To evaluate the impact of the Universal Neonatal Hearing Screening (UNHS) on the age at diagnosis, beginning of treatment, and first cochlear implant surgery.
MethodsA retrospective cohort study with children up to 12 years old with bilateral hearing loss were divided into two groups: patients who underwent UNHS and the ones who didn't. The groups were compared according to their age at the beginning of the evaluation at a specialized center, at the beginning of the intervention, and, for the ones who had indication, at the cochlear implant surgery. The group who underwent UNHS was divided between the ones who passed the screening test and the ones who didn't. They were compared according to their ages at the same moments as the first two groups.
Results135 patients were included. The median age at the first appointment in a specialized center was 1.42 (0.50 and 2.50) years, at the beginning of treatment 2.00 (1.00 and 3.52) years, and the cochlear implant surgery 2.83 (1.83 and 4.66) years. Children who underwent UNHS were younger than those who didn't, at the three evaluated moments (p < 0.001). In a subanalysis, children who passed the UNHS but were later diagnosed with hearing loss reached the first appointment with a specialist and started treatment older than those who failed the tests.
ConclusionPerforming UNHS interfered with the timing of deafness diagnosis and treatment. However, children who passed the screening but were later diagnosed with hearing loss were the category with the most important delay.
When deafness begins in the early years of life, it compromises the development of speech and language, interfering with the child's learning. In the long run, effects on interpersonal communication, psychological well-being, quality of life, and economic independence can be observed.1–3
The union of current concepts on neural plasticity of the auditory system with technological advances on neonatal hearing screening and treatment methods, as well as the advent of cochlear implant (CI), increased the pursuit for early diagnosis and rehabilitation. In Brazil, hearing screening was considered mandatory by law for all children born in Brazilian hospitals in 2010.4
It is recommended for hearing screening to be performed until the end of the first month of age. Children who fail the screening should be retested to have a confirmation on the hearing status by the age of three months and receive intervention by six months of age.5 In practice, many hearing health services find it difficult to achieve recommended rates of diagnosis, not only due to the low coverage of the UNHS but also due to the high rates of loss of follow-up and the delayed diagnosis of hearing impairment after screening.1,3,6,7 Thus, UNHS fails to comply with its original proposal: early diagnosis and intervention to minimize the effects of sound deprivation.
In Brazil, studies on the effectiveness of UNHS in the treatment of childhood hearing loss are lacking. This study aims to evaluate the impact of neonatal hearing screening on the age of diagnosis and onset of hearing loss treatment in Brazilian children. The authors believe that understanding the effects of screening can help professionals in the care of young children.
Material and methodsA retrospective cohort study was conducted at a referral outpatient clinic for child deafness in a tertiary public hospital in southern Brazil. Referrals occurred for two reasons:
- a)
Failure on neonatal hearing screening (NHS) or
- b)
Investigation and treatment of already diagnosed hearing loss
Inclusion criteria were children aged up to 12 years with bilateral hearing loss either congenital (knowingly acquired before birth, regardless of the time of hearing loss onset) or "acquired in the neonatal period" (ANP), which clusters causes of hearing loss related to neonatal conditions.8 Exclusion criteria were: a) infectious diseases (such as sepsis and meningitis) that happened after the neonatal period; b) children that did not have the child's health card (where the result of UHNS is registered) or whose parents or caregivers did not know information about the result of UNHS.
The first consultation of each patient was conducted approaching information to characterize the profile of the child, assessing the presence of risk factors for deafness, and defining the etiological diagnosis of hearing loss. Detailed information regarding neonatal hearing screening was carried out through the following sources:
- a)
information and results of exams (TOAE or ABR) provided by parents,
- b)
medical record data (if the child was born in the study's hospital) and
- c)
verification of the child's health card, which obligatorily requires the registration of the UNHS result.
Patients who underwent UNHS did it through the following process: transient otoacoustic emission (TOAE) test performed with automatic equipment (Madsen AccuScreen hand-held device with a non-linear click sequence considering stability above 80% and artifact below 20%). The test was performed at the maternity ward in the first days of the life of the newborn. The retest, in case of failure to respond on the first test, was also performed with TOAE, within 30 days after the first evaluation. The test results were classified as “pass” or “fail” and recorded by the audiologist on the child's card. If the child failed on both steps (test and retest) he/she was referred to a tertiary center for diagnosis.
