To compare the frequency of hospitalization in children with Inborn Errors of Immunity with antibody deficiency previous to intravenous immunoglobulin (pre- IVIG) with a one-year period after initial IVIG (post-IVIG).
MethodsMedical reports of 45 patients during an eight-year period were reviewed from 2018 to 2019. Wilcoxon-test was used for related samples.
ResultsForty-five children were included in the study, aged 29–249 months of age, and most of them (64.4%) were males. Median ages at onset symptoms and at diagnosis were 6 and 73 months old, respectively. Specific antibody deficiency and unclassified hypogammaglobulinemia were the predominant diagnoses (31.1% and 17.8%, respectively). X-linked agammaglobulinemia, Hyper IgE syndrome, Hyper IgM, transient hypogammaglobulinemia of infancy, and Common Variable Immunodeficiency (CVID) were also reported, in a low frequency. Forty-four (97.8%) patients were hospitalized before IVIG, and 10 patients (22.2%) after. Annual mean hospital admission reduced from 2.5 to 0.5, pre and post-IVIG, respectively (p < 0.0001). Mean length of stay (LOS) reduced from 71 to 4.7 days/year (p < 0.0001) in general ward and in the PICU from 17.2 days/year to zero (p < 0.0002). Pneumonia was the main cause of hospital admission with a reduction in the number of episodes per patient from an average of 2.2–0.1 per year (p < 0.001). Concomitant use of antibiotic prophylaxis did not influence the number of hospital admission.
ConclusionOne-year intravenous IVIG significantly decreased the number of hospitalizations and length of stay in children with impaired antibody production. Social and economic impacts would be required.
Inborn Error of Immunity (IEI) also referred to as primary immunodeficiency encompass more than 400 heterogeneous genetic diseases with various degrees of impairment of innate or adaptive immune systems.1 IEI usually manifests early in life with life-threatening or recurrent infections, susceptibility to autoimmunity, malignancy, and autoinflammatory diseases.1,2 Predominantly antibody deficiency (PAD) represents more than 50% of IEI1–3 and includes hypogammaglobulinemias, such as X-linked agammaglobulinemia (XLA), transitory hypogammaglobulinemia of infancy (THI), and common variable immunodeficiency (CVID),2,4 but also functional antibody defects, as specific antibody deficiency (SAD), which is characterized by an abnormal IgG antibody response to a pneumococcal vaccine with normal IgG, in patients older than two years of age.2–4 Other more genetically complex IEI, including combined immunodeficiencies, diseases of immune dysregulation, and combined immunodeficiencies with syndromic features, however also present with defects in the humoral system that contribute to an increased susceptibility to infections.1,5
Patients with PAD have frequent hospitalizations and immunoglobulin replacement therapy (IgRT), via intravenous (IVIG) or subcutaneous (IGSC), is currently the leading therapeutic approach.5–7 The usual loading dose of IVIG is 400 to 600 mg/kg/dose every 3–4 weeks, but higher doses between 600 and 800 mg/kg/dose (or up to 1200 mg/kg) may be required and are particularly indicated in case of chronic lung and/or sinus diseases.5–7
Many studies have reported the benefits of human Ig in reducing episodes of bacterial infections, hospital admission, as well as prevention of chronic pulmonary disease and survival in patients with IEI.6–10 In Brazil IVIG is provided by the government for patients with IEI6,7 and, as far as we know, there is no data focusing on the efficacy of IVIG in reducing hospital admission in pediatric Brazilian patients with IEI. The goal of this study was to assess hospital admission, as well as the length of stay (LOS) in children with IEI, pre and one-year post-intravenous immunoglobulin therapy initiation (IVIG) in a tertiary pediatric hospital. Major causes of hospital admission were also evaluated.
