The objective of this study was to evaluate the effect of Kangaroo Position (KP) in microcirculation (MC) of the flexor muscles of preterm newborns.
MethodA controlled clinical trial was conducted in the city of Recife, Brazil, with 26 preterm children randomized in the Kangaroo Group (13) and in the Control Group (13). Assessments of blood flow, temperature, and tissue oxygen saturation (SO2) were made at two different times and in the biceps brachii muscle and hamstrings muscle group: before the KP and after 24 h of KP. In the Control Group, the registrations were performed at the times corresponding to those of the Kangaroo Group. The mean values among the times were analyzed by paired t-test for repeated measures. The clinical trial was recorded in Clinical Trials (NCT03611088).
ResultsIn the Kangaroo Group there was an increase in tissue temperature and blood flow at the time evaluation periods (p < 0.05). In the control group, there was no statistical difference between the recording moments hamstring muscles group, but in the biceps brachii, there was a reduction in mean blood flow (p = 0.023).
ConclusionIn conclusion, the KP has effects on the microcirculation of the flexor muscles of preterm newborns.
The Kangaroo Mother Care (KMC) is defined as a continuous skin–to–skin contact (SSC) between the mother and her newborn, which is established at an early stage.1-3 The major component of KMC is the Kangaroo Position (KP), by which infants are placed with flexed limbs, vertically between the mother's breasts firmly attached to the thorax and underneath her clothes.4
Studies have shown several benefits of KMC, such as reducing mortality, severe infection/sepsis, hypothermia, hypoglycemia, pain, hospital readmission; as well as improvement in breastfeeding, weight gain, greater head circumference growth, and improvement in psychomotor development.2,5-7 Furthermore, they also have shown that KMC promotes an improvement in the vital signs and in physiological parameters, such as oxygen saturation and temperature.5,8,9
Only the macrocirculation effect on the KP was researched. Nevertheless, microcirculation (MC) is essential to provide oxygen and nutrients to the tissues and cells, and its development in healthy newborns, similar to macrocirculation, happens during the first weeks of life.10-13 At critical times, such as preterm birth, the authors know the macrocirculation alterations, such as a decrease in blood pressure, but prematurity also causes modification in the MC.11,14,15
Such modifications are due to the immaturity of vital organs and vessels, lack of self-regulation of blood flow to vital organs, and immaturity of protection mechanisms against infections, hypoxemia, hyperviscosity, and acidosis.14-17 An altered MC can negatively impact brain oxygenation, it also presents a correlation with low neurodevelopment in newborns, infections, hypertension, and premature death to stroke.15,17-20
Considering the MC dysfunction repercussions in newborns due to the absence of a fully developed vascular tree, as well as the electrophysiological alterations found after the KP stimuli in the given population, the authors considered the hypothesis of studying if the parameters of microcirculation would change after the KP.21-23 Therefore, the objective of this study was to assess the KP effect in the microcirculation of the flexor musculature of preterm newborns.
