Describe the device-associated infections in the NICUs in Cali – Colombia, a middle-income country, between August 2016 to December 2018.
MethodsObservational cross-sectional study evaluating reports of device-associated infections in 10 NICUs in Cali, Colombia, between August 2016 and December 2018. Socio-demographic and microbiological data were obtained from the National Public Health surveillance system, through a specialized notification sheet. The relationship of device-associated infections with several outcomes including birth weight, microorganisms, and mortality was evaluated using OR CI95%, using the logistic regression model. Data processing was performed using the statistical program STATA 16.
Results226 device-associated infections were reported. The rate of infection with central line-associated bloodstream infections was 2.62 per 1000 days of device use and 2.32 per 1000 days for ventilator-associated pneumonia. This was higher in neonates under 1000 g; 4.59 and 4.10, respectively. 43.4% of the infections were due to gram-negative bacteria and 42.3% were due to gram-positive bacteria. Time from hospitalization to diagnosis of all device-associated infections had a median of 14 days. When compared by weight, infants with a weight lower than 1000 g had a greater chance of death (OR 3.61; 95% CI 1.53–8.49, p = 0.03). Infection by gram-negative bacteria was associated with a greater chance of dying (OR 3.06 CI 95 1.33–7.06, p = 0.008).
ConclusionsThese results highlight the need to maintain epidemiological surveillance processes in neonatal intensive care units, especially when medical devices are used.
Device-associated infections correspond to the most frequent form of healthcare-associated infections (HAIs) in the neonatal intensive care unit (NICU). This remains an important cause of morbidity and mortality despite development in perinatal care in recent years.1,2
Neonates have specific characteristics that make them more vulnerable to developing health care-associated infections.3,4 Firstly, due to their developmental age, they present problems such as an immature immune system, prematurity, and associated diseases. Secondly, risk factors associated with medical care include invasive procedures, use of devices, antibiotic therapy, delayed start of enteral feeding, and requirement of parenteral nutrition.4,5 Devices play a main role in the care of critically ill neonates, as they are used to provide vital support, at the risk of increasing infection.4,5
The most common form of neonatal HAIs are those related to medical devices. At the top of the list are central line-associated bloodstream infections (CLABSI), followed by ventilator-associated pneumonia (VAP).1,5,6
HAIs have widely variable epidemiology, as their incidence depends on geographic location, available resources in that region, and risk factors in that certain population. Knowledge of the local epidemiology becomes an essential feature in order to generate a significant impact in the burden of disease, bacterial resistance, length of hospital stay, health costs, and mortality.2,7-9
As this data has been considered an indicator of the quality of care, the Centers for Disease Control and Prevention (CDC) recommends routine reports and active surveillance.10 In Colombia, public health surveillance protocols have been developed by the National Institute of Health and Ministry of Health which adopted the CDC recommendations and are established by SIVIGILA (National Public Health Surveillance System and Subsystem of Information).11
This study describes the demographic and microbiological characteristics of device-associated infections in all of the Neonatal Intensive Care Units in Cali, Colombia between 2016 and 2018. Identification of this information could encourage the implementation of strategies for prevention and management, antibiotic stewardship, and the strengthening of epidemiological surveillance systems.
Materials and methodsStudy design and populationA descriptive, observational, cross-sectional study of all neonates with diagnosis of device-associated infection reported in 10 NICUs in Cali-Colombia, from August 2016 to December 2018.
Data was obtained from SIVIGILA (National Public Health surveillance system and the subsystem of Information), through a mandatory notification sheet for device-associated infections. This information was collected by a group of trained professionals in each healthcare institution and was based on the clinical records. This notification sheet included central line-associated bloodstream infections (CLABSI), ventilator-associated pneumonia (VAP), and catheter-associated urinary tract infection (CAUTI).
During the period studied, there were 78.482 live births reported. Reports of neonates hospitalized for at least 48 h were included.
Rates of infections were calculated for each device based in data obtained from the same source in 2018. This was presented by a number of new infections for each device, over days of use for each respective device, and a multiplication coefficient (1000). This was stratified for each birthweight category: 〈 1000 g, 1001–1500 g, 1501–2500 g, 〉 2500 g.
According to World Bank Classification, Colombia is considered a middle-income country.