MethodsA retrospective study was carried out enrolling children with IEI under regular IVIG therapy for at least one year, over the period comprised between 2011 and 2019 in a pediatric tertiary hospital in Brazil. Data were collected from July-2018 to July-2019, by reviewing medical records and interviewing the patient's family members. The inclusion criteria were (i) age from 1 month of age to 18 years old during the therapy; (ii) diagnosis of IEI based on the diagnostic criteria of the American3,4 and European Societies for Immunodeficiency11; (iii) baseline laboratory tests showing a decrease in serum IgG (at least two standard deviations below the reference mean for age)12 in minimum two tests and/or impaired vaccine response (rubella, measles, hepatitis B and purified polysaccharide vaccine (23-PPV)13; (iv) regular IVIG replacement therapy for at least 12 months. Clinical data collected were: age at onset symptoms, age at diagnosis, IVIG period, lymphocyte counts and immunoglobulin (Ig) serum level pre and post-IVIG (12-months after IVIG initiation), age at IVIG initiation, diagnostic delay, number of hospital admission, length of stay at hospital and type of infection: a) pneumonia; b) upper respiratory infections (pharyngitis, tonsillitis, sinusitis, and otitis media), c) others (including gastrointestinal diseases and urinary tract infection). Pre-IVIG was defined as the period between the first infection and IVIG initiation, and post-IVIG assessment took place 12 months after initiation of IgG therapy. Patients with low IgG in the baseline were allocated into the hypogammaglobulinemia group, their clinical and laboratory data pre and post-IVIG therapy was analyzed independently on the others. Data on antibiotics and immunosuppressive drugs used in association with IVIG during the first year of IVIG were also studied. Diagnosis of SAD was done in children older than 24 months of age with impaired IgG production to the purified polysaccharide vaccine (23-PPV) antigens in the presence of normal immunoglobulin concentrations and normal antibody responses to protein.5,13 Patients were tested for a minimum of seven serotypes and 1.3 μg/mL was considered protective IgG concentration for each serotype, according to the literature.5 Normal antibody response to polysaccharide antigens is defined differently according to age: in children aged 2–5 years, >50% of concentrations tested are considered protective, with an increase of at least 2- fold observed, and in patients aged 6–65 years, >70% of concentrations tested are considered protective.5 Length of stay (LOS) was defined as the duration of hospital stay from the day of hospital admission until the patient´s discharge. Patients were excluded if the follow-up was less than 12 months or noncompliance. The study was approved by the Institutional Research Ethics Committee.
Statistical analysisData are described as frequency, median, mean, range, and interquartile range (IQR). Analysis was performed with Graph Pad Prism v.6.05® (GraphPad Software, La Jolla, USA) and IBM SPSS (Statistical Package for the Social Sciences) ® 23, 2015. Kolmogorov-Smirnov´s test was used as a normality test and the Wilcoxon test was used for quantitative and related samples. Mc Nemar test and U Mann–Whitney tests were used to compare qualitative and quantitative non-parametrical data, respectively. Statistical significance when p value < 0.05.
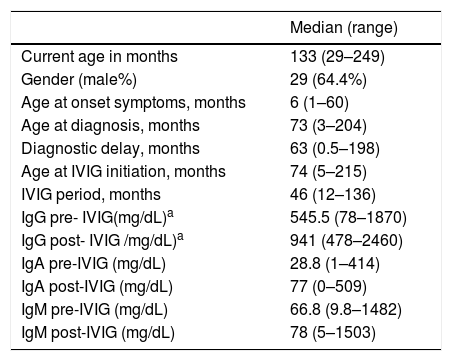
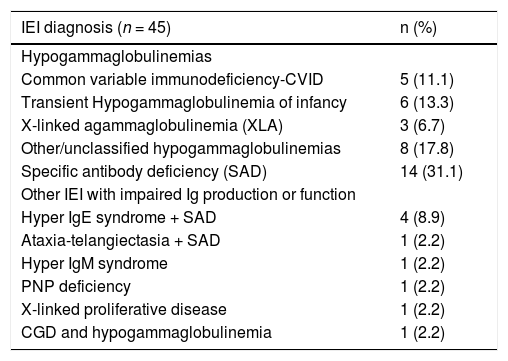
ResultsClinical features of all studied patientsIn total 45 children with IEI under IVIG therapy were studied, and most of them were male (n = 29/64.4%). The median age at onset of symptoms was six months old and at IVIG initiation was 74mo. Baseline characteristics and Ig levels pre and post-IVIG of all patients are summarized in Table 1. Most of the patients (80%) had hypogammaglobulinemia or functional antibody defect (Table 2).