The type and degree of hearing loss were defined by frequency-specific auditory evoked potential (Interacoustics Eclipse EP25 ABR system® [Denmark] with NB-chirps®) and/or audiometry (either visual reinforcement or tonal and vocal, according to age and ability of the child in answering the exam [audiometer Interacoustics AD 27 with or without supra-aural phones TDH-3]). Pediatric hearing loss was classified according to the World Health Organization (WHO),9 which uses a quadritonal mean between the hearing thresholds for the frequencies of 500 Hz, 1000Hz, 2000 Hz, and 4000 Hz. It classifies as mild (thresholds between 26 and 30 dB), moderate (31 to 60 dB), severe (61 to 80 dB), and profound (greater than 81dB).
For etiological diagnosis of hearing loss, all non-syndromic children underwent genetic examination for analysis of GJB2 gene mutations and deletion del(GJB6-D13S1830) of the GJB6 gene. In cases of suspected middle/inner ear malformations and CI candidates, an imaging exam was performed (computed tomography and/or magnetic resonance imaging).
The initial type of treatment was chosen according to the type and degree of hearing loss. Most children started treatment with hearing aids (HA) and, in cases where the response to treatment was not adequate, a multidisciplinary evaluation was performed to verify the indication of a CI.
From January 2015 to December 2017, 135 patients filled the inclusion criteria. The sample was then divided into two groups:
- 1.
Group 1: patients who underwent UNHS
- 2.
Group 2: patients who didn't undergo UNHS
Groups were compared regarding three main ages that implied the effectiveness of referral and treatment:
- a)
Age at the first consultation at a specialized center: when the diagnosis and first intervention were performed in a tertiary hospital (whether if it was in the hospital that performed the CI or another).
- b)
Age at the beginning of treatment: when the child underwent the first intervention. In most cases, HA.
- c)
Age at CI surgery: the authors used the age at the first CI in patients who underwent sequential bilateral surgery.
After comparisons between groups 1 and 2 regarding the ages above, another analysis was performed. The authors subdivided group 1 between children who passed (group A) and those who failed (group B) at the screening and compared their ages at the three moments described above. Comparisons regarding the degree of hearing loss, risk factors, the etiology of the hearing loss, and the provenance of the children were also made.
The present study's hospital admits children from all areas of the state of Rio Grande do Sul for hearing loss investigation. The total sample was analyzed according to the region of origin of each patient in order to compare these regions with the ages at the first appointment in a specialized center and the beginning of treatment. The groups were as follows:
- 1.
Porto Alegre, Rio Grande do Sul (RS)
- 2.
Metropolitan area of Porto Alegre
- 3.
State's (RS) countryside.
To compare the age variables at the first visit, at the beginning of treatment, and at the first CI between the various factors studied, the authors used the Mann-Whitney U test and Kruskal-Wallis. To compare the characteristics of patients who underwent or not UNHS, Fisher's exact test was used. Multiple linear regression was performed to verify whether the UNHS was independently associated with the other covariates (which had p < 0.20 in the previous analyzes) in the age at which children were diagnosed and treated. For this analysis, these variables had to be transformed by natural logarithm, and after adjusting the models, the normality of the residuals was verified by the Shapiro-Wilks. The SPSS 18.0 software package was used for statistical analysis (SPSS Inc. released in 2009. PASW Statistics for Windows, version 18.0. Chicago: SPSS Inc).
The project was approved by the Research Ethics Committee of the Hospital de Clínicas de Porto Alegre (HCPA) under protocol number 15-0445. All guardians of the children signed a free and informed consent form.
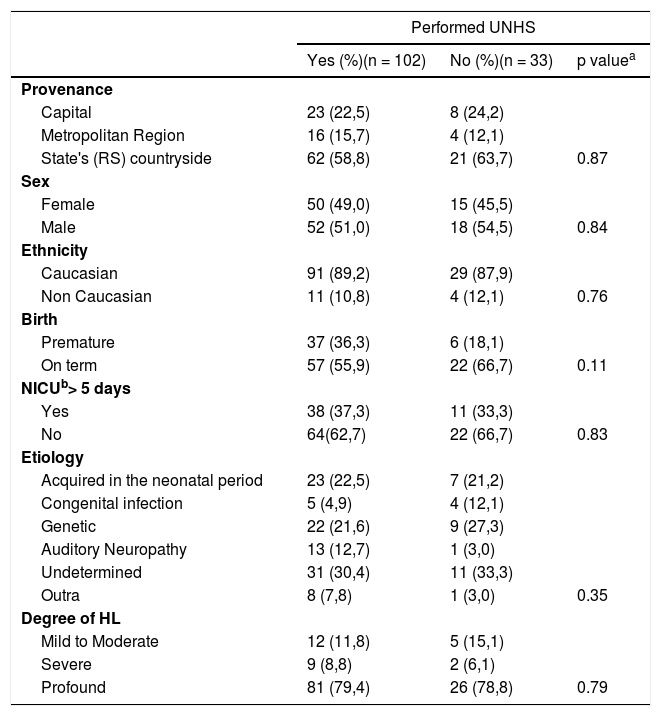
ResultsA total of 135 patients ranging from zero to 12 years old were included in the study. UNHS was performed on 102 children, which corresponded to 75.6% of the sample (group 1). Consequently, 33 children did not perform the test (group 2). They belonged to the same socioeconomic status and 96.3% of mothers had prenatal care. Other characteristics are described in Table 1.