Case definitionDevice-associated infections were defined and adjusted for neonates according to the criteria of the National Public Health surveillance system, through a Public Health Surveillance Protocol of the National Health Institute of Colombia, which adopt and translate the definitions from the Center for Disease Control and Prevention (CDC).10,11
Central line-associated bloodstream infection (CLABSI) was defined as a laboratory-confirmed bloodstream infection in the presence of a central line that had been in place for more than 2 consecutive days or one day after removal, with documentation of a recognized pathogen in at least one blood culture or a commensal pathogen in 2 separate blood cultures, in addition to clinical symptoms.10,11
Ventilator-associated pneumonia (VAP) was defined as the development of pneumonia in a patient who had remained on invasive ventilation for at least 2 days or one day after extubation. In clinically defined pneumonia for children under 1 year of age (including neonates), the patient has to have an impaired gas exchange, at least one radiological criteria, and three clinical criteria. For pneumonia with specific laboratory findings, the patient has at least one radiological criteria, one clinical criteria and one laboratory criteria.10,11
Catheter-associated urinary tract infection was defined as a laboratory-confirmed urinary tract infection in patients with a urinary catheter for more than 2 consecutive days or one day removed, and at least one clinical criteria.10,11
Patients with community-based infection cases or with documentation of contaminated cultures (Micrococcus, coagulase-negative staphylococci) in the absence of a device at the time the culture was taken, were excluded from the study. Contamination was excluded by using two or more blood cultures, taken from separate samples, positive for a commensal skin pathogen, plus clinical signs or symptoms in children under 1 year of age, including neonates.