Clinical and laboratory characteristics of studied patients (n = 45).
| Median (range) | |
|---|---|
| Current age in months | 133 (29–249) |
| Gender (male%) | 29 (64.4%) |
| Age at onset symptoms, months | 6 (1–60) |
| Age at diagnosis, months | 73 (3–204) |
| Diagnostic delay, months | 63 (0.5–198) |
| Age at IVIG initiation, months | 74 (5–215) |
| IVIG period, months | 46 (12–136) |
| IgG pre- IVIG(mg/dL)a | 545.5 (78–1870) |
| IgG post- IVIG /mg/dL)a | 941 (478–2460) |
| IgA pre-IVIG (mg/dL) | 28.8 (1–414) |
| IgA post-IVIG (mg/dL) | 77 (0–509) |
| IgM pre-IVIG (mg/dL) | 66.8 (9.8–1482) |
| IgM post-IVIG (mg/dL) | 78 (5–1503) |
Distribution of patients according to the Inborn Error of Immunity diagnosis (IEI) (n = 45).
PNP, nucleoside phosphorylase; CGD, Chronic Granulomatous disease.
Twenty-two patients were classified in the group hypogammaglobulinemia, most of them were males (81.8%), and median age of 10 months (3–180 months) (Supplementary Table 1). In this group were included patients with CVID (5/22), XLA (3/22), and THI (6/22) (Table 2). The authors also included patients with other hypogammaglobulinemias (OH) (8/22), which means patients without a well-defined diagnosis, who had a history of recurrent infections and low IgG and/or IgM or IgA, some of these patients had neutropenia (4/21), others had lymphopenia (3/7).
The patient with the highest LOS pre-IVIG (167 days/year) was admitted at the age of 2.7 years old, after several hospital admissions, complicated by neurologic sequelae (Supplementary Table 1). He had pre-IgG levels of 212 mg/dL with normal IgA and IgM. IVIG therapy was initiated in a loading dose of 508 mg/kg/monthly associated with antibiotic prophylaxis, and after 12-months-IVIG, he spent 24 days at the hospital and his IgG levels increased to 984 mg/dL.
The lowest IgG level post -IVIG (478 mg/dL) was observed in a 14-year-old-girl who had CVID phenotype and autoimmune anemia (AIA), this patient also had the lowest CD4+ T cell counts pre and post IVIG, 280 cells/µL and 240 cells/µL, respectively. Perhaps the concomitant use of corticosteroids to treat AIA has influenced her laboratory results, on the other way, she did not require hospital admission in that year.
More than half of hypogammaglobulinemic patients (12/54.5%), had nearly critical values of IgG serum levels (200 mg/dL) when they were admitted. As the study's data are retrospective, some patients are not under IVIG therapy anymore, e.g. patients with THI.
Patients with specific antibody deficiency and complex IEIIn total 14 patients received a diagnosis of SAD. All patients had severe infection leading to hospital admission, and in 10 patients, a chest computed (CT) scan was available, five patients (35.7%) of SAD patients showed some lung abnormality such as atelectasis, bronchiectasis and ground-glass opacity.
Nine patients with well-defined IEI, such as Ataxia-telangiectasia and Chronic Granulomatous Disease with hypogammaglobulinemia (one case each) and Hyper IgE syndrome, (4 cases) were included in this study (Table 2). Purine nucleoside phosphatase (PNP) deficiency and X-linked lymphoproliferative syndrome (XLP) were also diagnosed in two boys, who underwent hematopoietic cell transplantation afterwards.
IVIG dose ranged from 400 to 800 mg/kg (median 517 mg/kg) every three to four weeks, based on the clinical feature and the updated guidelines6,7,14 (Supplementary Table 2). The highest loading dose was observed in a patient with Hyper IgM syndrome with several pulmonary sequelae, including bronchiectasis and bronchiolitis obliterans with organizing pneumonia (BOOP), who was diagnosed at the age of 11yo11months, and onset symptoms at 3yo.
