Covid-19 had a direct impact on children's health. The aim of this review was to analyze epidemiological and clinical data, the consequences of the pandemic, and vaccination aspects in this group.
Sources of dataThe searches were carried out from January 2020 to November 2022, in the MEDLINE databases (PubMed) and publications of the Brazilian Ministry of Health and the Brazilian Society of Pediatrics.
Summary of findingsCovid-19 has a mild presentation in most children; however, the infection can progress to the severe form and, in some cases, to MIS-C. The prevalence of the so-called long Covid in children was 25.24%. Moreover, several indirect impacts occurred on the health of children and adolescents. Vaccination played a crucial role in enabling the reduction of severe disease and mortality rates. Children and adolescents, as a special population, were excluded from the initial clinical trials and, therefore, vaccination was introduced later in this group. Despite its importance, there have been difficulties in the efficient implementation of vaccination in the pediatric population. The CoronaVac vaccines are authorized in Brazil for children over three years of age and the pediatric presentations of the Pfizer vaccine have shown significant effectiveness and safety.
ConclusionsCovid-19 in the pediatric age group was responsible for the illness and deaths of a significant number of children. For successful immunization, major barriers have to be overcome. Real-world data on the safety and efficacy of several pediatric vaccines is emphasized, and the authors need a uniform message about the importance of immunization for all children.
More than two years after the start of the Covid-19 pandemic, the authors were able to see a profound direct impact on the health of children and adolescents. Data from the Centers for Disease Control and Prevention (CDC) of the United States of America (USA), which develops reliable surveillance, indicate that, until September 21, 2022, children in that country represented 17.4%% of all confirmed infections by SARSCoV-2 and that about a quarter of those hospitalized for Covid-19 required care in intensive care units (ICU).1 Up to November 9, 2022, in the USA, 1,853 children under 18 years of age of the US died from Covid-19, far exceeding the 249 deaths in this age group due to seasonal influenza in the same period, to make a parallel with another viral respiratory infection for which vaccines are available and routinely recommended.2,3
During the initial phase of the pandemic, considering the cases notified to the World Health Organization (WHO), covering the period from December 30, 2019, to October 25, 2021, it was observed that children under five represented 2 % (1,890,756) of cases and 0.1% (1,797) of global deaths. Children aged 5 to 14 accounted for 7% (7,058,748) of cases and 0.1% (1,328) of global deaths, while older adolescents and young adults (15 to 24 years) accounted for 15% (14,819,320) of cases and 0.4% (7,023) of the reported global deaths. However, the pandemic evolution has shown that the epidemiological scenario has undergone important changes. Reported cases of Covid-19 among children increased dramatically in the year 2022, during the rise of the Omicron variant and its various sub-lineages, at a time when most countries had discontinued the non-pharmacological measures to contain the pandemic. For example, in the USA, in July 2022, 14,003,497 cases were reported in children, which, at the time, represented 18.6% of all reported cases, corresponding to 18,605 cases per 100,000 children in the population. Globally, up to July 24, 2022, children under 5 and those aged 5 to 14 accounted for 2.47% and 10.44% of Covid-19 cases, respectively. Adolescents and young adults aged 15 to 24 years comprised 13.91% of all cases.4