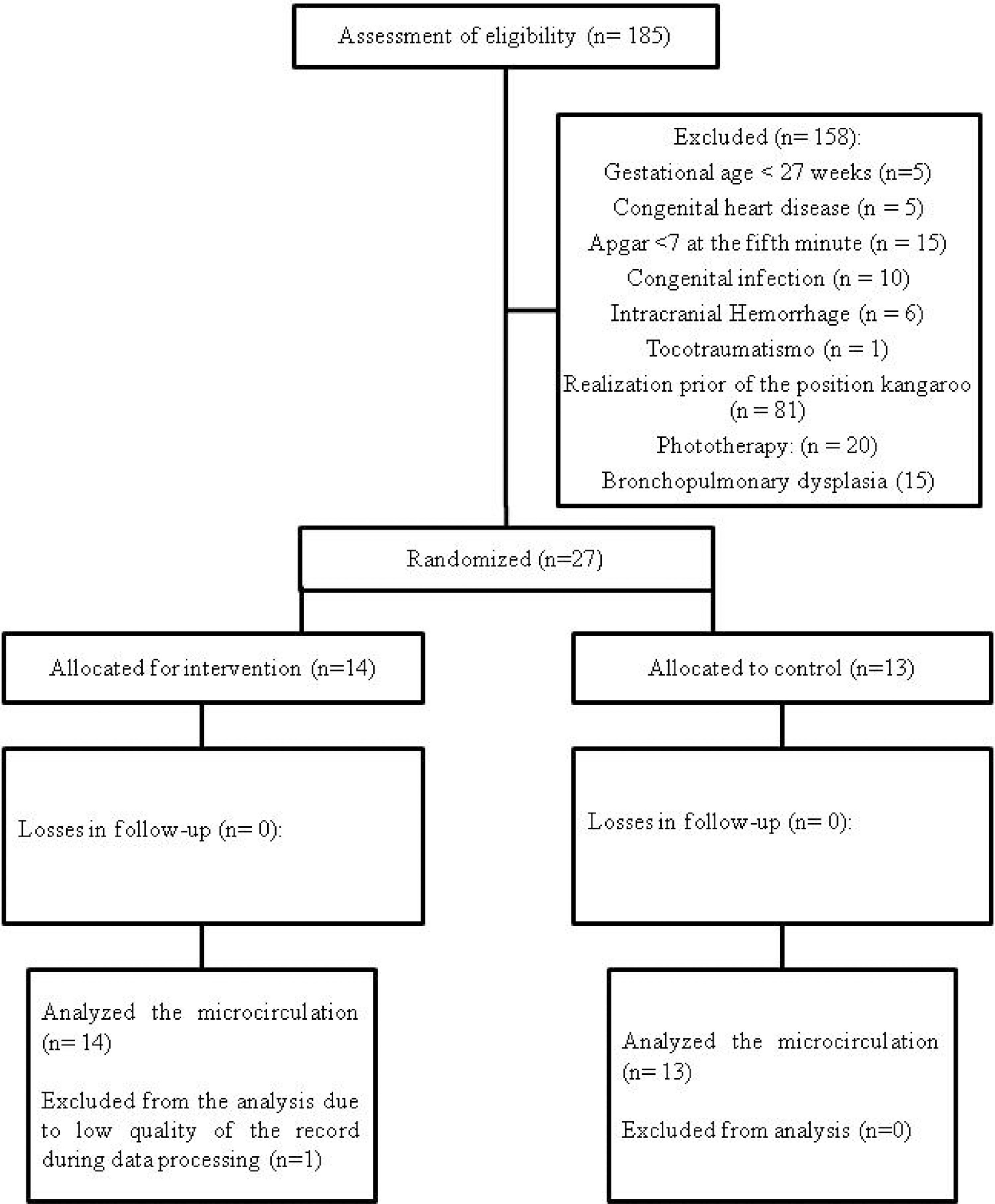
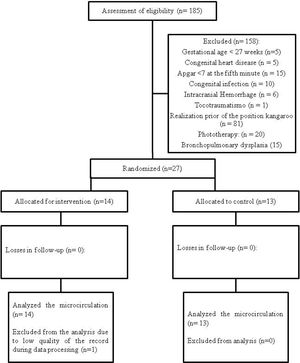
MethodsParticipantsA controlled randomized clinical trial study was carried out, from January to September 2018, at the Instituto de Medicina Integral Prof. Fernando Figueira (IMIP), in Recife, Brazil, with 27 preterm newborns, that were randomized into two groups: Kangaroo Group (n = 14) and Control Group (n = 13). They were hospitalized in the Kangaroo Unit at IMIP.
IMIP's Kangaroo Unit covers a surface area of 600 m2 and it has a ward with 22 beds for clinically stable preterm newborns (respiratory frequency of between 30–60 inspirations per minute, a heart rate of between 120–160 beats per minute, peripheral oxygen saturation of over 89%, absence of signs of respiratory distress, absence of cyanosis or pallor and pain). The newborns should tolerate food, breathe without the use of an apparatus, and weigh more than 1250 grams.