Demographic characteristics of patients included divided by groups according to UNHS status.
| Performed UNHS | |||
|---|---|---|---|
| Yes (%)(n = 102) | No (%)(n = 33) | p valuea | |
| Provenance | |||
| Capital | 23 (22,5) | 8 (24,2) | |
| Metropolitan Region | 16 (15,7) | 4 (12,1) | |
| State's (RS) countryside | 62 (58,8) | 21 (63,7) | 0.87 |
| Sex | |||
| Female | 50 (49,0) | 15 (45,5) | |
| Male | 52 (51,0) | 18 (54,5) | 0.84 |
| Ethnicity | |||
| Caucasian | 91 (89,2) | 29 (87,9) | |
| Non Caucasian | 11 (10,8) | 4 (12,1) | 0.76 |
| Birth | |||
| Premature | 37 (36,3) | 6 (18,1) | |
| On term | 57 (55,9) | 22 (66,7) | 0.11 |
| NICUb> 5 days | |||
| Yes | 38 (37,3) | 11 (33,3) | |
| No | 64(62,7) | 22 (66,7) | 0.83 |
| Etiology | |||
| Acquired in the neonatal period | 23 (22,5) | 7 (21,2) | |
| Congenital infection | 5 (4,9) | 4 (12,1) | |
| Genetic | 22 (21,6) | 9 (27,3) | |
| Auditory Neuropathy | 13 (12,7) | 1 (3,0) | |
| Undetermined | 31 (30,4) | 11 (33,3) | |
| Outra | 8 (7,8) | 1 (3,0) | 0.35 |
| Degree of HL | |||
| Mild to Moderate | 12 (11,8) | 5 (15,1) | |
| Severe | 9 (8,8) | 2 (6,1) | |
| Profound | 81 (79,4) | 26 (78,8) | 0.79 |
The initial treatment for 128 children was the use of HA because 6 were lost to follow-up and 1 had parents who chose not to use it. Of those, 67 (53,1%) had poor response to treatment and were evaluated by a multidisciplinary team (the remaining 61 had adequate hearing gain and remained with it). One was considered a candidate for an ABI and 66 for CI. Only 46 children performed CI surgery; 9 were still awaiting surgery, 5 were lost to follow-up and 6 had families who decided not to perform surgery.
The most prevalent etiology was genetic (23%), followed by ANP (22.2%), auditory neuropathy spectrum disorder (ANSD) (10.4%), congenital infection (6.7%), and other causes (6.7%). In 31.1%, the etiology remained undetermined. There was no difference between groups 1 and 2 regarding each of the etiologies. (p = 0.35). There was also no statistical difference when comparing each etiology with age at the first visit, at the beginning of treatment, and the CI surgery (Table 2).