Globally, it is estimated that 90% of pediatric deaths from Covid-19 have occurred in low- and middle-income countries, with the highest mortality rates among children being identified in the group of infants younger than one year, a reflection of the great social inequalities and their differentiated impact on human health.5 A systematic review study, which corroborated this evidence, concluded that a 20-year-old young individual was almost three times more likely to die at the beginning of the pandemic in a low- or middle-income country than in a high-income country. The higher mortality rates in low-income countries are probably due to poorer access to good-quality medical care, which would make the development of strategies to prevent SARS-CoV-2 infection even more relevant in this scenario of greater adversity.6
In Brazil, considering the first two years since the beginning of the pandemic, it is estimated that Covid-19 has killed two children under the age of five per day, comprising a total of 1,439 victims. The Northeast region accounted for almost half of these deaths. The analysis of this initial period of the pandemic in Brazil shows that children from 29 days to one year old are the most vulnerable ones. Infants in this age group accounted for almost half of the deaths recorded among children under five years of age, which suggests the importance of developing specific vaccines for this age group, so they can also be effectively protected. Until June 2022, data collected by the United Nations Children's Fund (UNICEF) in 91 countries show that Covid-19 was the underlying cause of death for 5,376 children under five years of age worldwide. Brazil, therefore, accounted for about one in five of these deaths.7,8
The direct impact of Covid-19 on the health of children and adolescentsAlthough Covid-19 has a mild presentation in the vast majority of children, generally being considered a milder disease when compared to adults, SARS-CoV-2 infection can have a severe evolution. Children with underlying medical conditions are at increased risk of severe disease, although some previously healthy children may also have this outcome. Current evidence suggests that children with special health care needs, including genetic conditions such as Down syndrome, neurological or metabolic conditions, congenital heart disease, chronic encephalopathy, prematurity, dependence on enteral nutrition, airway anomaly, severe uncontrolled asthma, are at greater risk of developing severe forms of Covid-19. Similar to what is observed among adults, children with obesity, diabetes, asthma, chronic pulmonary disease, sickle cell disease, or immunosuppression also constitute a group of patients at increased risk of severe disease.9,10
Although considered rare but serious, some children who had Covid-19 later developed Multisystem inflammatory syndrome in children (MIS-C). In the US, adjusted incidence estimates of the occurrence of this syndrome were approximately 1 to 10 cases per 1,000,000 person-months, with estimates varying with race/ethnicity, and age group.11 Patients with MIS-C usually present with persistent fever, cardiorespiratory and gastrointestinal symptoms, mucocutaneous lesions, and, in severe cases, hypotension and shock. Cardiac, cardiorespiratory, and gastrointestinal complications are the most common reasons for admission to intensive care units, indicating the severity of this condition.12
Moreover, children and adolescents aged 18 and under who have had Covid-19 are up to 2.5 times more likely to be diagnosed with diabetes 30 days or more after the infection, which highlights the importance of Covid-19 prevention strategies in this age group, including vaccination for all eligible persons and prevention and treatment of chronic diseases.13 In another more recent study, it was observed that new diagnoses of type 1 diabetes were more likely to occur among pediatric patients with prior COVID-19 than among those with other respiratory infections. It is known that respiratory infections have been associated with the onset of diabetes, but in this study this risk was even higher among those with Covid-19, raising concerns about long-term post-Covid-19 autoimmune complications among young people and highlighting the need for the development of specific treatments and preventive measures, such as vaccines.14