The Kangaroo Unit provides medical and nursing services, speech therapy, and physiotherapy for clinically stabled preterm newborns. In this unit, the newborns, when referred to medical services, are evaluated, and they undergo an early stimulation program (procedures and programs that facilitate the development or skill acquisition).
Newborns were included in the preterm groups if they had not previously been to the KP, and if they also had a gestational age of 28–34 weeks and a corrected age of a maximum of 37 weeks at the time of the first electromyographic examination.
The exclusion criteria were Apgar less than 7 in the 5th minute; previous history of intracranial hemorrhage (diagnosed by ultrasonography and registered in the medical record); previous convulsion history; congenital infection (cytomegalovirus, rubella, toxoplasmosis, syphilis, and vertically transmitted HIV); infections of the central nervous system (meningitis or encephalitis); malformations in the central nervous system (hydrocephaly and genetic syndromes); congenital cardiopathies; trauma during delivery (injuries to the brachial plexus, dislocation of the hip and pelvis fractures); gastroesophageal reflux disease disorder; be in phototherapy; bronchopulmonary dysplasia; and anemia. All of these conditions should be present in the medical record with a diagnosis defined by professionals in the hospital sector.
The sample size was calculated using a previous study24 that evaluated the blood flow in preterm newborns. The difference between the pre-and post-intervention flow averages of 345, with a standard deviation of 73 and 211, was used. With an alpha error of 0.05 and potency of 0.9, the resulting sample size was 10 individuals.
The random allocation in the Kangaroo Group or in the Control Group was performed by program R (version 3.3.1) from a list of 40 numbers (from 1 to 40). A random sample of 20 numbers was drawn, indicating the order of entry into the Kangaroo Group. The Control Group was defined by the undrawn numbers from the list. Once the order of the numbers was known, the newborns were allocated into groups according to their admission in the sector. The researchers responsible for the allocation of the newborns in the Kangaroo or Control Group were different from those who performed the evaluation of the microcirculation analysis and statistical analysis.
The nursing technicians at the Kangaroo Unit were previously trained to assist the progress of the research. These professionals were responsible for the newborns that were in the Kangaroo Group or in the Control Group.
A flowchart (Fig. 1) was completed according to the recommendations of the CONSORT Statement,25 it includes loss of data from a newborn (n = 1) due to poor record quality during data processing.
Data collectionThe evaluation was performed in a specific room for the exam, with temperature control at 20 – 23°C, absence of other equipment that could generate interference in the captured signal. To evaluate the microcirculation (tissue oxygen saturation - SO2 and tissue temperature), the white light spectroscopy method was used, using the MoorVMS-OXY® unit; the Laser Doppler Fluxometry (LDF) technique is performed by microcirculation devices that monitor the superficial blood flow to the skin and underlying tissues. The LDF signal is proportional to the number and rate of red blood cells in a given area of the skin and is expressed in perfusion units (PU). Was used two CP2T-1000® skin probes; two PH2® probe holders; double-sided adhesive discs to attach the probe to the skin; two PH1-V2® fiber optic cables; MoorVMS-PC 1.0® software for data processing; a USB and notebook cable to capture, process and interpret the signal.
During collection, the preterm newborn was positioned in a high dorsal decubitus position, with a slope of approximately 30 degrees, the right side of the body in contact with the mother's abdomen and the head resting on the left folded elbow of the mother. The microcirculation parameters were captured with the premature in Brazelton state 4 or 5 (inactive alert or activity alert) respectively.26 The probes (CP2T-1000®) were positioned in the anterior region of the left arm of the newborn, well fixed by specific double-sided adhesives, supplied by the same manufacturer, they were placed on the skin in the region related to the biceps brachii muscles and in the hamstring group. After the placement of the probe, the signal was captured for 1 minute.
