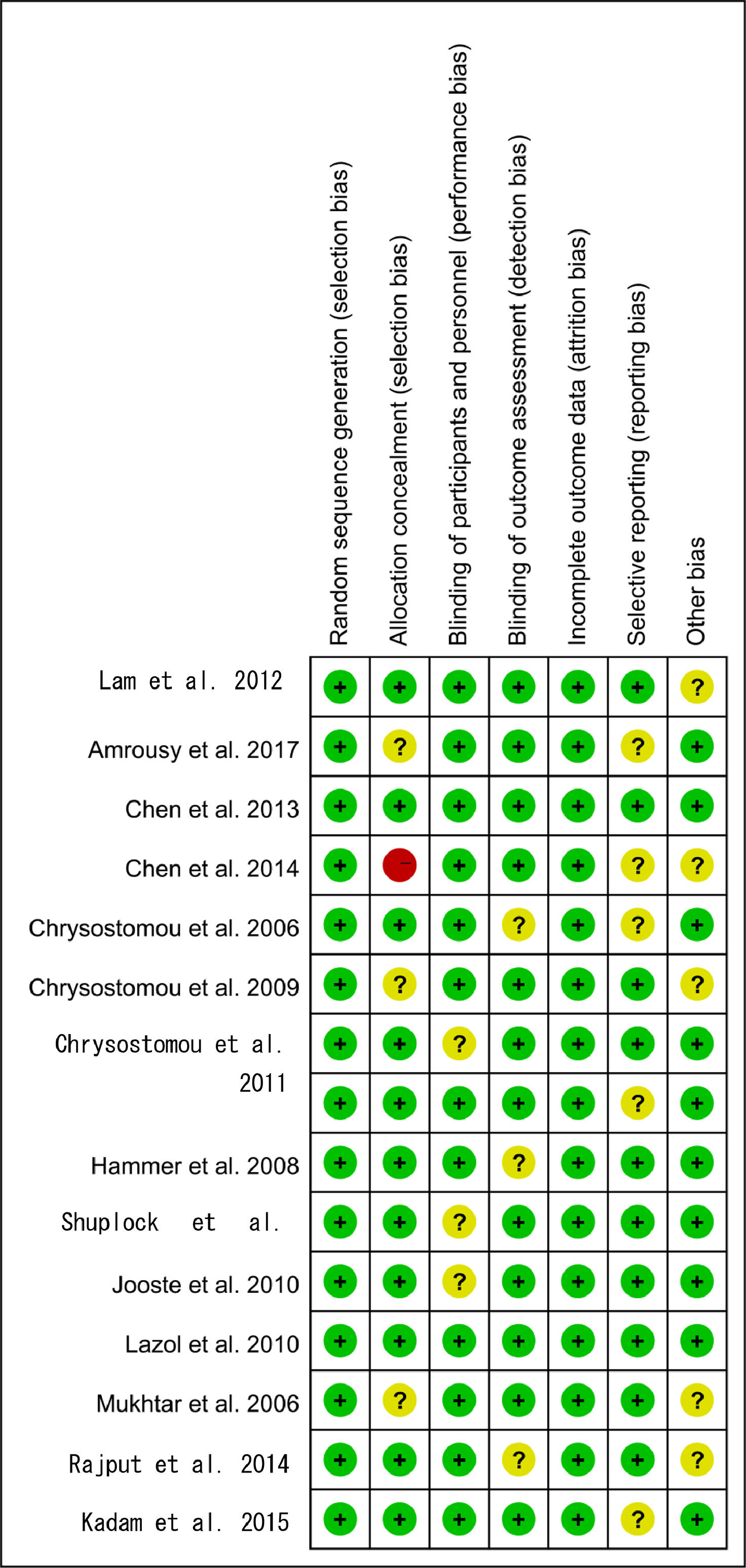
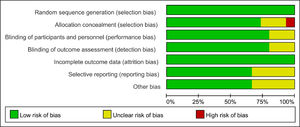
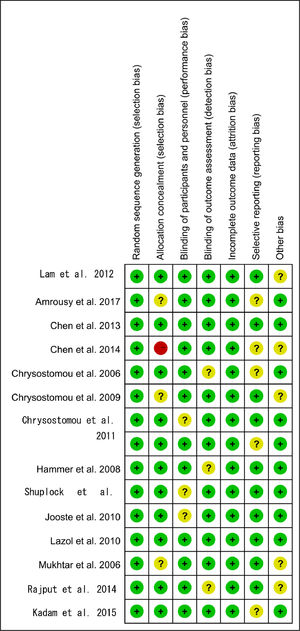
Based on the quality assessment part of the Cochrane handbook, a systematic evaluation was conducted to assess the bias risk of all included articles, such as selection bias, selective report bias, incomplete report bias, and publication bias. According to the Cochrane handbook, the quality of the literature was defined as low, moderate and high risk. All the included studies strictly followed the principle of random distribution, and before the beginning of the study, the subjects with chronic diseases who might cause adverse harm in the course of the study and those who were more likely to have complications in the course of the study were