To assess the BMI among children with Growth Hormone Deficiency (GHD) and Idiopathic Short Stature (ISS) and its correlation to ghrelin, Growth Hormone (GH), and Insulin-like Growth Factor-1 (IGF-1) levels.
MethodsA cross-sectional descriptive study in which 42 patients attending the Pediatric endocrine clinic were enrolled, allocated into two groups: group I: GHD children; group II: ISS children. Ghrelin, IGF-1 and GH in both groups were measured.
ResultsGhrelin was significantly higher among GHD group (p < 0.001). Overall, there was a strong negative correlation between IGF-1 and ghrelin (r = -0.977, p-value = < 0.001) while a moderate positive correlation between ghrelin and BMI (r = 0.419, p-value = 0.006). There was a weak positive non-significant correlation between IGF-1 and BMI (r = 0.276, p-value = 0.077). In GHD group, there was a weak positive non-significant correlation between ghrelin and GHmax measurement (r = 0.052, p-value = 0.824), while a weak negative non-significant correlation between both variables in ISS group (r = -0.243, p-value = 0.288). In GHD group, there was a moderate positive correlation between ghrelin and BMI (r = 0.500, p-value = 0.021), but weak negative non-significant correlation between both variables in ISS group (r = -0.255, p-value = 0.265).
ConclusionThere was a negative feedback loop between ghrelin and IGF-1, whereas a positive feedback between ghrelin and BMI. BMI was more affected in the ISS group but was non-significantly correlated with ghrelin. There was no significant compensatory response of ghrelin suggesting its contribution to the pathogenesis of ISS.
Idiopathic short stature (ISS) is recognized as a clinical description, not a disease. There is no identifiable pathological cause making it a normal variant of growth with height less than 2 SD below the mean height corresponding to the same age, sex and ethnicity.1 In addition, management remains challenging with many therapeutic options on an individual basis.
Ghrelin is a pleiotropic gut hormone controlling the gut-brain axis. It influences the secretion of growth hormone as one of its actions.2 it is considered as a potent appetite stimulator and believed it may affect growth through insulin-like growth factor-1 (IGF-1) production through growth hormone secretion as well as enhancing the food intake through the orexigenic effect.3
Variable results have been reported when trying to assess the negative feedback loop between GH/IGF-1 axis and ghrelin.4
The current study aimed at measuring the correlation between ghrelin, growth hormone (GH), and IGF-1 levels and the anthropometric measurements among children with short stature in order to find out whether ghrelin influences nutritional status through GH/IGF-1 axis.
MethodsA cross-sectional descriptive study was conducted at the Pediatric endocrine clinic, Suez Canal University Hospitals from December 2017 to October 2018.
Patients were enrolled in this study after History taking, clinical examination and laboratory tests. The proposal for this study was approved by the Research Ethics Committee, faculty of medicine at Suez Canal University. Written informed patient consent was obtained from each subject before the study.
Children presented with proportionate short stature according to the Egyptian growth chart, of both genders, aged under 10 years were included. Children with familial short stature, chronic diseases, clinical syndromes (e.g., Turner), hormonal deficiency other than growth hormone (thyroid…etc), or skeletal dysplasia were excluded.
Patients were subjected to auxological assessment of weight (using a Seca weighing scale), height (using a stadiometer), and body mass index (kg/m2). The results were plotted on the WHO Z-score available for the corresponding age and gender.
Bone age was also done and calculated according to Greulich-Pyle's standards.
Under complete aseptic conditions, peripheral venous blood was collected and all of the following were measured: ghrelin levels, IGF-1 and thyroid profile.
Growth hormone provocation was done initially by clonidine to all the patients, and if Peak GH concentration (GHmax) below 10 ng/mL, then the authors proceeded to insulin tolerance test (ITT).