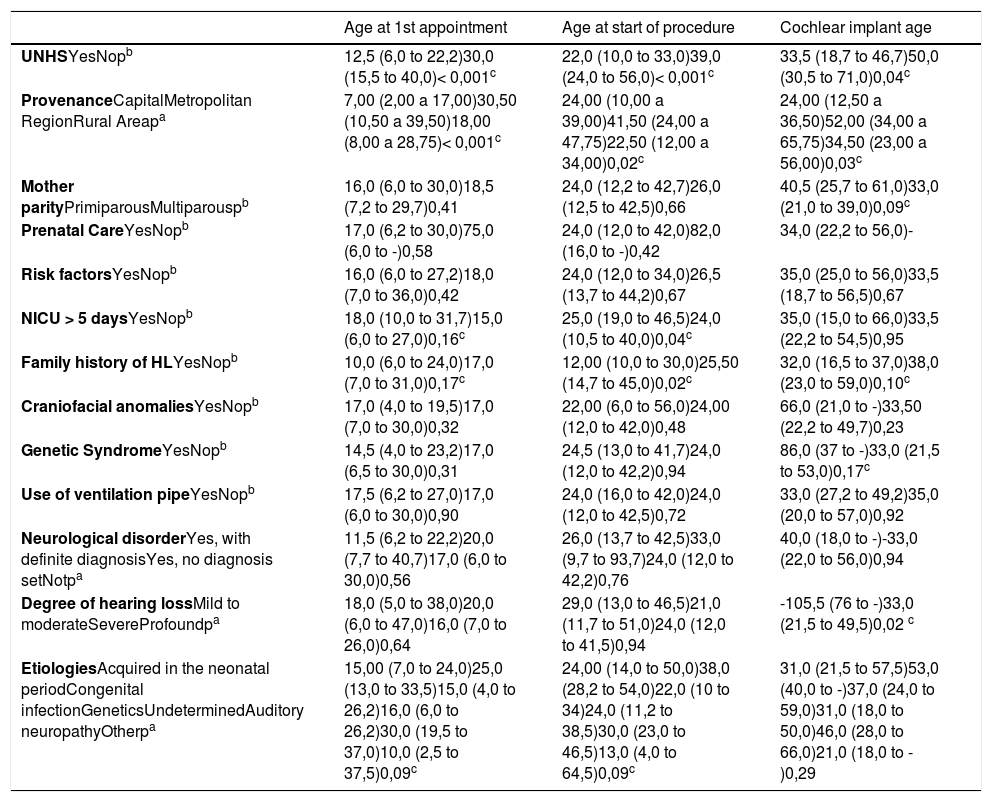
Median (25th and 75th percentiles) in months and p-value of the variables compared with age at first consultation at a specialized center, age at the beginning of treatment and age at the first cochlear implant.
| Age at 1st appointment | Age at start of procedure | Cochlear implant age | |
|---|---|---|---|
| UNHSYesNopb | 12,5 (6,0 to 22,2)30,0 (15,5 to 40,0)< 0,001c | 22,0 (10,0 to 33,0)39,0 (24,0 to 56,0)< 0,001c | 33,5 (18,7 to 46,7)50,0 (30,5 to 71,0)0,04c |
| ProvenanceCapitalMetropolitan RegionRural Areapa | 7,00 (2,00 a 17,00)30,50 (10,50 a 39,50)18,00 (8,00 a 28,75)< 0,001c | 24,00 (10,00 a 39,00)41,50 (24,00 a 47,75)22,50 (12,00 a 34,00)0,02c | 24,00 (12,50 a 36,50)52,00 (34,00 a 65,75)34,50 (23,00 a 56,00)0,03c |
| Mother parityPrimiparousMultiparouspb | 16,0 (6,0 to 30,0)18,5 (7,2 to 29,7)0,41 | 24,0 (12,2 to 42,7)26,0 (12,5 to 42,5)0,66 | 40,5 (25,7 to 61,0)33,0 (21,0 to 39,0)0,09c |
| Prenatal CareYesNopb | 17,0 (6,2 to 30,0)75,0 (6,0 to -)0,58 | 24,0 (12,0 to 42,0)82,0 (16,0 to -)0,42 | 34,0 (22,2 to 56,0)- |
| Risk factorsYesNopb | 16,0 (6,0 to 27,2)18,0 (7,0 to 36,0)0,42 | 24,0 (12,0 to 34,0)26,5 (13,7 to 44,2)0,67 | 35,0 (25,0 to 56,0)33,5 (18,7 to 56,5)0,67 |
| NICU > 5 daysYesNopb | 18,0 (10,0 to 31,7)15,0 (6,0 to 27,0)0,16c | 25,0 (19,0 to 46,5)24,0 (10,5 to 40,0)0,04c | 35,0 (15,0 to 66,0)33,5 (22,2 to 54,5)0,95 |
| Family history of HLYesNopb | 10,0 (6,0 to 24,0)17,0 (7,0 to 31,0)0,17c | 12,00 (10,0 to 30,0)25,50 (14,7 to 45,0)0,02c | 32,0 (16,5 to 37,0)38,0 (23,0 to 59,0)0,10c |
| Craniofacial anomaliesYesNopb | 17,0 (4,0 to 19,5)17,0 (7,0 to 30,0)0,32 | 22,00 (6,0 to 56,0)24,00 (12,0 to 42,0)0,48 | 66,0 (21,0 to -)33,50 (22,2 to 49,7)0,23 |