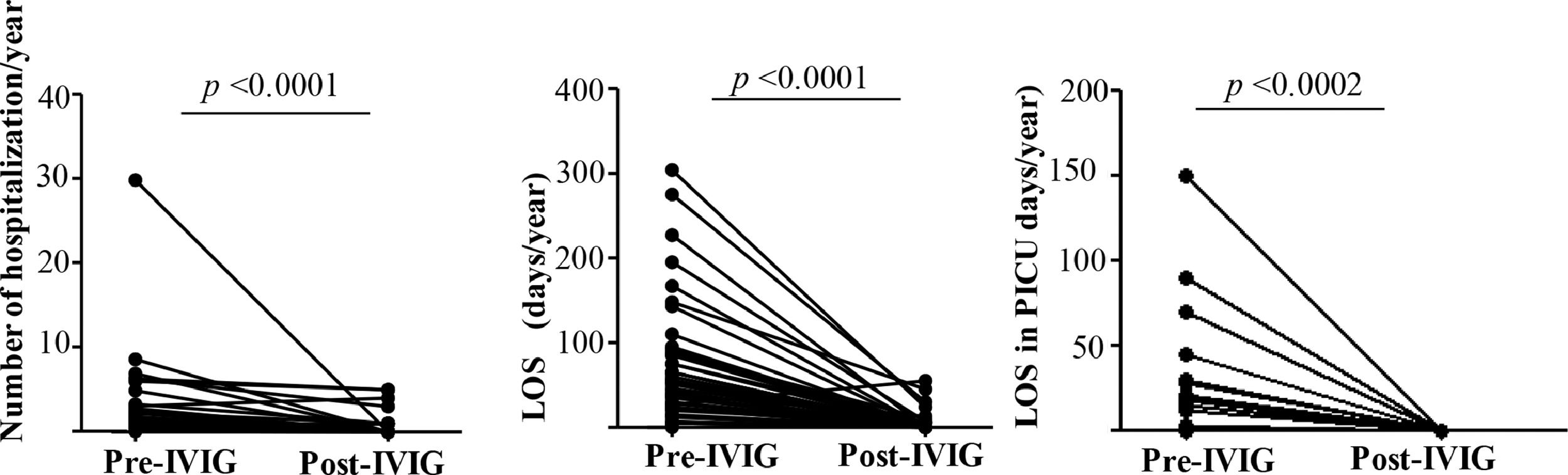
Number of hospitalization and length of stay (LOS) after IVIG therapyAlmost all patients (44/97.8%) had documented hospital admission prior to IVIG and only 10 patients (24%) post-IVIG. The annual number of hospital admission decreased significantly from 2.5 (median:1.2, range: 0–30) to 0.5 (median: 0, range:0–5). The LOS reduced from 71 days (range: 0–304) to 4.7 (median: 54, range: 1–55) per patient (p < 0.0001) Fig. 1A e 1B. The number of children who required PICU was also significantly reduced from 17 (37.7%) with a mean of LOS of 17.2 days/year (range:1–150) to zero (p < 0.0002) Fig. 1C.
Comparative analyses of 45 patients previously (Pre) and one-year post-Immunoglobulin therapy initiation (post-IVIG) in pediatric patients with IEI. In total 44 and 10 patients were hospitalized pre and post-IVG respectively. Figure (1A) represents the number of hospital admission/patient/year pre-IVIG (n = 44 patients) and post-IVG (n = 10 patients). Length of stay (LOS)/patient/year in general ward, Pre-IVIG (n = 44 patients), post-IVG (n = 10 patients) (1B). Figure 1C describes the LOS in Pediatric Intensive Care Unit -PICU per year /patient, Pre-IVG (n = 17 patients) and post-IVG (n = 0 patient). Wilcoxon-test. Statistical significance when p < 0.05.