The study population was divided into 2 groups:
- •
Group I: Growth hormone deficiency group (GHD) children
Defined as height more than 2 standard deviation below the mean of the corresponding age and gender, with delayed bone age more than 1 year and GHmax below 10 ng/mL in two growth hormone provocation tests.5
- •
Group II: idiopathic short stature (ISS) children
Defined as height more than 2 standard deviation below the mean of the corresponding age and gender, and provoked growth hormone (GHmax ≥ 10 ng/dL) without other causes of short stature.1
The collected data were organized, tabulated and statistically analyzed using statistical package for social sciences (SPSS) version 21 (SPSS Inc, Chicago, USA), running on IBM compatible computer. For qualitative data, frequency and percent distributions were calculated. For quantitative data, mean and standard error (SE) were calculated.
ResultsThis study included a total of 42 short stature children under the age of 10 years old. They were divided into two equal groups, the GHD group (group I) and the ISS (group II). Both groups were matched regarding age in years (7.48 ± 1.66, 7.43 ± 1.47 respectively) as well as gender and family history. On the other both groups differed significantly regarding weight p ≤ 0.001, height p = 0.04, body mass index (BMI) p = 0.01 and bone age (p = 0.002)
GHD was more affected in bone age (4.57 ± 1.16 years), the SDS of height, and weight (-3.17 ± 0.17, -2.92 ± 0.27) respectively. However, BMI-SDS (-0.63 ± 0.07) was higher than the ISS group (-0.68 ± 0.06).
The ISS bone age, the SDS of height and weight were (5.71 ± 1.13 years, -2.59 ± 0.15, -2.75 ± 0.25) respectively.
There were statistically significant differences (p ≤ 0.001) between all of the growth hormone measurements after clonidine induction. The Peak GH concentration (GHmax) for the ISS group was nearly five times higher than the corresponding value in the other group (12.3 ± 0.62, 2.2 ± 0.25) ng/mL respectively.
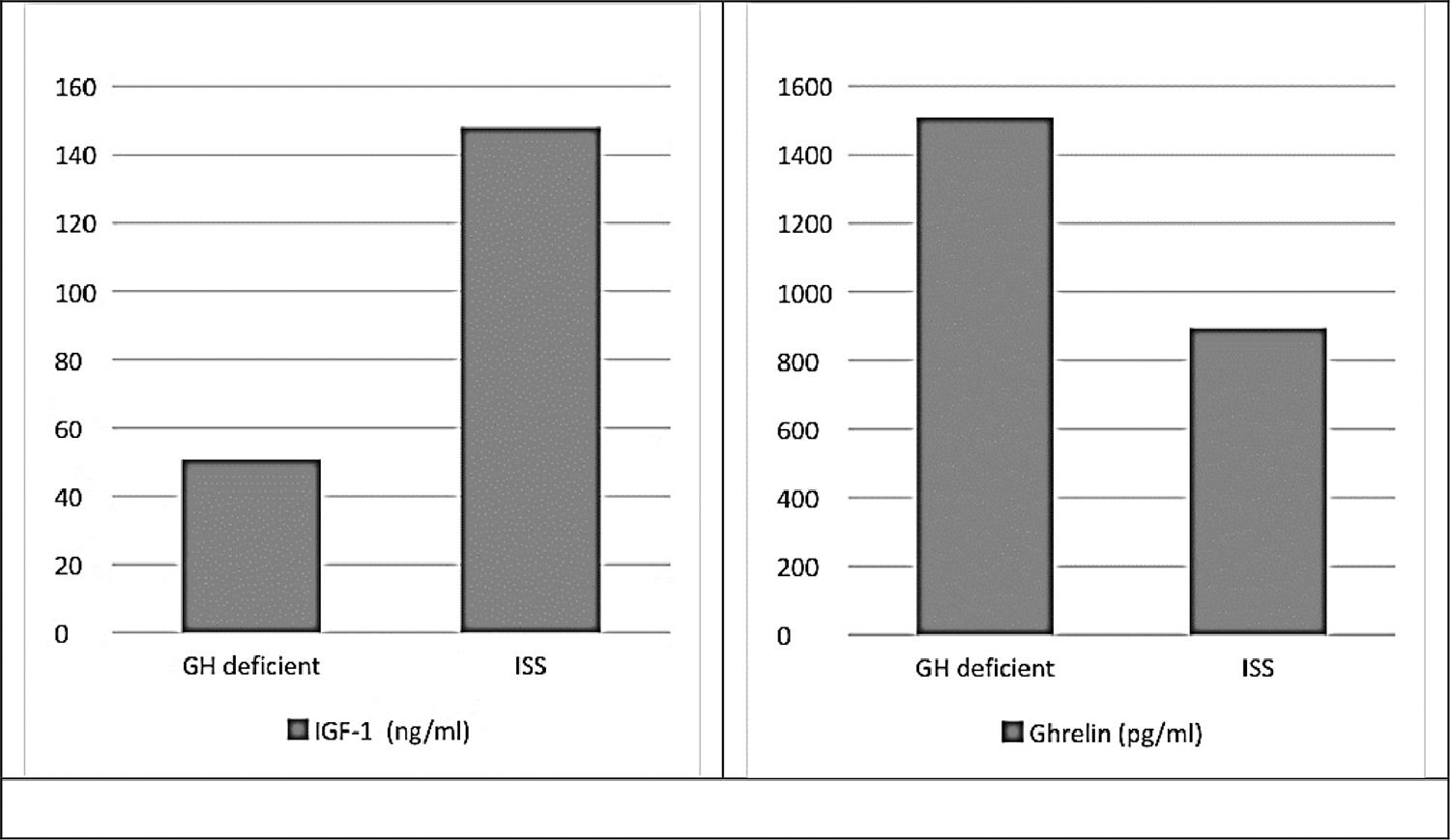
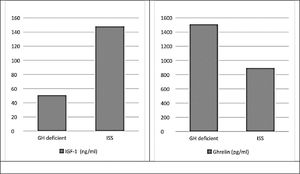