| Genetic SyndromeYesNopb | 14,5 (4,0 to 23,2)17,0 (6,5 to 30,0)0,31 | 24,5 (13,0 to 41,7)24,0 (12,0 to 42,2)0,94 | 86,0 (37 to -)33,0 (21,5 to 53,0)0,17c |
| Use of ventilation pipeYesNopb | 17,5 (6,2 to 27,0)17,0 (6,0 to 30,0)0,90 | 24,0 (16,0 to 42,0)24,0 (12,0 to 42,5)0,72 | 33,0 (27,2 to 49,2)35,0 (20,0 to 57,0)0,92 |
| Neurological disorderYes, with definite diagnosisYes, no diagnosis setNotpa | 11,5 (6,2 to 22,2)20,0 (7,7 to 40,7)17,0 (6,0 to 30,0)0,56 | 26,0 (13,7 to 42,5)33,0 (9,7 to 93,7)24,0 (12,0 to 42,2)0,76 | 40,0 (18,0 to -)-33,0 (22,0 to 56,0)0,94 |
| Degree of hearing lossMild to moderateSevereProfoundpa | 18,0 (5,0 to 38,0)20,0 (6,0 to 47,0)16,0 (7,0 to 26,0)0,64 | 29,0 (13,0 to 46,5)21,0 (11,7 to 51,0)24,0 (12,0 to 41,5)0,94 | -105,5 (76 to -)33,0 (21,5 to 49,5)0,02 c |
| EtiologiesAcquired in the neonatal periodCongenital infectionGeneticsUndeterminedAuditory neuropathyOtherpa | 15,00 (7,0 to 24,0)25,0 (13,0 to 33,5)15,0 (4,0 to 26,2)16,0 (6,0 to 26,2)30,0 (19,5 to 37,0)10,0 (2,5 to 37,5)0,09c | 24,00 (14,0 to 50,0)38,0 (28,2 to 54,0)22,0 (10 to 34)24,0 (11,2 to 38,5)30,0 (23,0 to 46,5)13,0 (4,0 to 64,5)0,09c | 31,0 (21,5 to 57,5)53,0 (40,0 to -)37,0 (24,0 to 59,0)31,0 (18,0 to 50,0)46,0 (28,0 to 66,0)21,0 (18,0 to -)0,29 |
UNHS, universal neonatal hearing screening; NICU, neonatal intensive care unit; HL, hearing loss.
Concerning provenance, 23.1% of the patients were from Porto Alegre, 14.9% from the metropolitan area, and 61.9% from the countryside. Patients coming from Porto Alegre were younger at the first consultation in a specialized center when compared to those from the metropolitan area (p < 0.001) and the countryside (p < 0.001) Patients from Porto Alegre had a lower age at the first CI when compared with those coming from the metropolitan area (p = 0,02) and the countryside (p = 0.03).