excluded. Therefore, the lack of data that could damage the effectiveness of the test was not possible. Both items were rated as low risk and the rest included in the overall evaluation of RCT were considered low risk of bias, which indicated that the quality of the meta-analysis was favorable and the result of the meta-analysis was reliable (Figs. 2 and 3).
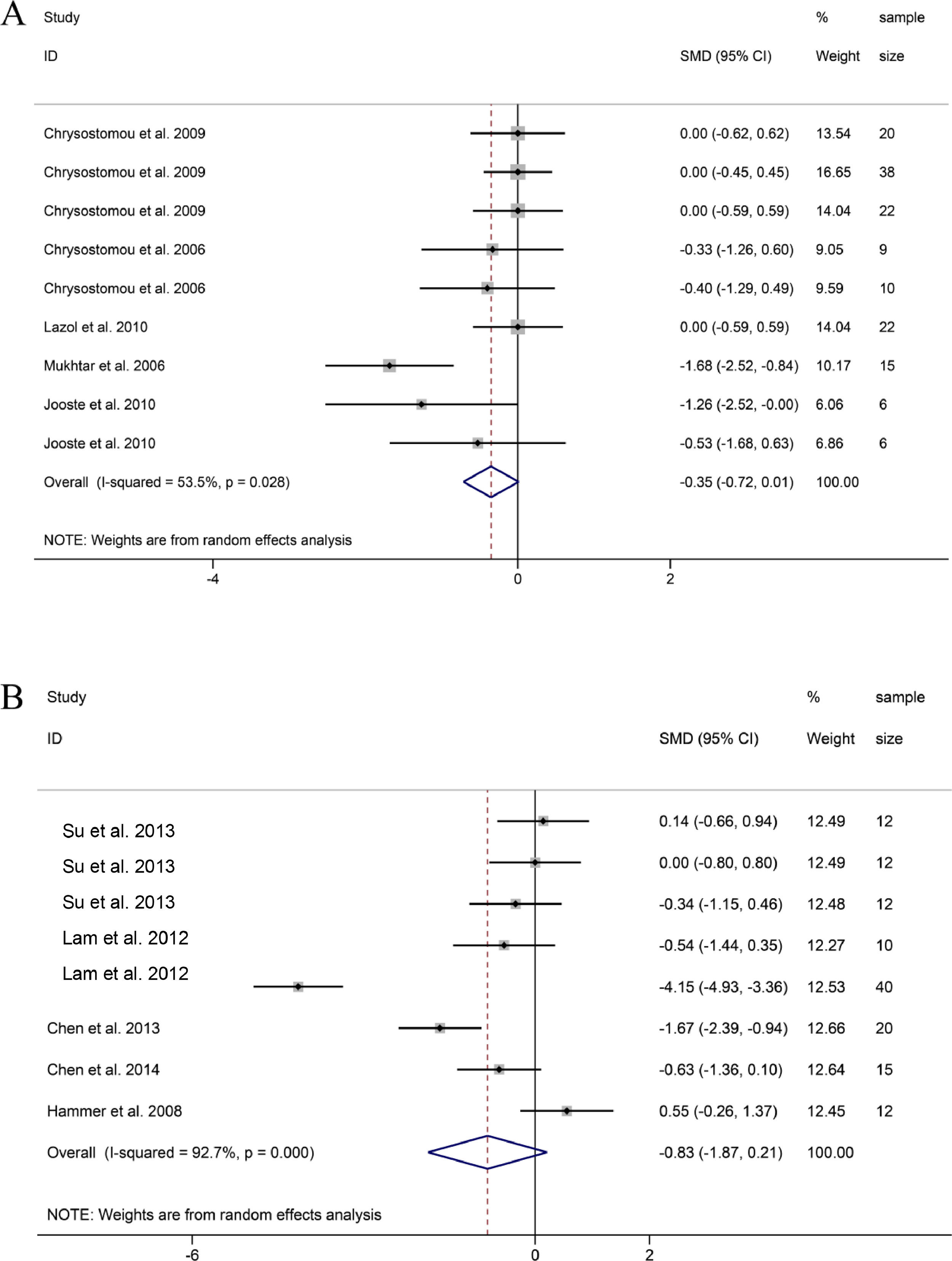
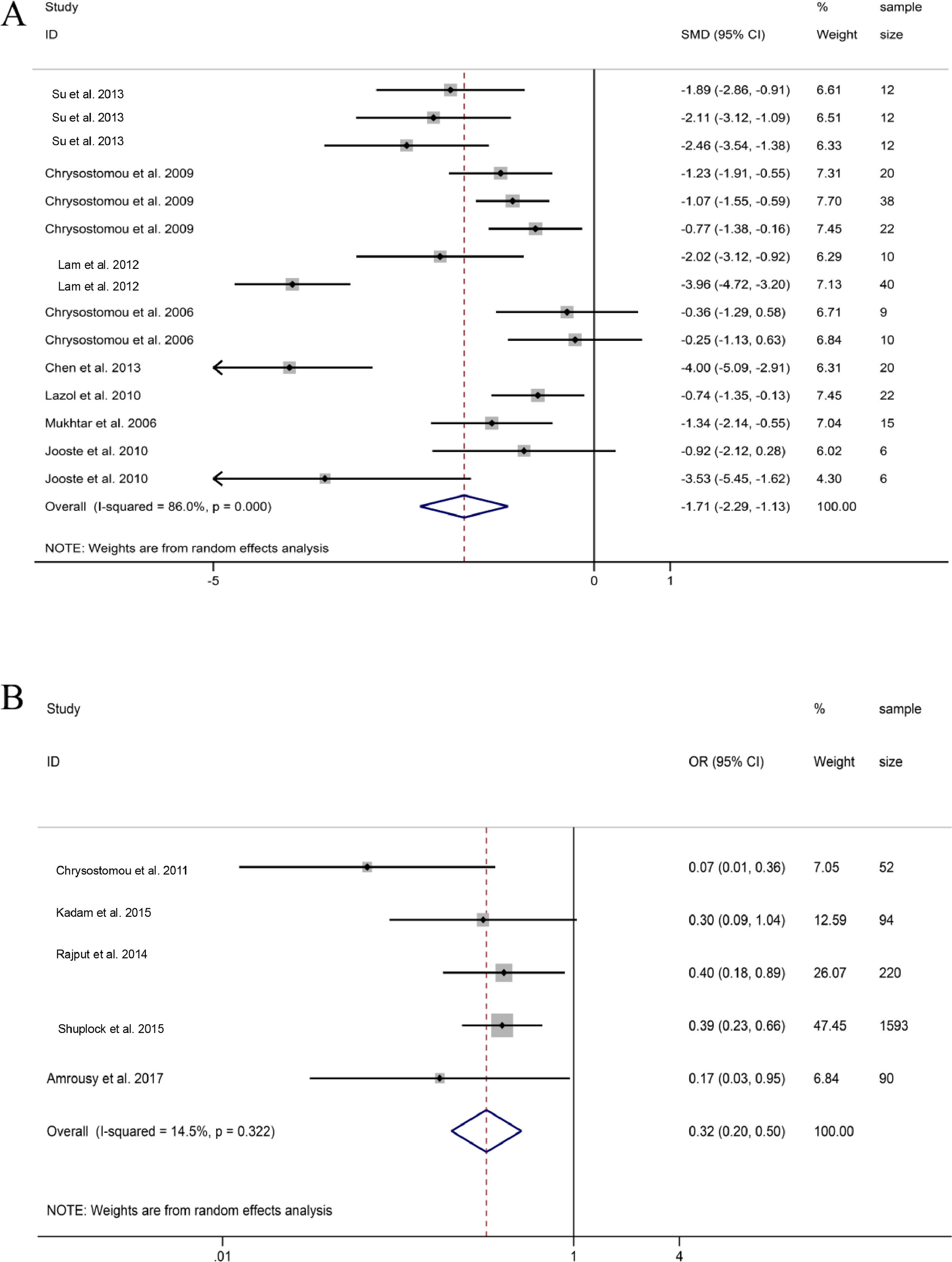
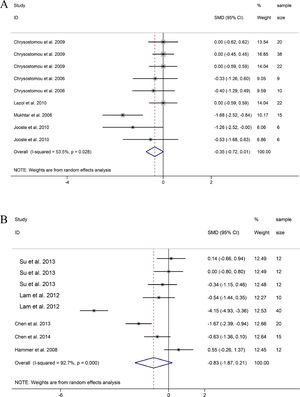
SBP. 9 groups on the changes of SBP before and after DEX treatment in pediatric cardiac surgery, it was found that the SBP of patients treated with DEX during or after cardiac surgery decreased compared with the baseline SBP, but the difference was not statistically significant (SMD = -0.35, 