In a systematic literature review study, the prevalence of the so-called long Covid in children was 25.24%, with the most prevalent clinical manifestations of this condition being mood swings (16.50%), and fatigue (9.66%). and sleep disorders (8.42%). Moreover, children infected with SARS-CoV-2 were at increased risk of persistent dyspnea, anosmia/ageusia, and/or fever compared to controls. Although with limitations, such as the lack of standardized definitions, loss of follow-up, and heterogeneity of the included studies, this meta-analysis illustrates possible impacts of this condition, which may be underestimated, on child development and school performance, for instance.15
Indirect impact of Covid-19 on the health of children and adolescentsIn addition to direct health problems, children and adolescents have been disproportionately affected by Covid-19 control measures. The most important indirect effects are related to the closing of schools interrupting the provision of educational services and increasing emotional distress and mental health problems. When unable to attend school and socially isolated, children are more prone to abuse, sexual violence, teenage pregnancy, and child marriage, increasing the likelihood of dropping out of school. Moreover, there was an interruption in physical activities and routines and a loss of access to a wide range of services provided by the school, such as school lunches, health, nutrition, clean water, sanitation and hygiene, and services aimed at children with special needs, such as support for learning, speech therapy, and social skills training. That is, there was a significant worsening of pre-existing inequalities.16
Role of children and adolescents in Covid-19 transmissionOutbreaks of Covid-19 have occurred in secondary schools, summer camps, and daycare centers, demonstrating the transmission of SARS-CoV-2 in settings where pediatric patients predominate. Some preliminary evidence seems to indicate that younger children may be less infectious, considering secondary attack rates, than adolescents and adults.17
The relationship between age, viral load, and transmission across the symptom spectrum of SARS-CoV-2 infection have not been fully investigated because people with no or mild symptoms are rarely systematically tested. Thus, the relative transmissibility of SARS-CoV-2 at different ages remains uncertain, largely due to the challenges involved in pinpointing the influences of different factors, such as biological, host, virus and its variants, and environmental factors.18
More than two years after the start of the pandemic, a better understanding of the role of children in the transmission of Covid-19 has been the subject of studies and the data gathered so far seem to indicate that they are less likely to transmit Covid-19 when compared to adults. Household transmission remains the most prominent source of child-to-adult and child-to-child transmission. However, more studies are needed to better understand how childhood transmission of Covid-19 has been impacted by the reopening of schools and the advancement of vaccines, as well as the emergence of new variants of SARS-Cov-2.19
Inclusion of the pediatric population as a target audience for vaccines against Covid-19Vaccination has played a crucial role in the fight against Covid-19, allowing the reduction of rates of severe illness and mortality, in addition to restricting the spread of the disease, which has been the main factor in controlling the pandemic.20 If the disease in the pediatric population is not controlled, children may become a reservoir of the virus, leading to epidemics in the community.21-23