One male patient had 30 hospital admissions pre-IVIG, his first immunologic visit was at nine years of age, with a history of recurrent infections since two. He had low IgG, impaired vaccine response, and low CD4+ T cells and B cells. After IgRT he had just one hospital admission.
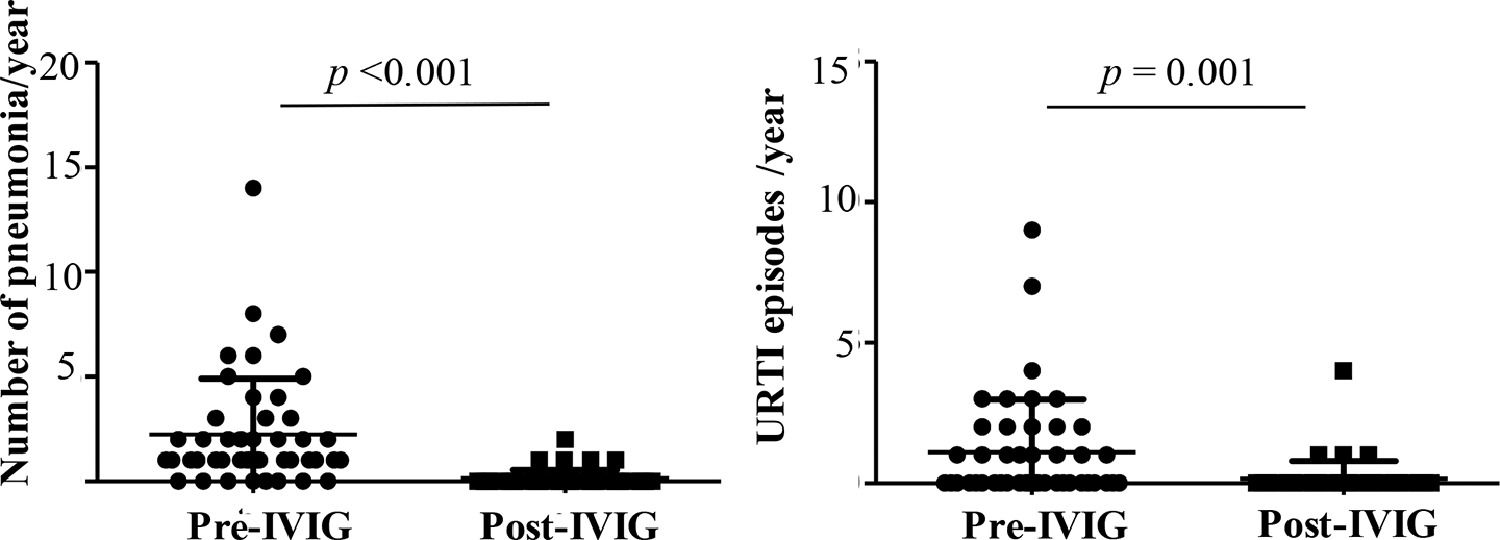
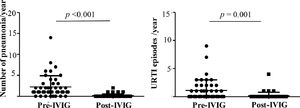
Reduction in respiratory infection episodes after IVIGThe authors observed a significant reduction in the number of patients hospitalized due to pneumonia, the main cause of hospital admission, from 84.4% to 5%. The number of episodes per patient decreased from an average of 2.2 (median:1, range:0–14) to 0.1 per year (median:0, range: 0–2) (p < 0.001) (Fig. 2A). Upper respiratory tract infections (URTI) episodes also reduced, from a mean of 1.1 to 0.1 episode annually, pre and post-IVIG, respectively (p = 0.001) (Fig. 2B). Other causes of hospital admission, such as gastroenteritis, urinary infections, surgery were also noted, but in less frequency and not described.
A) In 45 patients with Inborn Error of Immunity, the number of patients who had pneumonia reduced from 37 to 5, pre and post-IVIG therapy, respectively, and Fig. 2A illustrates the number of pneumonia episodes per patient per year, pre-IVG (n = 37 patients) and post-IVIG (n = 5 patients) and; 2B) Number of upper respiratory tract infection (URTI) episodes per patient per year, pre–IVIG (n = 20 patients) and post-IVIG (n = 5 patients). Wilcoxon-test. Statistical significance when p < 0.05.