95% CI: -0.72, 0.01; Fig. 4A).22,24,26–28 Sensitivity analysis was used to study the sources of heterogeneity. Then, it was found that heterogeneity may be related to one group of research Mukhtar et al. and research Jooste et al., respectively.27,28
MAP. 8 groups reported MAP data before and after DEX treatment during or after pediatric cardiac surgery. The combined results of 8 groups showed that MAP also observed a downward trend, and the difference was not statistically significant (SMD =-0.83, 95% CI: -1.87,0.21; Fig. 4B)(21, 23, 25, 29, 30). Through sensitivity analysis, it was found that the main source of heterogeneity may be related to a group in the study of Lam et al. and research in the study of Hammer et al.23,30
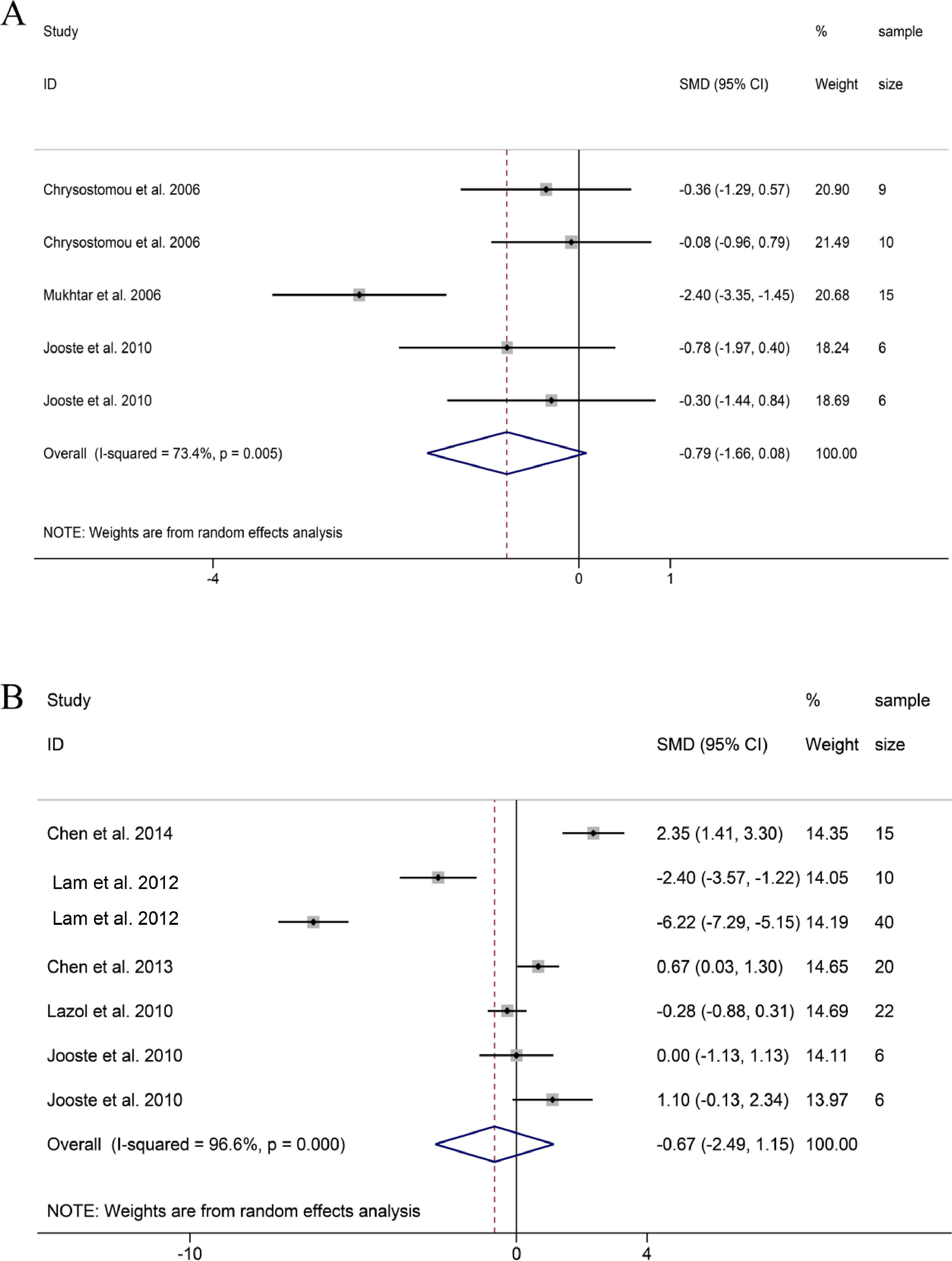