With the shared effort of government authorities, pharmaceutical companies, and the scientific community, a number of Covid-19 vaccines have been widely used in the adult population. Children and adolescents, as a special population, were generally excluded from the initial clinical trials and, therefore, the vaccination was introduced later in this group.24 For example, in Brazil, the inactivated virus vaccine by the pharmaceutical company Sinovac Biotech (CoronaVac) received conditional approval for the immunization of the pediatric population aged over three years, and the messenger RNA (mRNA) vaccine BNT162b2, developed in collaboration with Pfizer and BioNTech laboratories, was successively authorized for use in adolescents aged 12 to 18 years, later children aged five to 11 years with a special presentation containing one-third of the standard dose and which subsequently received authorization from the US regulatory agency Food and Drug Administration (FDA) and the Brazilian National Health Surveillance Agency (ANVISA) for another pediatric presentation, corresponding to one-tenth of the adult dose, for use in children aged 6 months to 4 years.23-27
Despite the crucial importance of vaccines in controlling the pandemic, there have been difficulties in efficient vaccine implementation.23,24 The introduction of effective vaccines against SARS-CoV-2, initially in school-aged children and adult populations, combined with the emergence of new variants of SARS-CoV-2, with greater transmission capacity, resulted in a proportional increase in infections in younger children.27 Moreover, unfortunately, inequalities in the distribution of vaccines, vaccine hesitancy, misinformation, and political complexities, make vaccination coverage insufficient to contain the pandemic.21
Immunogenicity of vaccines against Covid-19 in the pediatric populationOverall, evidence of good immunogenicity has been observed for vaccines against Covid-19 in children. In most studies, comparable humoral antibody responses were observed regarding neutralizing antibody titers in children and adults. The BNT162b2 vaccine, in adolescents aged 12-15 years, showed that the geometric mean of antibodies was 1.7-fold higher than that observed in the group aged 16 to 25 years; among children aged 5 to 11 years, the geometric mean of antibodies was identical to that observed in the group aged 16 to 25 years. Even the group of infants aged 6 to 23 months reached geometric means identical to those obtained in the group aged 16 to 25 years, bearing in mind that the infants received one-tenth of the dose used in the group aged 16 to 25 years. The only exception was the group aged 2 to 5 years, which required 3 doses to reach non-inferiority.28-30
Regarding the need for booster doses, data from Zhu et al.31 indicated that there was no increase in cellular immunity after an additional vaccine dose. This result, however, must be interpreted with caution, due to the limited data on the cellular immune response and the observation that, after the circulation of the Omicron variant, the need for booster doses also in adolescents became more evident.32
The initial phase I and II studies of the CoronaVac vaccine, involving children and adolescents aged three to 17 years of age, demonstrated that antibody seroconversion rates were greater than 96%.33
Efficacy and effectiveness of vaccines against Covid-19 in the pediatric populationThe published initial results regarding the BNT162b2 vaccine (Pfizer) in adolescents were based on a phase III study, which included 2,126 participants aged 12 to 15 years.34 Most participants were white (85%), 90% of adolescents had had no previous contact with SARS-CoV-2, and immunocompromised adolescents were not included in the study. the randomization was 1:1 (vaccine and placebo) with a 21-day interval between the doses. The geometric mean ratio of neutralizing antibodies after the second dose in adolescents aged 12 to 15 years, compared to the group aged 16 to 25 years, was 1.76 (95% confidence interval [CI], 1.47 to 2 .10), thereby proving non-inferiority (lower limit of 95% CI greater than 0.67).