Two groups were designed: 1) preterm newborns in kangaroo position (Kangaroo Group); 2) preterm newborns not in kangaroo position (Control Group). In the Kangaroo Group, the microcirculation was measured before being in KP (0h). Immediately after taking the first evaluation, they were placed for the first time in KP, which was performed according to the orientation of the IMIP service: using a band (made of cotton) as a support to the newborn, against the adult's thorax, in a prone and vertical position, and dressed with few pieces of clothes keeping skin-to-skin contact with the mother. Subsequent readings were taken after 24h of the KP. The newborns were kept in the kangaroo position for 8-12 hours per day, until the second evaluation, after 24h the first recording. In the Control Group, the newborns were not submitted to the Kangaroo Position (remaining resting in the crib), but the records were performed during the periods of time corresponding to those of the Kangaroo Group. The records were always made by the same researchers (NPSF, AAF and JTBL).
During the collection of data, the researchers ensured that the newborns were not performing motor physical therapy. The newborns did not, therefore, undergo any kind of early motor stimulation during data collection, except for oral stimulation by speech therapists, when necessary. The observance of the children's permanence in the appropriate conditions for both the Kangaroo Group and the Control Group was supervised by the researchers.
Data processingFor the data processing, a 30-second selection (windowing) was performed for each registry of each variable: blood flow, SO2 and tissue temperature, through moorVMS-PC 1.0® software.
Statistical analysisFor the comparison between the variables of Gestational Age (GA), Corrected Gestational Age (CGA), and birth weight, after verifying the normal distribution of the data (Kolmogorov-Smirnov test), the t-test was used to compare the Kangaroo and Control Groups. To compare the Apgar, the Mann-Whitney Rank Sum Test was used and the Chi-square test was used to compare the sex.
Comparison of the means of the times was performed by paired t-test, after establishing the normality of the distribution (Shapiro-Wilk test) and the homogeneity of variance (Levene Test). The alpha error for rejection of the null hypothesis was 0.05.
Ethical aspectsThe study was submitted to the IMIP's Ethics Committee for Research involving Human Beings and was approved (N° 69847917.7.0000.5201). The parents or guardians who agreed to participate signed terms of free informed consent. The clinical trial was recorded in the ClinicalTrials.gov (NCT03611088).
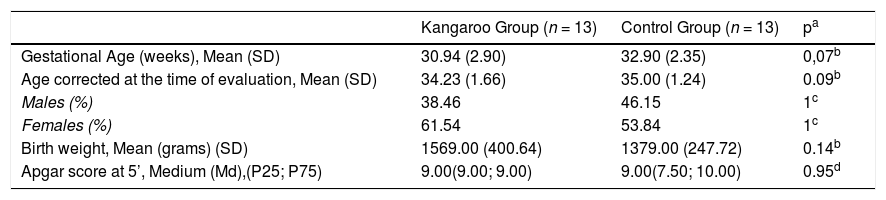
ResultsThe clinical and biological characteristics of the newborns in each group are presented in Table 1.
Clinical and biological characteristics of the newborns.
| Kangaroo Group (n = 13) | Control Group (n = 13) | pa | |
|---|---|---|---|
| Gestational Age (weeks), Mean (SD) | 30.94 (2.90) | 32.90 (2.35) | 0,07b |
| Age corrected at the time of evaluation, Mean (SD) | 34.23 (1.66) | 35.00 (1.24) | 0.09b |
| Males (%) | 38.46 | 46.15 | 1c |
| Females (%) | 61.54 | 53.84 | 1c |
| Birth weight, Mean (grams) (SD) | 1569.00 (400.64) | 1379.00 (247.72) | 0.14b |
| Apgar score at 5’, Medium (Md),(P25; P75) | 9.00(9.00; 9.00) | 9.00(7.50; 10.00) | 0.95d |
SD, Standard Deviation; n, number; P, percentile.