Statistical analysisA univariate and bivariate statistical analysis was performed. The distribution of continuous variables, frequency, qualitative variables, loss of data, and the validity of the information was explored. Measures of frequency, central tendency, and dispersion were used, according to the classification of each of the variables and their distribution. Bar diagrams and measures of central tendency were used for the nominal variables. The distributions of the characteristics of interest were compared, using the statistical tests chi-square and Fisher's exact test. For continuous variables, the student's t-test was used. For comparison of means, the strength of the association of OR and its CI (95%) was determined. The data was recorded in a .xls file to be worked in Excel® and then exported to the STATA 14 software (Statacorp Inc. Texas) for statistical analysis.
All patients with device-care-associated infections were correlated with weight category, type of microorganisms, and death.
Ethical considerationsThis study was undertaken with approval by the Ethics Committee at the University Hospital, the Cali municipal health secretariat and Universidad del Valle according to Helsinki international ethical regulations.
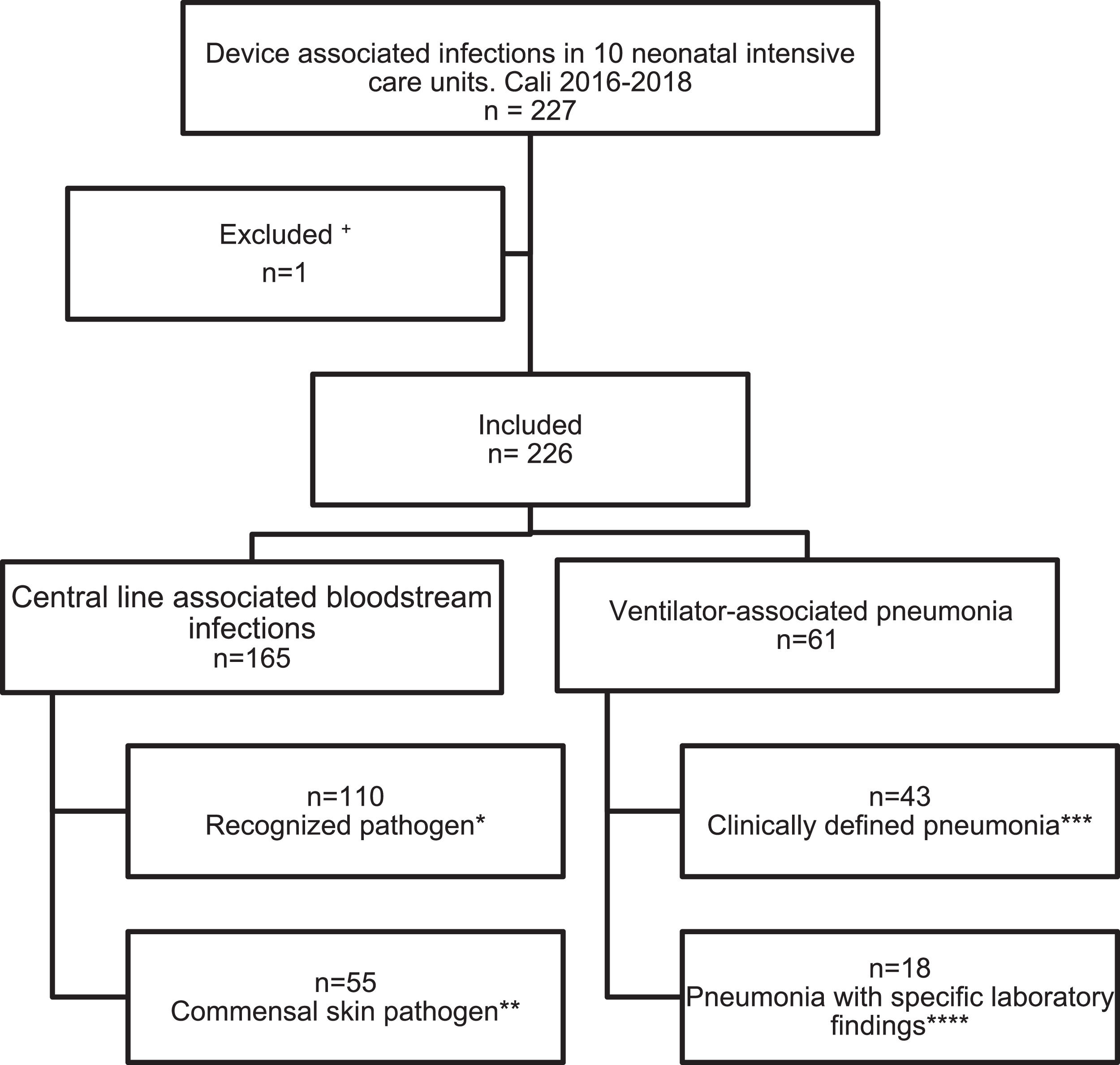
ResultsBetween August 2016 to December 2018, 226 reports of device-associated infections were identified in a total of 10 NICUs in Cali, Colombia.
52.7% (n = 119) of the reports correspond to males and 47.3% to females (n = 107). Distribution by birth weight was: 16.1% (n = 36) for those neonates > 2500 g, whereas 48.2% (n = 108) were extremely low birth weight (< 1000 g) (17.4% 1001–1500 g; 18.3% 1501–2500 g).
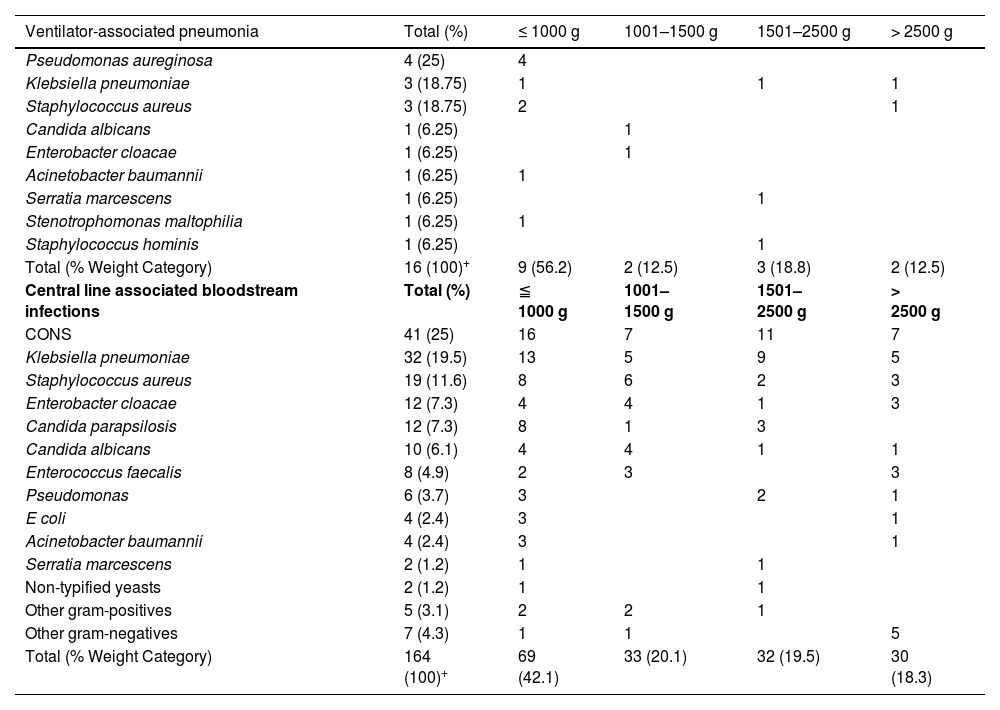
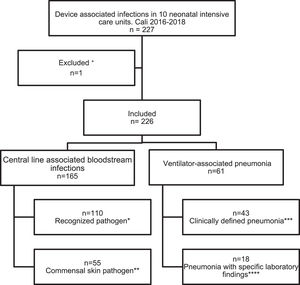
Regarding devices used, n = 165 (73%) were central line-associated bloodstream infections (CLABSI) and n = 61 (27%) ventilator-associated pneumonia (VAP), infections associated with other medical devices were not documented (Fig. 1). In n = 183/226 cases a positive blood culture was documented. The identification of one pathogen was missed. In general, the gram-negative pathogens were most frequent (43.41%), within them Klebsiella pneumoniae was identified in 19.78% (n = 36), followed by coagulase-negative staphylococci (CoNS) with 23.08% (n = 42) as the most frequentgram-positive (Supplemental material 1).