There were statistically significant differences (p ≤ 0.001) between study groups regarding levels of insulin-like growth factor-1 and ghrelin, which was the expected physiological response (Fig. 1).
The whole study population showed a strong negative statistically significant correlation between insulin-like growth factor-1 and ghrelin (r = -0.977, p-value ≤ 0.001) as well as between ghrelin and GHmax measurement in the whole study population (r = -0.993, p-value ≤ 0.001). However, there was a strong positive statistically significant correlation between IGF-1 and GHmax in the whole study population (r = 0.976, p-value ≤ 0.001).
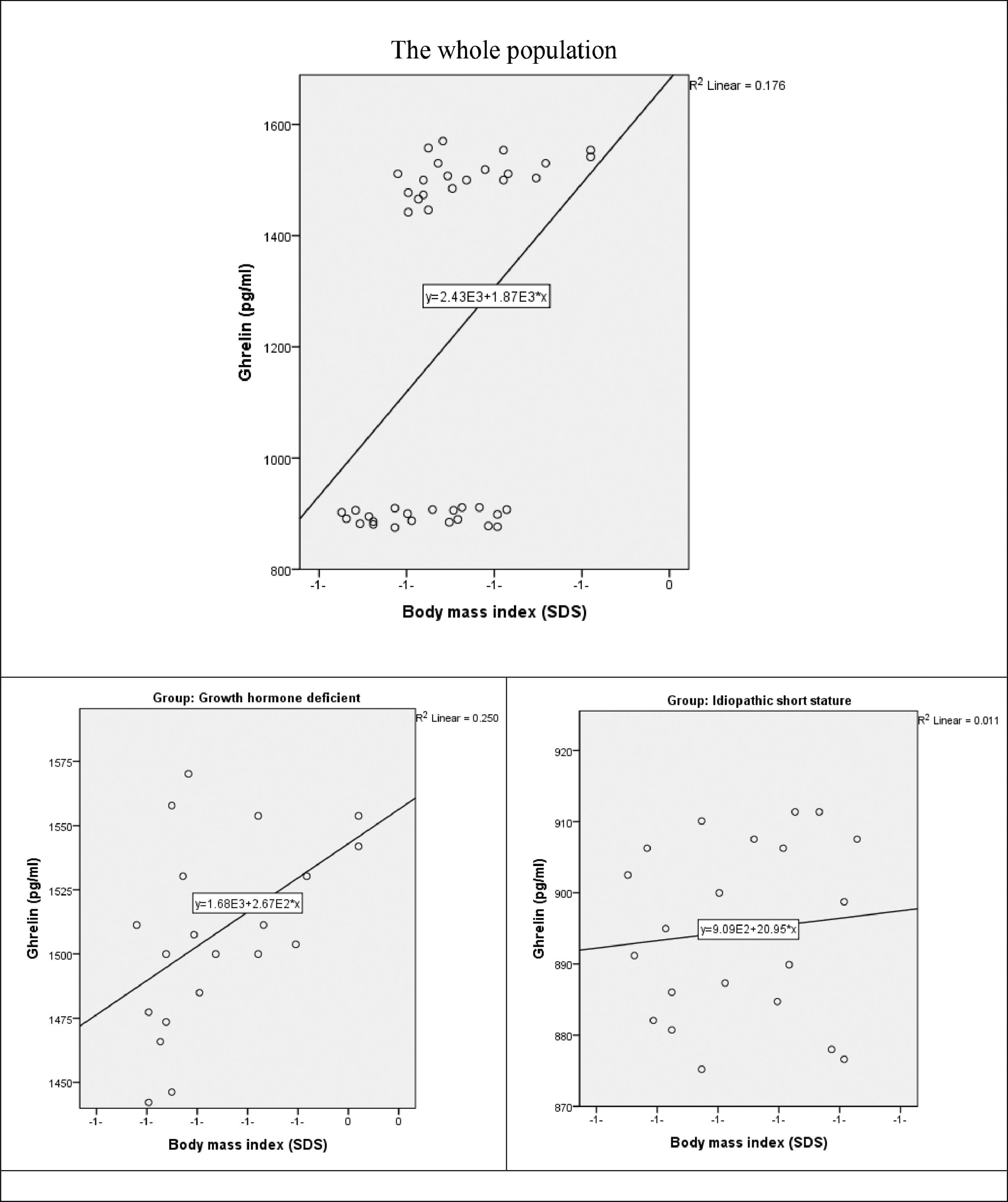
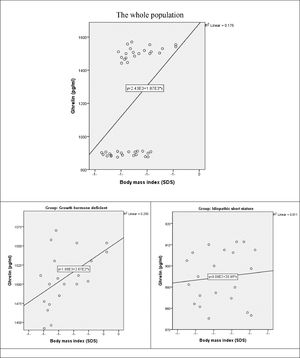
There was a moderate positive statistically significant correlation between ghrelin and BMI in the whole study population (r = 0.419, p-value = 0.006). the correlation was preserved in GHD group (r = 0.500, p-value = 0.021). on the contrary in ISS group, there was a weak positive non-significant correlation between both variables (r = 0.106, p-value = 0.648) (Fig. 2).