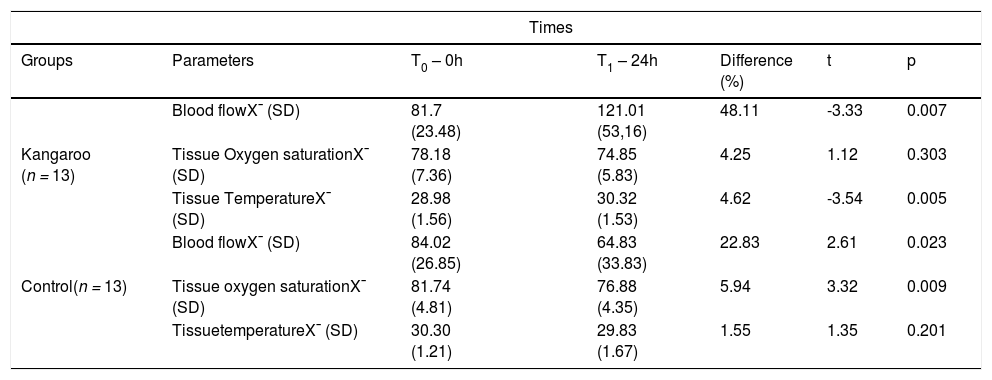
Regarding the brachial biceps muscle (Table 2), the authors observed that in the Kangaroo Group, there was a variation of the blood flow (t = -3.33; p = 0.007) and tissue temperature (t = -3.54; p = 0.005) between the two recording moments, with the mean unity of T1 increasing in relation to T0. The tissue oxygen saturation (SO2) remained constant (t = 1.12; p = 0.303). In the Control Group, in the blood flow (t = 2.61; p=0.023) statistical differences were observed between the two moments, there was a decrease in the mean; the SO2 decreased (t = 3.32; p = 0.009); in the tissue temperature (t = 1.35; p = 0.201) no statistical differences were observed between the two moments.
Microcirculatory parameters of the brachial biceps muscle of preterm newborns submitted and not submitted to the Kangaroo Position at different times.
X¯, mean; SD, Standard Deviation; Blood flow: PU; Tissue Temperature:°C; Tissue Oxygen Saturation (SO2): %; T0 and T1 (First and second moments of register); Kangaroo Group x Control Group: Normality Test (Shapiro-Wilk): p > 0.05; and Paired t-test.
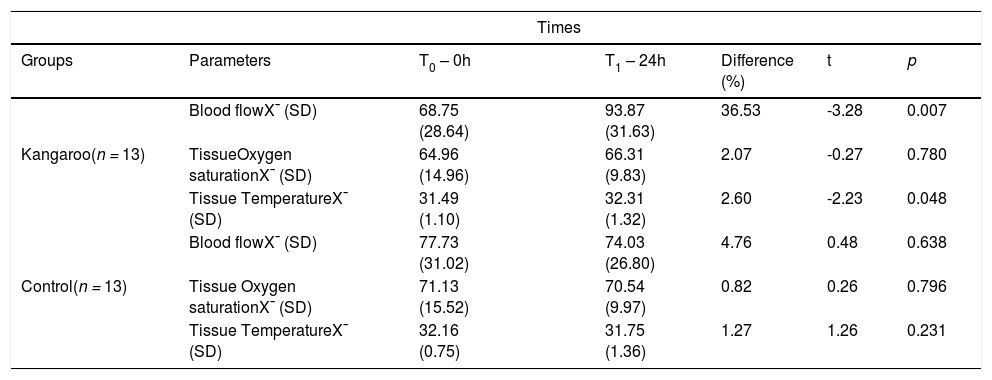
In relation to the hamstrings muscle group (Table 3), the authors observed that in the Kangaroo Group, there was a variation of the blood flow (t = -3.28; p = 0.007) and tissue temperature (t = -2.23; p = 0.048) between the two recording moments, with the mean unity of T1 increasing in relation to T0. The SO2 remained constant (t = -0.27; p = 0.780). In the Control Group, at blood flow (t = 0.48; p=0.638), SO2 (t = 0.26; p = 0.796) and tissue temperature (t = 1.26; p = 0.231), no statistical differences were observed between in the two moments.