Events reported according to epidemiological surveillance guidelines of the Colombian Ministry of Health based on the CDC surveillance system.
+Loss of germ typing in a registry.
* At least 1 positive blood culture for a recognized pathogen.
** Two or more blood cultures, taken from separate samples, positive for a commensal skin pathogen, plus clinical signs or symptoms in children under 1 year of age, including neonates.
*** At least one alternative radiological criteria and one clinical criteria for children under 1 year of age including neonates.
**** At least one radiological criteria, one clinical criteria and one laboratory criteria. In this study the laboratory criteria in the 18 cases were a positive blood culture.
Data of birthweight was obtained in 16/18 reports of VAP and in 164/165 reports of CLABSI. A total of 3 data were missed. The extremely low birth weight (< 1000 g) with VAP corresponds to 56.2% (n = 9/16), with the most frequent pathogen identified in this group being Pseudomona aeruginosa (n = 4/9; 25%). Likewise, the extremely low birth weight (< 1000 g) with CLABSI corresponds to 42.1% (n = 69/164), with CoNS being the pathogen most frequently isolated in 23.18% (n = 16/69) (Table 1).
Microorganisms* in device-associated infections according to weight category.
| Ventilator-associated pneumonia | Total (%) | ≤ 1000 g | 1001–1500 g | 1501–2500 g | > 2500 g |
|---|---|---|---|---|---|
| Pseudomonas aureginosa | 4 (25) | 4 | |||
| Klebsiella pneumoniae | 3 (18.75) | 1 | 1 | 1 | |
| Staphylococcus aureus | 3 (18.75) | 2 | 1 | ||
| Candida albicans | 1 (6.25) | 1 | |||
| Enterobacter cloacae | 1 (6.25) | 1 | |||
| Acinetobacter baumannii | 1 (6.25) | 1 | |||
| Serratia marcescens | 1 (6.25) | 1 | |||
| Stenotrophomonas maltophilia | 1 (6.25) | 1 | |||
| Staphylococcus hominis | 1 (6.25) | 1 | |||
| Total (% Weight Category) | 16 (100)+ | 9 (56.2) | 2 (12.5) | 3 (18.8) | 2 (12.5) |
| Central line associated bloodstream infections | Total (%) | ≦ 1000 g | 1001–1500 g | 1501–2500 g | > 2500 g |
| CONS | 41 (25) | 16 | 7 | 11 | 7 |
| Klebsiella pneumoniae | 32 (19.5) | 13 | 5 | 9 | 5 |
| Staphylococcus aureus | 19 (11.6) | 8 | 6 | 2 | 3 |
| Enterobacter cloacae | 12 (7.3) | 4 | 4 | 1 | 3 |
| Candida parapsilosis | 12 (7.3) | 8 | 1 | 3 | |
| Candida albicans | 10 (6.1) | 4 | 4 | 1 | 1 |
| Enterococcus faecalis | 8 (4.9) | 2 | 3 | 3 | |
| Pseudomonas | 6 (3.7) | 3 | 2 | 1 | |
| E coli | 4 (2.4) | 3 | 1 | ||
| Acinetobacter baumannii | 4 (2.4) | 3 | 1 | ||
| Serratia marcescens | 2 (1.2) | 1 | 1 | ||
| Non-typified yeasts | 2 (1.2) | 1 | 1 | ||
| Other gram-positives | 5 (3.1) | 2 | 2 | 1 | |
| Other gram-negatives | 7 (4.3) | 1 | 1 | 5 | |
| Total (% Weight Category) | 164 (100)+ | 69 (42.1) | 33 (20.1) | 32 (19.5) | 30 (18.3) |
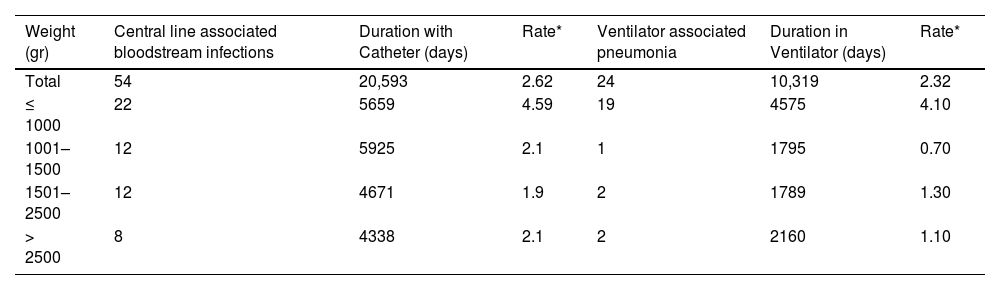
The incidence rate for device-associated infections was 2.62 per 1000 catheter days for CLABSI and 2.32 per 1000 ventilator days for VAP, whereas for those with birthweight under 1000 g the incidence rate was 4.59 per 1000 catheter days and 4.10 per 1000 ventilator days respectively (Table 2).