Effectiveness studies have been published, with real-world data after the introduction of the BNT162b2 vaccine (Pfizer), in some countries such as the USA, Israel, and the United Kingdom. In Israel, a retrospective cohort study estimated the vaccine effectiveness in adolescents aged 12 to 15 years vaccinated with two separate doses, with a 21-day interval between them. The results showed a 91.5% effectiveness (CI 88.2%-93.9%). At the follow-up, none of the vaccinated adolescents was hospitalized after the second dose of the vaccine.35,36
In the USA, after the introduction of the vaccination in adolescents, a case-control study showed the effectiveness of two doses of the Pfizer/BioNTech vaccine against hospitalization due to Covid-19. Similar to the data from Israel, the effectiveness of two doses of the Pfizer/BioNTech vaccine against hospitalization for Covid-19 was 93% (95% CI = 83%-97%). During the study period, the Delta variant was the predominant one and there were 77 ICU admissions. The 29 adolescents were considered to have severe cases of the disease and the two deaths occurred in the non-immunized group.37-39 As for MIS-C, a US study demonstrated a Pfizer/BioNTech vaccine effectiveness of 91% (95% CI = 78%-97%).40
After its successful use in the 12-17 age group, the CDC released, in November 2021, a presentation of the Pfizer vaccine containing one-third of the standard dose, therefore 10 mcg, aimed at children aged five to 11 years. The approval of this presentation was supported by the analysis of real-world data in this age group during the circulation of the Omicron variant, after the initial studies that defined the dose for this age group and the safety profile.41 In phase II/III studies, the objectives were to evaluate safety, tolerability, and immunogenicity by demonstrating the non-inferiority of neutralizing antibody response. As secondary objectives, the effectiveness of disease prevention was verified. A 90.7% efficacy (95%CI = 67.7 to 98.3%) was demonstrated for the prevention of Covid-19, at least seven days after the second dose. Regarding adverse events, the vaccine showed a good safety profile.41-43
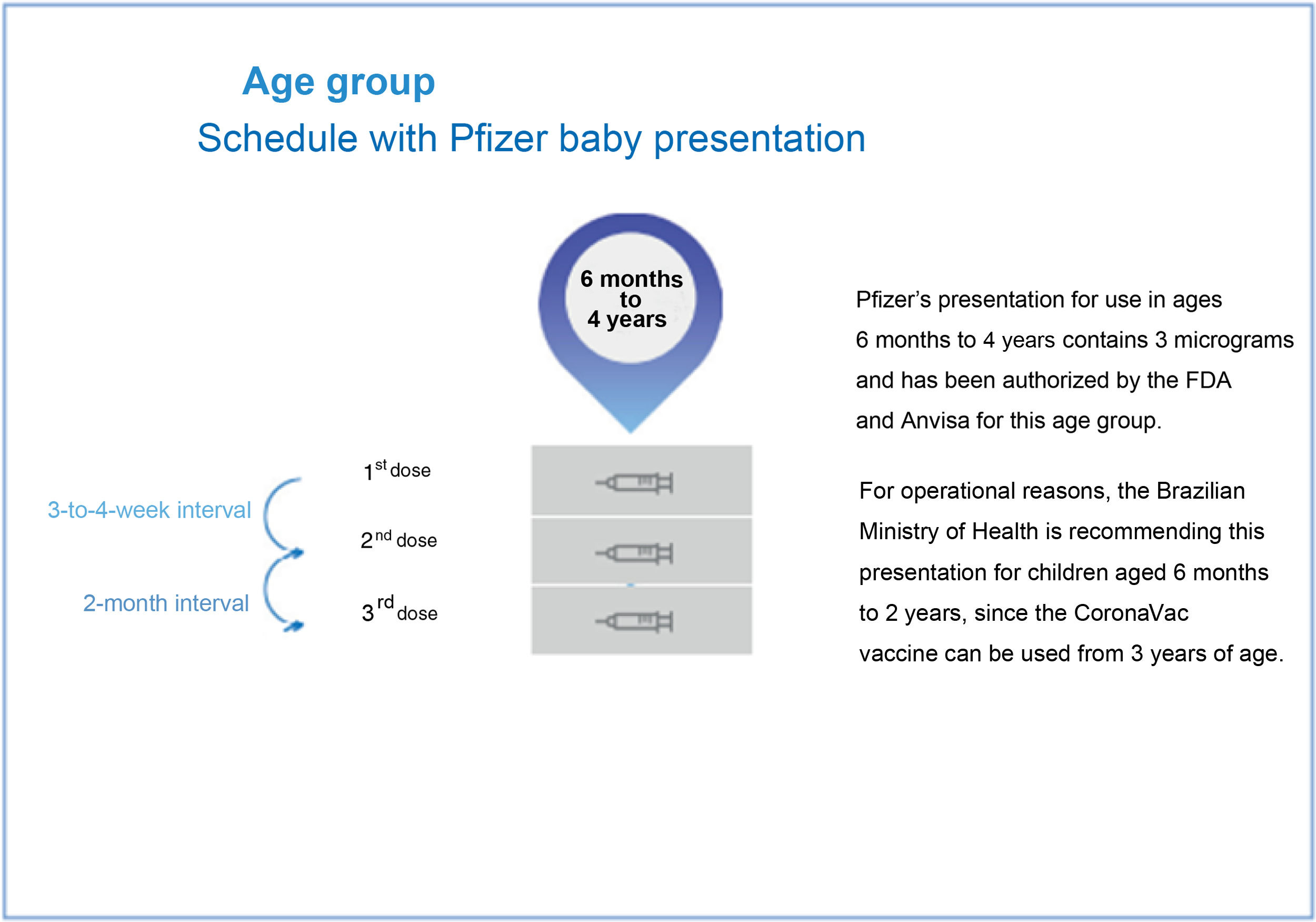
Subsequent studies evaluated the use of a presentation with one-tenth of the standard dose in children aged six months to four years, which was released for this group in a primary schedule with three doses with 21-day intervals between the first and second doses and two months between the second and the third dose. In September 2022, ANVISA, based on prior authorization from other regulatory agencies such as the FDA, authorized the use of this new presentation also in Brazil.44 The Ministry of Health started the vaccination with this pediatric presentation in November but has currently restricted the use for children with comorbidities only. Scientific societies such as the Brazilian Society of Pediatrics (Sociedade Brasileira de Pediatria) have vehemently opposed the vaccination of children with comorbidities only, a strategy that is difficult to implement, especially in the poorest regions of the country, precisely where vaccine protection is most needed. The delay in starting vaccination for all children in this age group, especially at times of increased viral circulation, has enormous impacts on the children's health. The imminent risk of Covid-19 in the pediatric population is evident.45
The authors emphasize that tens of thousands of hospitalizations due to Covid-19 in children and adolescents and among the thousands of deaths, as well as among cases of MIS-C, the majority occurred in previously healthy children, highlighting once again the need not to restrict the availability and recommendations of vaccines to higher risk groups only.