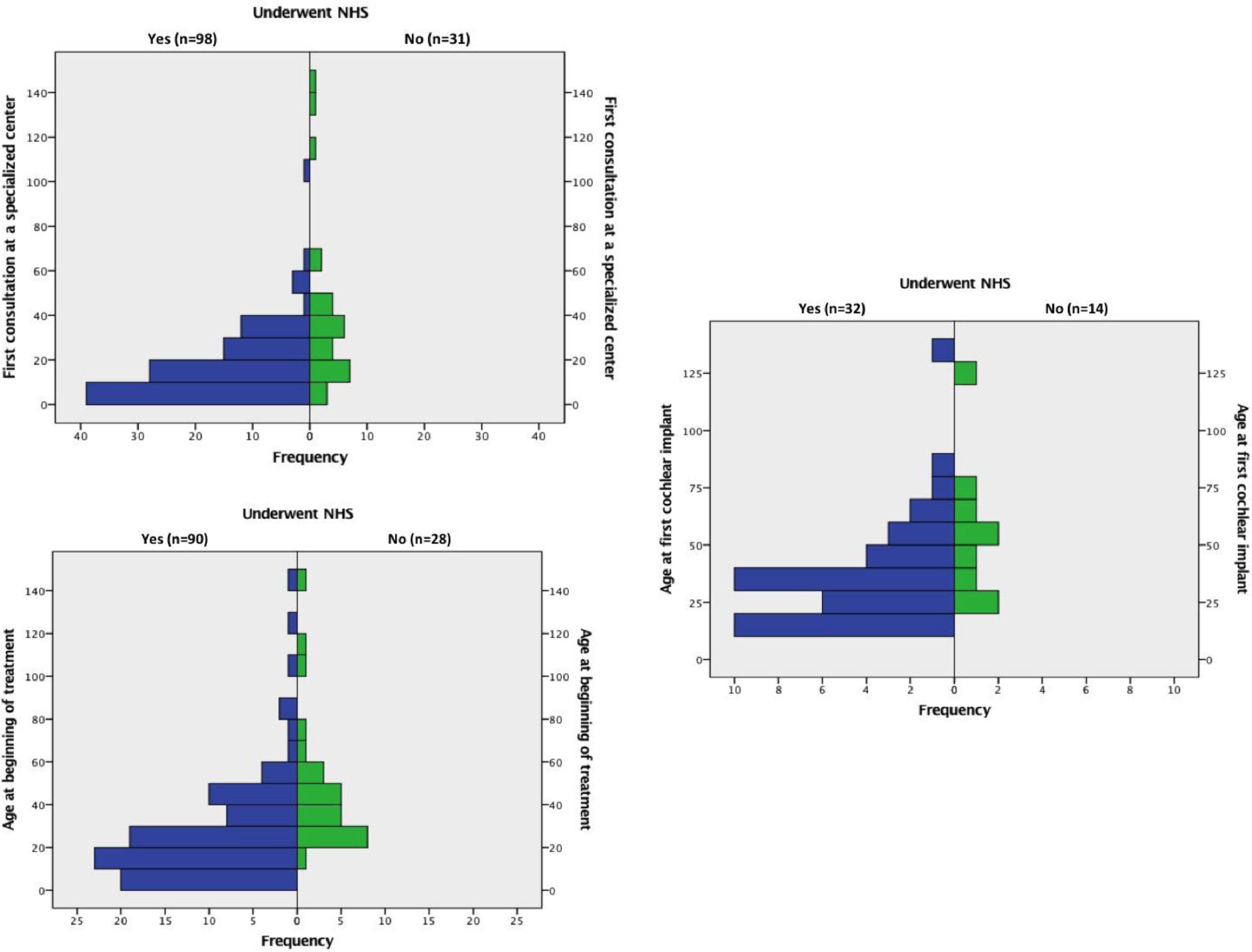
Comparisons between groups 1 and 2The median ages at the beginning of treatment and at the time of the first CI for the whole sample were 2.00 (1.0 and 3.52) and 2.83 (1.83 and 4.66) years, respectively. The median ages at which the children in each group had their first appointment at a specialized center, started treatment and underwent CI surgery are shown in Table 2. From the total of implanted patients, 38 had undergone UNHS (30 failed and 8 passed) and 8 hadn't undergone UNHS.
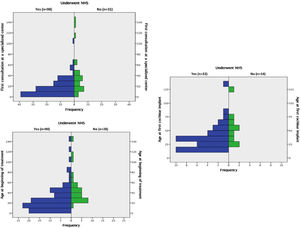
Children who underwent UNHS (group 1) were younger than those who did not (group 2), both at the first consultation in a specialized center (p < 0.001), at the beginning of treatment (p < 0.001), and at CI (p = 0.04). Histograms with these results can be viewed in Fig. 1.
In multiple linear regression, the covariables used to analyze the association of UNHS with the age of the first consultation and at beginning of treatment were: provenance, NICU > 5 days, family history of hearing loss (HL) and etiologies. For determination of the age at the first cochlear implant, variables were: provenance, mother parity, family history of HL, genetic syndrome, and degree of hearing loss. Multiple linear regression showed that performing UNHS was the only covariable that interfered with the age of the first consultation at a specialized center (p < 0,001 and beta = 0.35), the age at the beginning of treatment (p < 0,001 and beta = 0.42), and the age of the first CI (p < 0,05 and beta = 0.33), independently.
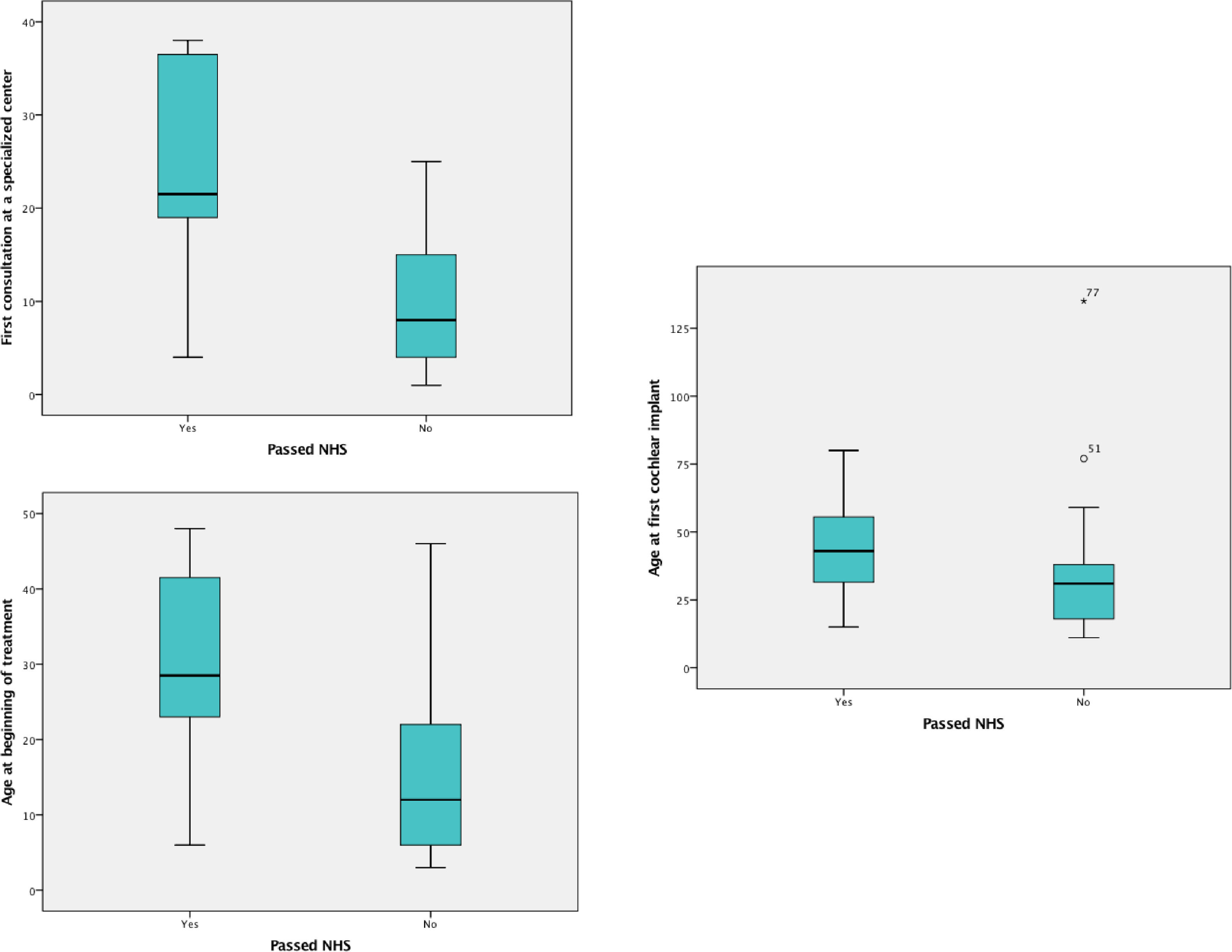
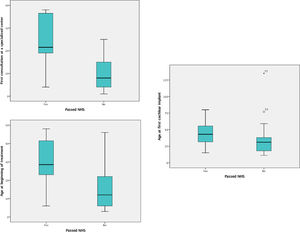