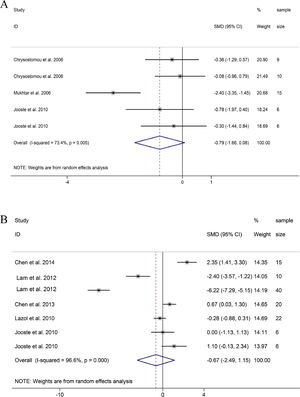
DBP and CVP. Similar to SBP and MAP, DBP and CVP also showed a downward trend, and the difference was not statistically significant (DBP: SMD = -0.79, 95%CI: -1.66, 0.08; CVP: SMD = -0.67 95%CI: -2.49, 1.15; Fig. 5A and B).23–29 Through sensitivity analysis, it was found that the heterogeneity of DBP came from the study of Mukhtar et al.27 Frustratingly, the hemodynamic index of CVP had strong heterogeneity and no obvious trend. Therefore, the guiding significance of this index remains to be further discussed.
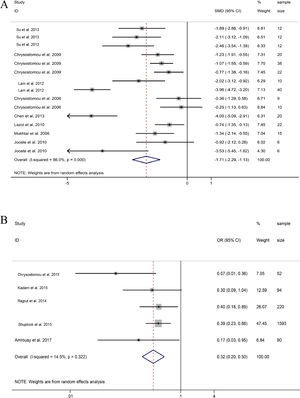
HR. 15 groups reported HR data before and after DEX treatment during or after pediatric cardiac surgery. The combined results of 15 groups found that HR showed a significant downward trend, and the difference was statistically significant (SMD = -1.71, 95%CI: -2.29, -1.13; Fig. 6A).21–28 Obviously, the decrease of HR in a group in Lam et al., and the study of Chen et al. and a group in Jooste et al. were significantly stronger than those in the other groups.23,25,28
Comparison between dexmedetomidine and control (Placebo)Junctional Ectopic Tachycardia (JET). Five studies revealed that the probability of JET during and after cardiac surgery treated with DEX was significantly lower than those of placebo groups (OR = 0.32, 95%CI: 0.20, 0.50; Fig. 6B).31-35 The five studies showed low heterogeneity, indicating that DEX can reduce the heart rate during and after pediatric cardiac surgery and effectively reduce the incidence of JET.