Microcirculatory parameters of the hamstrings muscles of preterm newborns submitted and not submitted to the Kangaroo Position at different times.
X¯, mean; SD, Standard Deviation; Blood flow: PU; Tissue Temperature:°C; Tissue Oxygen Saturation (SO2): %; T0 and T1 (First and second moments of register); Kangaroo Group x Control Group: Normality Test (Shapiro-Wilk): p > 0.05; and Paired t-test.
In the present study, the authors observed an increase in the mean blood flow in the Kangaroo Group from T0 (0 h) to T1 (24 h after the first evaluation), both referring to brachial biceps muscle (increasing 48.11%) and in the hamstrings muscle group (increasing 36.53%). However, in the Control Group from T0 to T1, the values of the blood flow in the brachii biceps muscle decreased (decreasing 22.83%) and in the hamstring muscles group, they did not change. It is the first time that research has shown an increase in tissue blood flow in the region of the flexor muscles the appendicular skeletal, particularly as an outcome caused by the Kangaroo Position (KP) stimulation - after 24 h of intervention.
There is a gap in the scientific literature regarding the evaluation of the microcirculation of premature infants submitted to the KMC and/or placed in KP. However, a study24 that may serve as a comparison with the present study's findings has found a significant increase in blood flow to the extremities in preterm infants placed at a temperature-controlled incubator, termed comfort temperature (a temperature of minimal difference between the abdominal wall and the extremities less than 2°C), taking into account a temperature of the abdominal wall 36.5°C. These findings may support the present study's results, in the light of the effects on KP already proven in body temperature7 - as an analogy to the incubator. Moreover, a decrease of blood flow occurred in the evaluation of the brachial biceps muscle of the Control Group strengthens the cause-effect relationship between KP and flow.
The association between blood flow and temperature seen in the aforementioned study24 corroborates the present study's results since this study also showed an increase the temperature, but it is important to note that the increase in temperature here identified refers to the tissue, particularly related to microcirculation. In the brachii biceps muscle increased 4.62% and in hamstrings 2.60%. In the Control Group, the temperatures remained stable after 24h, both the brachial biceps muscle and the hamstring muscle group.
The researches on premature infants submitted to the KMC has shown consistent results regarding the increase in body temperature, which made it necessary to better understand them and other effects related to the improvement of nutrients and oxygen supply to organs and tissues, such as improved motor development, arterial saturation, cerebral functioning, cardiorespiratory stability, among others.5,8,9,27
Another variable in the present study's research that changed and that deserves to be highlighted was tissue oxygen saturation (SO2). In the Kangaroo Group, both in the brachial biceps muscle and in the hamstring muscle group the SO2 remained stable. Meanwhile, in the Control Group of the brachial biceps muscle, there was a statistically significant reduction after 24h (from 81.74 to 76.88, reducing 5.94%); in the hamstring muscle group there was a reduction, although it was not statistically significant (from 71.13 to 70.54; reducing 0.82%).
Researches on premature infants inserted in the KMC show an improvement in oxygen saturation, however, such information refers to microcirculation.2,5,7,9 The information that the SO2 remains constant in the first 24 h compared to a mean decrease in the control group strengthens the present study's results and suggests a better "physiological behavior" of the evaluated tissues, the maintenance of the microcirculatory function, as stability in SO2, suggests the organ and tissue “homeostasis”.
Despite the dearth of researches investigating temperature, blood flow, and SO2 in preterm newborns submitted to KMC, the randomized clinical trial cited earlier24 found the association between increased temperature and blood flow in preterm infants placed in an incubator. Once this association is perceived, the authors can hypothesize that this bigger "nutrient flow" may have maintained stable oxygen saturation in the tissues. Knowing that an increase in muscle activity is associated with an increase in total peripheral blood flow, the effects on microcirculatory parameters found in the present study's research can be attributed to KP (main characteristic of KMC) and its capacity to produce stimuli (postural, vestibular, synesthesic and tactile).