In a study on the effectiveness of the SinoVac vaccine conducted in Chile, the estimated effectiveness of the CoronaVac vaccine in children aged 3 to 5 years during the Omicron outbreak was 38.2% (95%CI 36.5-39.9) for the prevention of COVID-19, 64.6% (95%CI, 49.6-75.2) for prevention of hospitalization and 69.0% (95% CI, 18.6-88.2) for prevention of ICU admission related to COVID-19.46
In July 2022, ANVISA authorized the use of the CoronaVac vaccine in the pediatric population in Brazil, for use in children aged three years or older, in a two-dose schedule with a 28-day interval, without a history of immunosuppression, a situation in which mRNA vaccines should be used.27
There is evidence that vaccination can reduce the risk of MIS-C. One study reported that post-vaccination MIS-C in adolescents aged 12 to 17 years occurred at a rate of 1.5 per million total vaccine recipients (95%CI 0.8 - 2.6).47 In contrast, 113 cases of MIS-C were observed among one million SARS-CoV-2 infections in the same age group.48 MIS-C prevention is important because affected infants and adolescents may face long-term health sequelae that are not yet known.
Vaccination of pregnant and lactating women against Covid-19 and child protectionCoV-2 infection prevention methods in infants also include protection induced by maternal vaccination. Although they were excluded from clinical trials, effectiveness studies have shown that pregnant women benefited significantly from the vaccination.49-51 Moreover, mothers vaccinated before or during pregnancy transfer specific antibodies against SARS-CoV-2 to their babies via transplacental transmission and through breast milk with the possibility of additional protection in young infants.52,53 However, this passive protection will only protect the baby for the first six to 12 months of life before maternal antibodies decline, and with the low durability and continued evolution of the virus, maternal antibodies are likely to protect for a few months. Even when breastfeeding is continued until the second year of life, the decline in antibodies induced by the vaccine and the limited uptake of antibodies from breast milk into the circulation would likely result in titers that are too low to protect the infant against infection.49 It is noteworthy that young infants are one of the most frequently hospitalized pediatric populations for SARS-CoV-254 infection and most of these children do not have comorbidities. Recent studies55-57 evaluated maternal-child protection for the fetus.
Thus, it is likely that in the near future, protection against severe Covid-19 for all children will require new studies with vaccination in children under six months of age.
Safety and adverse events of Covid-19 vaccines for children and adolescentsConcerns about safety and the appearance of adverse events are always present regarding any new vaccine. The rare cases of myocarditis and pericarditis that were observed in adolescents and young adults, especially those receiving mRNA platform vaccines, seem to be dose- and interval-dependent, and pharmacovigilance data have identified occurrences of rarely reported cases in the age group of 5 to 11 years.58-60
A follow-up study of adolescents in Israel indicated that BNT162b2 vaccine-induced myocarditis in adolescents seems to be a rare adverse event, occurring predominantly in male individuals after the second dose of the vaccine. The clinical course seems to be mild and benign over a six-month follow-up period, and cardiac imaging findings suggest a favorable long-term prognosis61 and, although some patients still had minor cardiac abnormalities, they occurred at a significantly lower rate than after SARS-CoV-2 infection.62,63
The number of participants in clinical trials conducted in young children (< 5 years) is too low to detect rare events, but considering the lower doses being used in this population and the relatively rare occurrence of myocarditis in this age group in general, the associated risk of myocarditis in infants aged six months to 5 years is unlikely to be identified.