Incidence rate of device-associated infections according to birth weight.
| Weight (gr) | Central line associated bloodstream infections | Duration with Catheter (days) | Rate* | Ventilator associated pneumonia | Duration in Ventilator (days) | Rate* |
|---|---|---|---|---|---|---|
| Total | 54 | 20,593 | 2.62 | 24 | 10,319 | 2.32 |
| ≤ 1000 | 22 | 5659 | 4.59 | 19 | 4575 | 4.10 |
| 1001–1500 | 12 | 5925 | 2.1 | 1 | 1795 | 0.70 |
| 1501–2500 | 12 | 4671 | 1.9 | 2 | 1789 | 1.30 |
| > 2500 | 8 | 4338 | 2.1 | 2 | 2160 | 1.10 |
The median diagnosis according to the days of hospitalization corresponded to 13 days (IQR = 8, 23) for CLABSI and 22 (IQR = 10.5, 38.5) days for VAP.
In the cases of CLABSI, a greater number of reports (n = 69;41.82%) were identified in neonates of less than 1000 g, compared to those greater than 2500 g (n = 30; 18.18%). However, this difference was not significant. For ventilator-associated pneumonia, 39 cases weighed less than 1000 g (61.10%), vs only 6 cases (10.17%) for those greater than 2500 g (OR 2.82 95% CI 1.08–7.38. p = 0.034), which is statistically significant.
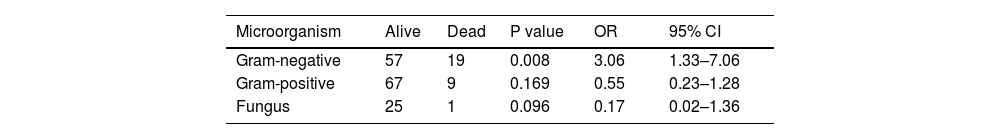
The probability of dying was higher in those under 1000 g vs over 1000 g, (OR: 3.61 95% CI: 1.53–8.49 p = 0.03). When evaluated mortality according to the type of bacteria, gram-negative are associated with a greater probability of death (OR 3.06 CI 95 1.33–7.06 p = 0.008) (Table 3).
Outcomes according to the type of microorganism* in device-associated.
Incidences of type device-associated infection and microorganisms showed no statistically significant differences within each birth weight category.
Antibiotic use was reported in 156/226 cases. In 64.2% of the reports a combined therapy with at least 2 antimicrobials was used. The most frequent antibiotic was Vancomycin, Meropenem and/or Cefepime.
DiscussionDevice-associated infections are an important cause of morbidity and mortality in the NICU. Control measures require epidemiological knowledge for the adequate implementation of strategies to reduce the burden of the disease. 7-9
DAIs vary significantly according to the region. In a recent international report between 2012 and 2017, which included 45 countries from Latin America, Asia, and Europe, the rate of CLABSI was 12.7 per 1000 catheters day and 7.5 per 1000 ventilator days.8 This is much higher than the one found in the present study, which corresponds to 2.62 and 2.32, respectively. In the last report from the United States, 2013, the rates for DAIs were even lower than the previously mentioned data. For CLABSI the rate was 1.22 for 1000 days catheter and 0.83 for 1000 days ventilator for VAP.12 This could be explained by the implementation of active surveillance protocols led by the CDC, along with differences in infrastructure, availability of high-tech equipment, antibiotic stewardship, etc., that differ between low-, middle- and high-income countries.