Eleven patients (24.4%) received prophylaxis with antibiotics (azithromycin, amoxicillin and cotrimoxazol) and/or antifungals (itraconazole) during the study period. The authors also noted that four patients (8.9%) received immunosuppressive drugs, such as corticosteroids, cyclosporine and azathioprine. Pulmonary sequelae, autoimmune cytopenia as well as conventional management of some IEI, as HIES and CGD were the reason to prescribe these medications (Supplementary Table 3).
None data on drugs used previously with IVIG therapy were collected. However, in order to see whether the concomitant use of antibiotics or immunosuppressive drugs associated with IVIG therapy, could have influenced the present study's results, a comparative analysis was performed. Neither number of hospital admission nor hospital stay was influenced by antibiotics use or immunosuppressive drugs (Supplementary Table 3). Data on the number and type of infections and laboratory results were also studied and, higher IgG and IgM levels post-IVIG in patients taking antibiotics were observed compared to patients without this medication, p = 0.02 and p = 0.03, respectively.
DiscussionThe benefit of human Ig in patients with primary antibody deficiency and in diseases with immune dysregulation related to a genetically defined IEI, is unquestionable and has been well described in other countries.5,8–10 The main purpose of this study was to describe the efficacy of IVIG in reducing the number of hospital admission, LOS, and the number of pneumonia cases in the first year of IVIG replacement therapy and, as far as we know, it is the first study evidencing the impact of Ig therapy in a pediatric cohort with IEI in Brazil.
Forty-five children were studied with a predominance of males, as observed by others and not well understood.8,15 All patients had recurrent infections, mainly pneumonia, early in life (median 6 months of age). However, the median duration of diagnostic delay was 63 months, almost the half median age of studied patients (133 months). It is late considering the advances in techniques to diagnosis IEI in the last decades and also higher than data from Peru and Mexico.16,17 Previous studies have shown that the earlier the diagnosis of IEI and establishment of suitable treatment, the less is the number and severity of infectious complications and hospital admission.9,17 The present study's data suggest a difficulty for general physicians and pediatricians to conceive IEI diagnosis in children with recurrent pneumonia, as well as low awareness of management of IEI, as observed previously in Brazil.18,19
Human Ig preparations are derived from a plasma pool and contain 95–99% protective titers of IgG against a large number of pathogens with traces of IgM and IgA, depending on the purification and fractionation process.7,14,20 Higher IgG serum levels after Ig therapy is expected and correlated with low frequency of infections and hospital admission,8,21,22 as the authors observed in patients into the group hypogammaglobulinemia. The authors also noticed a significant reduction in the annual LOS, probably influencing other clinical and social aspects, i.e. use of intravenous antibiotics, growth, and number of absent school days as mentioned by others.8,23
Replacement therapy for some groups of IEI is crucial and life-saving, such as XLA, CVID, and severe combined immunodeficiency (SCID).5 Other genetically complex IEI, such as Wiskott-Aldrich syndrome, Ataxia-telangiectasia, XLP, Hyper E and IgM syndromes, also have defects in antibody production or function that contribute to an increased susceptibility to infections and the benefits of IgRT has been described.7
Consensus recommendations for the use of human Ig replacement are: serum IgG < 200 mg/dL, which is always an indication, except for patients with transient hypogammaglobulinemia of infancy with no severe infections; serum IgG between 200 and 500 mg/dL is an indication in case of antibody production deficiency or recurrent and/or severe infections; serum IgG > 500 mg/dL is an indication for Ig replacement only when abnormal production of specific antibodies is verified, and recurrent and severe infections are present.7,14
In young children, especially infants, with low antibody production, the definitive diagnosis can be very difficult. It can result from an immaturity of the immune system or transient impaired production.2,11 Nevertheless, patients with IgG levels below 200 mg/dL with a previous history of severe infections requiring hospital admission, are a candidate for Ig replacement due to the high risk of complications.7 According to Schartojé and colleagues, it is impossible to predict which child will recover and which one will suffer from a more or less severe disease in the future.15 Recently Janssen and others, evaluating patients (adults and children) with unclassified hypogammaglobulinemia (unPAD), alert that these patients can have comorbidities similar to other well-defined IEI, influencing their quality of life.24
Diagnosis of SAD is also not so easy to establish, it involves a careful clinical and laboratory evaluation. In Brazil it has been a challenge to perform this diagnosis in the public health system. According to Costa-Carvalho et al. in a survey involving 17 Brazilian public reference centers of Immunology, fewer than 10% of clinical immunologists were able to evaluate the response to the pneumococcus polysaccharide vaccine. In the present study, the diagnosis was done evaluating a minimum of seven serotypes. Perhaps the authors have lost some diagnosis during the eight-year period, but patients with diagnosis SAD described in this study had documented recurrent or severe respiratory infections and poor response to pneumococcal polysaccharide vaccination; besides, most of them had pulmonary sequelae on chest CT scan.