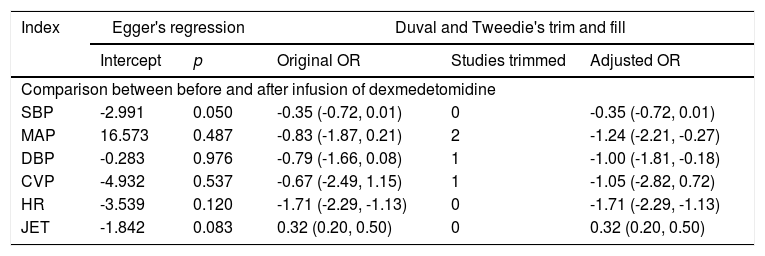
Publication bias assessment and sensitivity analysisEgger's test was used to analyze publication bias. It suggested that there was a publication bias in indices SBP and JET, but no publication bias was observed in other indexes. Duval and Tweedie's Trim and Fill Test of indexes MAP and DBP found that the effect quantity was not stable, and their guiding significance needs to be further discussed (Table 2).
Evaluation of publication bias and sensitivity analysis.
Evaluation of publication bias for the meta-analyses containing at least six studies.
SBP, Systolic Blood Pressure; MAP, Mean Arterial Pressure; DBP, Diastolic Blood Pressure; CVP, Central Venous Pressure; HR, Heart rate; JET, Junctional Ectopic Tachycardia.
In quantitative analysis, the high heterogeneity of meta-analysis results did not have a serious impact on the interpretation of the stability of hemodynamic indexes, which truly reflected the situation that may be faced in the clinical application of DEX. It was obvious that in the forest map, the mean and standard deviation of each group in the research were not merged, but regarded as one of the merged objects in the form of study, which was the main source of high heterogeneity in this study. The reason why the data were processed in that manner was that nowadays, DEX is used in patients, whether prophylactic medication before operation or recovery medication after an operation, to bring stable hemodynamic effects to patients, and it is nothing new to effectively reduce the heart rate and blood pressure of patients.13 Hence, it was only necessary to reconfirm the effect. Meanwhile, those studies that could not provide effective and analyzable data for meta-analysis studies also showed the same hemodynamic effects. The specific hemodynamic indicators were listed in supplemental Table 1.36–44 Based on the evidence of quantitative analysis and qualitative description, it was confirmed that DEX was able to reduce heart rate, blood pressure, stabilize hemodynamics and reduce the frequency of JET in preoperative preventive medication and postoperative recovery of pediatric cardiac surgery.
It was assumed that the publication bias of the indicator SBP observed by Egger's test mainly came from the fact that the group was used as a unit of analysis, and research Chrysostomou et al.22 and research Chrysostomou et al.24 of the groups accounted for 62.87% of the total analysis of the index SBP. The software could not identify this process, and the effects in the two studies were very similar, so it was concluded that the index had a nonnegligible publication bias. Based on the analysis of the publication bias detected by the index JET, it was undeniable that there lacked small-sized sample study in the JET index. Nevertheless, it was obvious that all five studies showed that the frequency of JET in the DEX group was less than those in the placebo group, which has clinical significance. As for the sensitivity analysis of Duval and Tweedie's trim and fill, it was assumed the indexes MAP and DBP showed a downward trend after DEX treatment, and the effect of maintaining hemodynamic stability during and after cardiac surgery was confirmed. With part of the study cut-off, the difference changed from no statistical significance to statistically significant for clinical guidance, which was only related to the statistical analysis method.