Regarding other adverse events in different age groups, it is important to highlight that, in general, they were more frequent in children aged 12 to 17 years than in children under 11 years of age for the Pfizer vaccine, with the vast majority being mild local and systemic reactions.24
Subsequently, pharmacovigilance data on adverse events in children in China and Chile were shown. Among the data from Chile, when more than three million doses of the vaccine had already been administered, only 319 adverse events were reported and most of them were considered non-severe.
For inactivated vaccines (CoronaVac),33 in general, adverse reactions were also more important in older children, similar to the results of comparisons of RNA vaccines.
To evaluate the adverse reactions to different doses of vaccines, two randomized controlled studies33,64 showed information on children and adolescents aged three to 17 years after receiving different doses of inactivated vaccines. The data suggest acceptable safety and tolerability profiles for several used doses of inactivated vaccines.
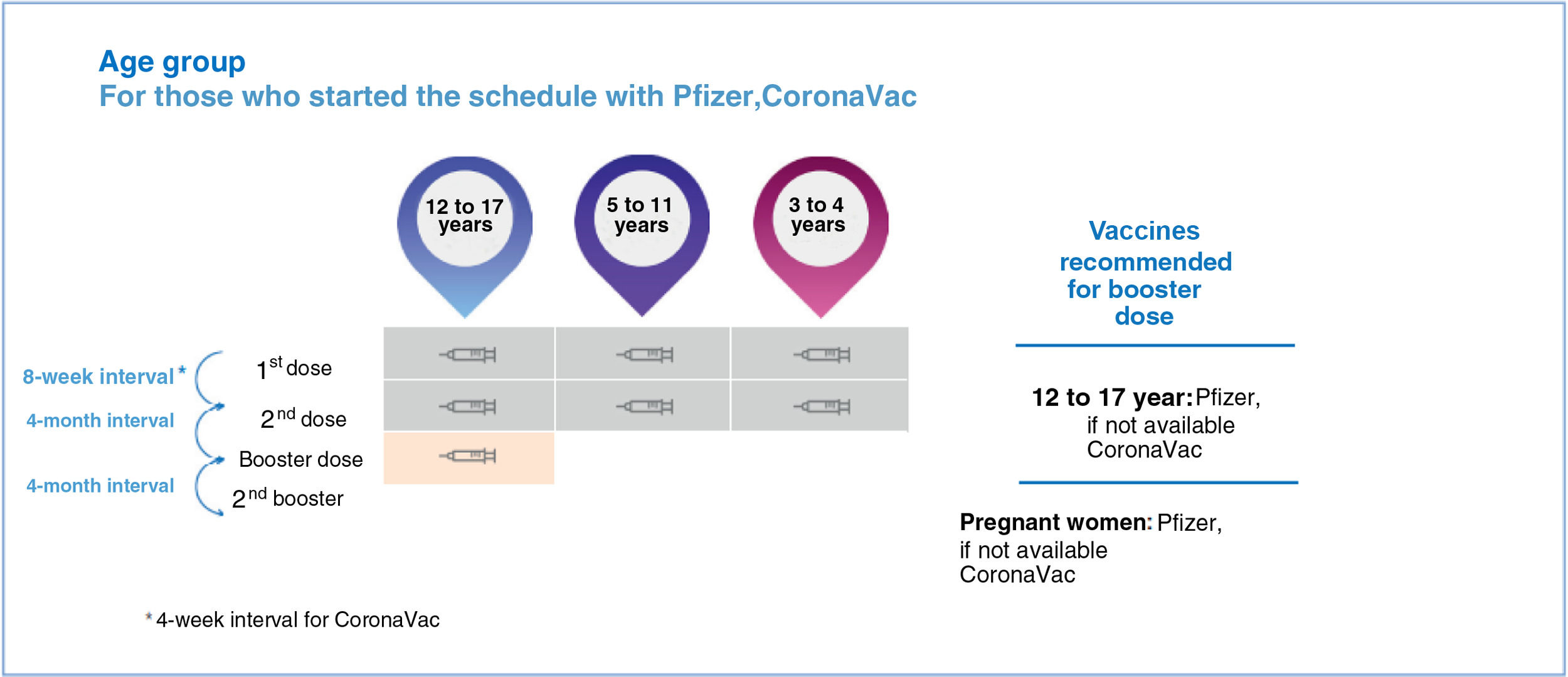
Covid-19 vaccination for children and adolescentsIn Brazil, the vaccines approved in the pediatric group, with their respective ages, are described in Figure 1.65Figure 2 depicts the vaccination schedule recommended for the administration of the Pfizer “Baby” vaccine in children aged six months to 4 years.45 As expected, there may be changes in the recommendations depending on the epidemiological scenario and the accumulation of new knowledge.