Comparisons between groups A and BAmong the 102 patients who underwent UNHS in the study's sample, 20 (19.6%) passed the screening tests (group A). The median age at the first visit, at the beginning of treatment, and at the CI for these patients was 1.79 (1.00 and 2.83) years, 2.66 (1.91 and 3.87) years, and 3.58 years, (2.47 to 4.89), respectively. The results of the same variables for those who underwent UHNS and didn't pass the screening tests (group B) were, respectively, 0.83 (0.33 and 1.66) years, 1.33 (0.72 and 2.54) years, and 2.58 (1.5 to 3.29) as shown in Fig. 2.
Boxplot comparing children who passed with those who failed at the UNHS (groups a and b) in regard to ages (in months) at first specialist consultation, at beginning of treatment and at first cochlear implant surgery.
Comparing the two groups regarding these variables, the authors identified that the children from group A reached the first appointment with a specialist and started treatment older than those from group B (p=0.03 and p=0,01 respectively). The difference between them regarding the CI age was not significant (p=0.06). Patients in group A had the following etiologies for deafness: 9 cases of ANSD, 4 cases of ANP, 2 cases of congenital cytomegalovirus (cCMV), 2 cases of syndromic genetic disease, and 3 undetermined.
The presence of at least 1 risk factor for hearing loss (11) was identified in 82 patients (60.7%) of the total sample. Of the patients who did not have risk factors for hearing loss, seven (13.2%) passed the UNHS screening. For these patients, the median age at the first visit to a specialized center was 3.11 years, at the beginning of treatment was 3.98 years and at the first CI, 4.93 years. The age distribution of those who passed UNHS was higher than those who failed for the three age variables (p < 0.05).