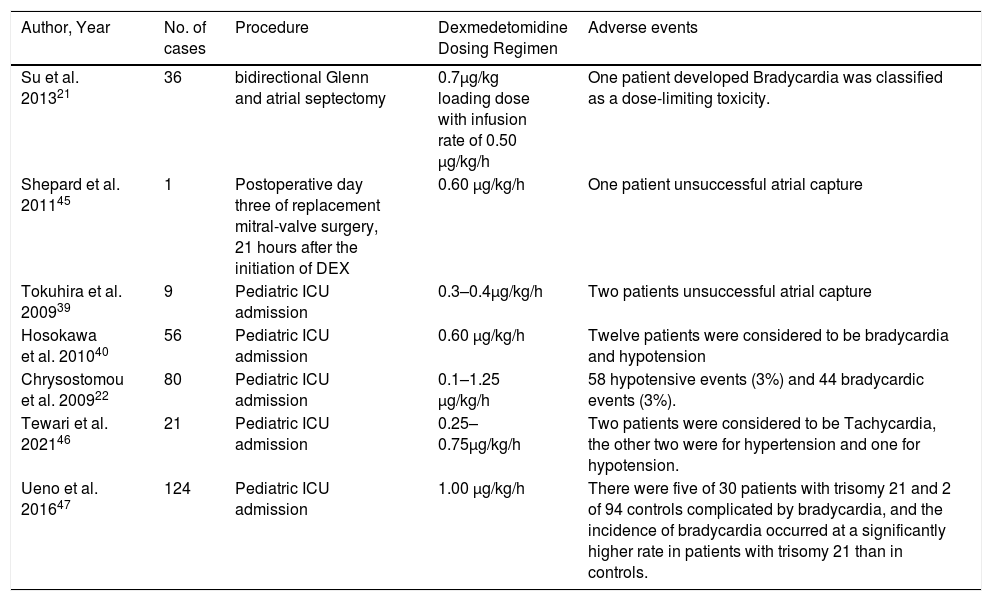
The adverse events caused by preoperative prophylaxis and postoperative recovery of DEX in pediatric cardiac surgery were presumed to be related to individual differences. All the adverse events reported in the study and after the administration of DEX in pediatric cardiac surgery were sorted out in Table 3.21,22,39,40,45–47 First, age had no significant effect on the pharmacokinetics of DEX, and the equilibrium half-life of different age groups was similar. But the decrease of tolerance to DEX may be caused by renal hypoplasia in some infants, which was not discussed in detail.23,37 This study focused on the dose and mode of treatment of DEX. The main purpose of using the group as the unit of the meta-analysis was to observe the stability of hemodynamic indexes and whether it was related to the frequency of adverse events with different doses of DEX and different treatment methods in the same study. Observed from the forest map, it was found that different DEX treatments in the same study produced different hemodynamic effects, but there was no good consistency in the same incremental dose treatment between different studies (Figs. 4–6, Table 1). Second, as the original data of the study could not be obtained, the authors cannot analyze the correlation between the dose and effect index of different DEX treatments statistically. Third, what could be confirmed was that there were only a few cases of adverse events such as bradycardia, hypotension, and transient hypertension among the doses collected for preoperative prophylaxis and postoperative recovery of DEX for pediatric cardiac surgery (Table 3). However, the incidence of bradycardia due to DEX treatment in children with trisomy 21 syndrome was significantly higher than that in normal children.47 Fourth, in addition to the commonly used method of slow injection of DEX in the application of DEX in children's cardiac surgery, rapid injection of DEX within 5 s to deal with emergencies was discussed. The hemodynamic indexes would temporarily increase within one minute after injection, but the hemodynamic indices would soon decrease to a stable level at 5 min.28,37 All in all, it was likely that the adverse events in the process of preoperative prophylaxis and postoperative recovery of DEX in pediatric cardiac surgery were related to individual differences.