Additionally, observational studies21-23 suggest an association between KP and an increase in the electromyographic activity of the flexor muscles of premature infants, therefore there was a gap regarding the mechanism by which these effects were verified. Thus, the modifications in the parameters of the microcirculation strengthen the hypothesis that the increase of this electric activity may be related to this bigger "nutrient flow". Bearing in mind that the homeostasis of the physiological phenomenon of muscular contraction is related to the delivery of ions, energy substrates, and oxygen, the authors can consider that the increase in the parameters of the microcirculation, after KP, strengthens the hypothesis that this increase in the electromyographic activity is related to the microcirculation mechanisms.
Very early exposure to events such as premature birth may contribute to the development of epigenetic modifications and subsequent adaptations of microvascular development. Such events influence the regulation of organs and systems, inducing a disadvantageous microvascular phenotype (capillary rarefaction, endothelial dysfunction).15,28
Consequently, there are a number of different perinatal “windows of opportunity” during which the maternal, fetal, and infant environment may influence the development of the microvasculature and its maturational shift.15 Considering the effects on microcirculation parameters found in the present study's research, the authors suppose that KP can be considered one of these "opportunities windows" aforementioned. Therefore, based on the present study's results, the authors suggest that this fragile population of newborns may benefit in the short and long term from the stimuli provided by KP. In addition, it emphasizes the relevance of the evaluation method that was used in this present research.
Evaluation of the cutaneous microcirculation, as the authors have done in the present study's research, can help us understand peripheral perfusion in a non-invasive manner, the physiological difference between premature and full-term babies, and especially interventions that can be performed to minimize the repercussions of immature development of the vascular tree of preterm newborns,13,10 such as the KP.
One limitation of this research is the lack of mothers' blindness regarding the intervention. Nevertheless, because KP is a mother-baby posture, this methodological tool was unfeasible. It is also important to highlight that although this study was performed with 13 individuals, a previous sample calculation was performed to support the generalization of the outcomes and to minimize the risk of bias. In addition, the authors do not collect information regarding the newborns’ race and weight at the time of data collection.
Another limitation is related to the absence of a stratification of the sample by age. It is suggested that further studies use this statistical tool, but in the present study's, the lack of stratification could be minimized by researching the standard deviation value found.
In conclusion, KP has effects on the microcirculation of the flexor muscles of preterm newborns. Furthermore, the innovative results of this study contribute to a better understanding of the mechanisms by which previous studies verified an increase in the electromyographic activity of the flexor muscles in preterm infants submitted to KP. The authors may suggest that this increase in normally hypotonic muscles may be related to better functioning of the microcirculation. However, future mechanistic and correlation studies may help to clarify more securely the relationship between electromyographic and microcirculatory variables. The association of the microcirculation parameters with the electromyographic activity and macrocirculation parameters should be examined in further studies. Longer follow-up studies may help clarify the evolution over time of microcirculatory parameters. Obtaining improved microvascular function in newborns submitted to KP can produce benefits (improved tissue function/organ/systems, such in the skeletal muscle) in childhood (engine development) and in adulthood (prevention of cardiovascular diseases) of this fragile population.
FundingKaísa Trovão Diniz - Postgraduate Scholarship by Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE). -Rafael Moura Miranda - Postgraduate Scholarship by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). José Eulálio Cabral Filho - Research productivity grant by O Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); and Chamada Universal 2014/ 458163/2014-7.
The authors thank the babies, parents, and the nursing technicians at the Kangaroo Unit at the Instituto de Medicina Integral Prof. Fernando Figueira (IMIP), Recife, Brazil. They would also like to thank the translator M.A. in Linguistics Técio Oliveira Macedo for editing the text in the English language.
1Study conducted at the Instituto de Medicina Integral Prof. Fernando Figueira – IMIP.