Covid-19 vaccines are the subject of some controversies and discussions. One of the most frequent questions is whether children or adults who have already had the disease need to receive the vaccine, since, theoretically, they have natural antibodies after the infection. It has been shown that people with a previous history of Covid-19 have enhanced immune responses when vaccinated, concluding that, in this situation, having had the disease and subsequently being vaccinated, is the combination that would result in a more robust immune response.21
In the presence of mild to moderate SARS-Cov-2 infection, the Covid-19 vaccine can be administered four weeks after the infection. When the condition is severe (including MIS-C), the Covid-19 vaccine should be administered three months after the resolution of the condition, after assessing the individual's health conditions.66
Regarding the other vaccines in the vaccination calendar and previous infection by SARS-CoV-2, the current guideline is to vaccinate the child with routine immunizations immediately after clinical improvement, and currently there may be simultaneous administration of Covid-19 vaccines with other vaccines included in the children's routine calendar.30
Considering the general benefits for society and the children themselves, it is imperative to improve measures to support Covid-19 vaccination in the pediatric population and expand the protected groups.63 One situation that requires more information is the use of these vaccines in immunocompromised pediatric populations. Immunosuppressed adolescents aged 12 to 17 years have a special schedule with the Pfizer vaccine, with an additional dose two months after D2 and a booster dose four months after the additional dose.65
The benefits of vaccinating children with the SARS-CoV-2 vaccines far outweigh the risks of acquiring the infection when unvaccinated, making the vaccine as important for children's health as other routine vaccines.66-69
The advantages of early immunization against SARS-CoV-2 include the following: (1) protection against SARS-CoV-2 infection and severe acute Covid-19 in infants and children; (2) protection against Covid-19 post-infectious inflammatory syndromes such as MIS-C; (3) potential protection against long-lasting Covid-19 sequelae and long Covid; (4) reduced transmission between families and in schools; (5) Long-lasting and strong humoral responses can be obtained early in life through existing SARS-CoV-2 vaccines at lower doses, reducing side effects and adverse events.70,71
Moreover, parental fear of Covid-19 is an important factor that influences the decision to vaccinate children.72 Therefore, to enhance valid parental perceptions of Covid-19, governments should proactively provide scientific communication and share data in a timely manner. Commitment to child health must begin with the elimination of vaccine hesitancy.
Effective vaccines have been developed, and it is up to government agencies to devise and implement the means to ensure the production and distribution of sufficient vaccines to populations of all ages in high-, middle-, and low-income countries, in urban and rural areas, and at an accessible cost. To succeed in these efforts, the authors also need to overcome vaccine hesitancy, including for children. Therefore, politicians, health professionals, and scientists must work together and provide critical real-world data on the safety and efficacy of vaccines, as well as a uniform message on the importance of vaccination to ensure protection against the effects of the Covid-19 pandemic for all populations.73
The authors must clearly communicate the characteristics of the available pediatric vaccines, how they work, what their limitations are, and why the benefits outweigh the risks.74 The authors must take advantage of the global successes of the well-established pediatric vaccine regimens used in routine vaccination schedules, providing a better chance of success in overcoming the unprecedented global effects of this pandemic.