Adverse events reported in the study and after the administration of DEX in pediatric cardiac surgery.
| Author, Year | No. of cases | Procedure | Dexmedetomidine Dosing Regimen | Adverse events |
|---|---|---|---|---|
| Su et al. 201321 | 36 | bidirectional Glenn and atrial septectomy | 0.7μg/kg loading dose with infusion rate of 0.50 μg/kg/h | One patient developed Bradycardia was classified as a dose-limiting toxicity. |
| Shepard et al. 201145 | 1 | Postoperative day three of replacement mitral-valve surgery, 21 hours after the initiation of DEX | 0.60 μg/kg/h | One patient unsuccessful atrial capture |
| Tokuhira et al. 200939 | 9 | Pediatric ICU admission | 0.3–0.4μg/kg/h | Two patients unsuccessful atrial capture |
| Hosokawa et al. 201040 | 56 | Pediatric ICU admission | 0.60 μg/kg/h | Twelve patients were considered to be bradycardia and hypotension |
| Chrysostomou et al. 200922 | 80 | Pediatric ICU admission | 0.1–1.25 μg/kg/h | 58 hypotensive events (3%) and 44 bradycardic events (3%). |
| Tewari et al. 202146 | 21 | Pediatric ICU admission | 0.25–0.75μg/kg/h | Two patients were considered to be Tachycardia, the other two were for hypertension and one for hypotension. |
| Ueno et al. 201647 | 124 | Pediatric ICU admission | 1.00 μg/kg/h | There were five of 30 patients with trisomy 21 and 2 of 94 controls complicated by bradycardia, and the incidence of bradycardia occurred at a significantly higher rate in patients with trisomy 21 than in controls. |
JET can lead to significant hemodynamic instability in the postoperative period after cardiac surgery. As a preventive and therapeutic drug, shown in the results, dexmedetomidine can effectively reduce the frequency of JET. JET is a tachyarrhythmia related to surgery for congenital heart disease which could cause serious hemodynamic instability in the postoperative period. JET is a narrow QRS complex tachycardia with atrioventricular dissociation and a slower atrial rate as compared to ventricular rate. Atrioventricular asynchrony causes loss of atrial contraction contribution to cardiac output, resulting in hemodynamic instability. At present, the exact mechanism of JET is unknown and may be related to proximal conduction tissue injury associated with indirect stretch injury or suture surgery. The incidence of JET has been reported to range from 8% to 24%, depending on the patient's choice and surgical procedure.48,49 While dexmedetomidine exerts sedative, analgesic and antianxiety effects through the pharmacological effects of α-2 adrenergic receptor agonist, the results of meta-analysis also showed that the use of dexmedetomidine in pediatric cardiac surgery can reduce heart rate and reduce the occurrence of JET.
The authors investigated the indirect results of cardiac output in 9 articles included in the meta-analysis and 15 articles included in the qualitative analysis. The reduction in heart rate and blood pressure caused by the efficacy of dexmedetomidine for maintaining hemodynamic stability in pediatric cardiac surgery does not support the reduction in cardiac output. The above studies included at least one of the following indicators that did not support decreased cardiac output: absence of acidosis, unchanged inotropic requirement, central venous pressure, and left ventricular function. Therefore, the authors do not believe that the decrease in blood pressure is due to low cardiac output syndrome, but is the result of sedation, analgesia, and possibly the result of activation alpha-2 adrenergic receptors in the central nervous system.
This study inevitably had some limitations: except for trisomy 21 syndrome, all the patients included in this review were patients without chronic diseases of kidney, liver or other organs, which had limitations in guiding the clinical application of DEX to prevent adverse events.
In conclusion, the application of DEX for preoperative prophylaxis and postoperative recovery in pediatric cardiac surgery patients is effective in maintaining hemodynamic stability with reduced blood pressure with no statistical significance. Furthermore, the clinical dose of DEX is not significantly related to the occurrence of pediatric adverse events which may be related to individual differences.
Ethics approval and consent to participateEthical approval was not needed because this is a meta-analysis.
Data for referenceThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
FundingThis paper is supported by the Hainan Natural Science Foundation Youth Project (Grant No:819QN394).