Contreras-Cuellar et al.,13 published data on DAIs from a NICU in Bogotá-Colombia in 2005. It shows the most frequent pathogens were gram-positives, present in 60% of the cases, followed by gram-negative with 36.3%. The present study found, there were similar rates of both gram-positives and gram-negatives. However, the majority of the reports correspond to gram-negatives in 43.4% vs 42.3% for gram-positives. Also, there was an important difference between the prevalence reported by Contreras-Cuellar, who found fungal infections corresponded to 3.03%, whereas in this current study to 14.3%. This shows an important change in the microbiology of these infections through the years, in the same country.
Yet, CONs infections head the list with 23%, as same as the distribution reported in high-income countries.14,15
Over the years, the advent of technology and medical knowledge has allowed an increased survival of premature neonates of lower weight and gestational age.16 These neonates have greater requirements for invasive procedures, use of devices, prolonged parenteral nutrition, and delayed enteral feeding, among other factors that make them a highly vulnerable population for the acquisition of infections associated with medical care.4,5 As reported by the current study, about half of the events reported correspond to neonates of less than 1000 g (48.2%), and the incidence rates for both catheter-associated infections and ventilator-associated pneumonia have an inversely proportional behavior regarding birth weight, with rates 2 to 4 times higher for those under 1000 g than for those in other weight groups. This data is consistent with those reported in other studies. 12,17
Birth weight has been a factor related to the microbiological profile of these infections. Studies have shown an increase in infections caused by gram-negative bacteria in premature infants with extremely low birth weights.18,19 This is consistent with the data reported by the current study, where the frequency is the highest at 43.7% for this weight group. Tsai et al. report that infections by gam-negative microorganisms are related to a higher risk of death (OR = 2.32, 95% CI [1.48–3.64], p ≤ 0.001). 22 Data collected in the current study report an odd 3.06 times higher (OR = 3.06 95% CI [1.33–7.06], p = 0.008).20 However, no events of meningitis or urinary infection were reported in this investigation
The use of broad-spectrum antibiotic therapy is observed in a high percentage of cases (64.20%) in this current study. North American studies report that the use of broad-spectrum antibiotic therapy in neonatal units can be as low as 5%.21 There is a higher concern about the link of this practice with the increasing rates of fungal infections in the studied clinical fields, as a factor that contributes to antimicrobial resistance.
This study has some limitations. First, the information comes from the database SIVIGILA (National Public Health surveillance system and the subsystem of information) through a notification sheet for device-associated infections. As it is a retrospective recollection of data, it could have led to missing relevant information for the analysis and interpretation. Secondly, the demographic and clinical data were limited. The registration of this information began in 2016, which limited the size of the sample.
There is no recent data in the literature that describes the behavior of device-associated infections at a local level, based on epidemiological surveillance systems. Thus, the strengths of this work highlight the contribution in order to identifying the behavior of these types of infections locally. This may lead to the development of public policies that contribute to improving the quality of health care and decreasing neonate morbidity and mortality in our centers.
ConclusionThe rising rates of survival in extremely preterm newborns, which require medical devices such as catheters and mechanical ventilation, call for the implementation of medical and technological strategies to improve health outcomes. An approach towards early enteral nutrition, short-term parenteral nutrition, administration of prenatal steroids, and noninvasive ventilation could make a starting point for care in our units.
The results obtained highlight the need to maintain epidemiological surveillance processes in neonatal intensive care units since it allows the generation of local and recent information on important public health events. Studies should continue to identify the clinical and microbiological behavior of these infections in the studied population which allows us to implement adequate control and prevention measures.
Statement of ethicsThis study protocol was reviewed and approved by the Institutional Committee for the Review of Human Ethics (CIREH) of the Universidad. Being a database of the National Public Health Surveillance System-SIVIGILA, it did not require written informed consent.
Data availability statementThe data of this study were obtained from the database of the Public Health Surveillance System of Colombia (SIVIGILA) where restrictions on their availability may be applied. This set of data can be requested from the Public Health Surveillance and Epidemiological Surveillance group at the Cali Municipal Public Health Secretariat.