DiscussionChildren who underwent UNHS were referred to a specialized center and started the treatment of hearing loss younger than those who didn't. These results reiterate the importance of UNHS to achieve the maximum language and learning potential of hearing-impaired children since without early diagnostic confirmation and intervention this initiative becomes irrelevant.8,10
Although children who underwent UNHS were significantly younger at the three evaluated moments (the first consultation at a specialized center, beginning of treatment, and first CI) than those who didn't, they were still older than recommended by JCIH.8 Literature shows that the delay in arriving at the reference center for diagnostic confirmation and intervention is not uncommon. Barbosa et al, in a study with 115 Brazilian CI users, found a mean age of 3.8 years in the first consultation at a referral center.11 For Canale et al, the average age at diagnosis was 6.8 months for patients who underwent UNHS and 2.44 years for those who didn't.12 Delays such as these are known to impact the speech and language development of children.13-15
According to the US Centers for Disease Control and Prevention (CDC), from 2009 to 2010, 97% of newborns were screened for hearing loss and 93% before the first month of life. Of the children who failed the screening tests, only 70% were diagnosed before 3 months of age and 56% started the intervention before 6 months.16
After the implementation of UNHS, it was observed that some children with hearing loss passed the neonatal hearing screening – most had auditory neuropathy and other types of progressive hearing loss. The fact that a child has passed the UNHS may convey a false guarantee that there is no hearing impairment, which often delays further the diagnosis and intervention of these children.1,17 In this study, the authors found that children who passed the screening tests were older at the first appointment with a specialist and at beginning of treatment than those who didn't. Although there was no statistical difference regarding age at the CI surgery, this might be because the sample of patients who reached this stage of treatment was small.
The 2000 JCIH recommendations suggested follow-up of children who had a risk factor for hearing loss but passed the UNHS, with the concern to early diagnose progressive hearing loss.18 However, children without risk factors who passed the UNHS were overlooked. Thus, their diagnosis occurred only after the emergence of signs of hearing impairment (as speech delay), which usually occurs after two years of life.17,18 In the study's sample, 13.2% of patients are part of this group - they have undergone UNHS and had no risk factors for hearing loss. The age at the first consultation at a specialized center, at the beginning of treatment, and at the CI, compared to those who didn't undergo UNHS, was higher. Due to these findings, the 2007 JCIH recommends for all children (regardless of whether or not they have risk factors for hearing loss) to be followed for hearing skills and language development during routine consultations with the pediatrician.8
The most common etiologies of late-diagnosed hearing loss are progressive (usually genetic and infectious) hearing loss and ANSD. cCMV may be a cause of progressive hearing loss, with studies describing incidences from 0 to 50%.19 In the present study, 45% of children who passed UNHS had ANSD. The authors also identified two cases of cCMV and two cases of syndromic diseases.
It is noteworthy that in this study all children were evaluated with TEOAE, both in the test and retest, regardless of the presence or absence of risk factors. The use of ABR is recommended because of the capacity to detect ANSD at the screening - therefore it could prevent false-negative screening in about half of the patients who had a hearing loss and passed the UNHS in the present study. Nevertheless, cCMV can lead to progressive hearing loss and is not identified in UNHS. These results demonstrate that moreover than being mandatory and performed in the vast majority of newborns, UNHS should also be performed following the appropriate protocols.
Newborn hearing screening programs changed the picture of congenital deafness in terms of age at diagnosis and at the beginning of the intervention. However, there is still a considerable delay in the time it takes for these children to reach specialized centers. For patients in this study, the delay occurred, in most situations, due to the parents' delay in taking their children for examinations and consultations, as well as a delay on referral. The authors believe there is a lack of knowledge of general practitioners, pediatricians, and even otolaryngologists about the importance of early intervention.
Parent's acceptance of the diagnosis and the need for intervention is another obstacle for early intervention. One reason is that the diagnosis occurs in the neonatal period when parents still can't realize the impact of hearing loss.17 The percentage of newborns that fail the initial test and miss follow-up retesting may exceed 50%. According to Nikolopoulos, the factor that most contributes to this loss of follow-up is parental, due to delays and missed consultations or reluctance for evaluations or surgeries.1
Socioeconomic factors and parent's education level can also influence the effectiveness of UNHS. According to Holte et al, of several predictor variables, only higher levels of maternal education were significantly associated with earlier confirmation of hearing loss and fitting of hearing aids.20 Improvements in health policies, tracking systems, and public awareness are crucial to the successful implementation of the program.1,3,6
The authors describe how UNHS interferes with deafness treatment in a sample of children from the only hospital that provides CI in the analyzed state, and more studies are needed to understand its impact within the Brazilian population. The authors hope this study can help to raise awareness of all professionals in the care of young children, especially in developing nations. The authors believe that laws to ensure UNHS in all hospitals are very important, but as they can depict from this present study, professionals who follow young children should be very alert to confirm if children who fail the screening are correctly referred and also if children who pass end up showing signs of late-onset hearing loss.