It´s important to keep in mind that Ig therapy is not recommended in all patients with SAD. The therapeutic strategies depend on the clinical manifestation and include an additional dose with conjugated pneumococcal, antibiotic prophylaxis, and Ig replacement therapy. Among them, antibiotic prophylaxis has been described as the mainstay of therapy for patients with SAD.13,25
Perez et al. described some features to consider Ig therapy in patients with SAD, including: i. severe or very frequent recurrent infections; ii. inability to tolerate antibiotic prophylaxis due to multiple hypersensitivities, severe side effects or complications such as Clostridium difficile colitis, etc.; or iii. failure to respond to prophylactic antibiotics 25. In this study, the authors also took into account abnormalities on chest CT scan as recommended in the Brazilian consensus on IgRT.7
Prior to IVIG 17 children (37.8%) required intensive care, suggesting a poor clinical status associated infections with a high risk to develop sequela, especially chronic pulmonary disease. The efficacy of IVIG in reducing the number as well the severity of infections was remarkable and it may have had a positive impact on the economic burden to the Brazilian health care system, taking into account the high daily cost of an intensive care unit day and, also that none patient was admitted in the PICU in the first 12 months of Ig therapy. Further studies on these results and economic impacts would be required.
Most of the patients (84.4%) had experienced at least 1 episode of pneumonia leading to hospital admission prior to IVIG. The occurrence of bacterial respiratory infections is the main clinical presentation of IEI, and many patients experience multiple episodes before the diagnosis.6,18 Orange and colleagues described that the prevalence of pneumonia and the pneumonia risk was inversely correlated with high IgG levels (up to at least 1000 mg/dL) in patients with hypogammaglobulinemia.22 On the other hand, Quinti et al., correlated the high number of pneumonia with low IgA levels pre and post- IVIG in CVID patients.21 In this study, no difference was noted between IgA levels pre and post-treatment, and as the study cohort included complex IEI and SAD patients, the significant reduction in pneumonia episodes after IVIG treatment suggest that not only IgG levels per se, but also IgG function were balanced in the first year of Ig therapy.
This study has some limitations, as the heterogeneity of IEI diseases, absent detailed data about chronic lung disease, and infections others than the respiratory tract. However, the authors highlight for the first time the efficacy of IVIG replacement therapy in Brazilian children with IEI, in reducing hospital admission, LOS, and number of pneumonia. The authors expect that the present study data can help physicians, especially pediatricians, to be alert of a possible diagnosis of IEI in cases of recurrent or severe pneumonia leading to hospital admission, and to consider IgRT if necessary.
FundingLMA, Scholarship (August.2018 to July.2019) has been provided by Instituto do Câncer Infantil e Pediatria Especializada/ Hospital da Criança de Brasília José Alencar.
The authors thank Prof. Beatriz T. Costa-Carvalho (in memorian) for her contribution to clinical immunology studies in Brazil and Larissa Souza for statistical analysis support.