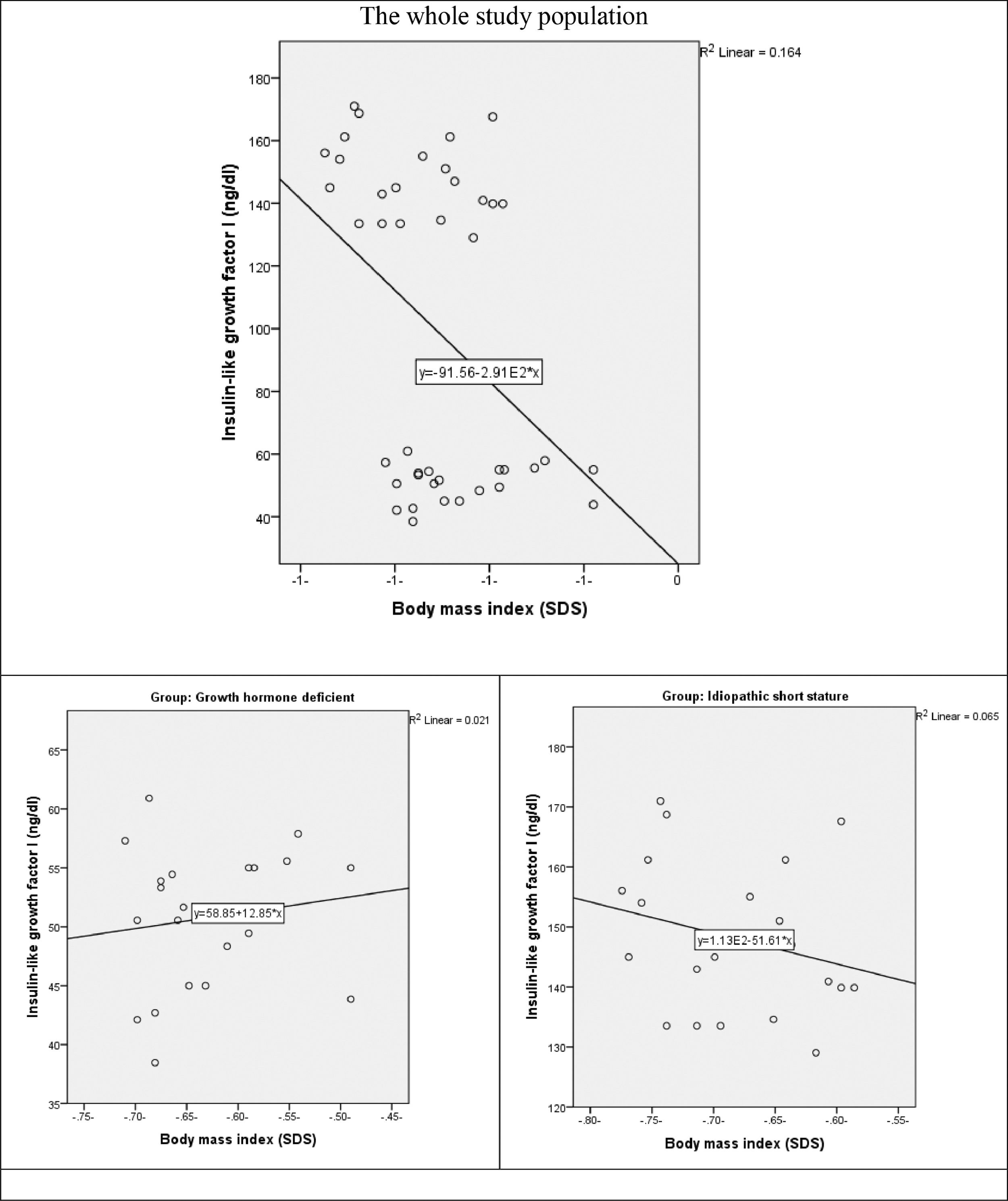
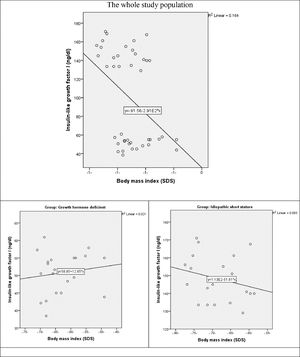
The correlation between IGF-1 and BMI revealed a weak positive non-significant correlation the whole study population (r = 0.276, p-value = 0.077). as well as in GHD group (r = 0.144, p = 0.534) There is a weak negative non-significant correlation between both variables in ISS group (r = -0.255, p-value = 0.265) (Fig. 3).
The blunted response of ghrelin with no significant compensatory response may play a role in the pathogenesis of ISS.
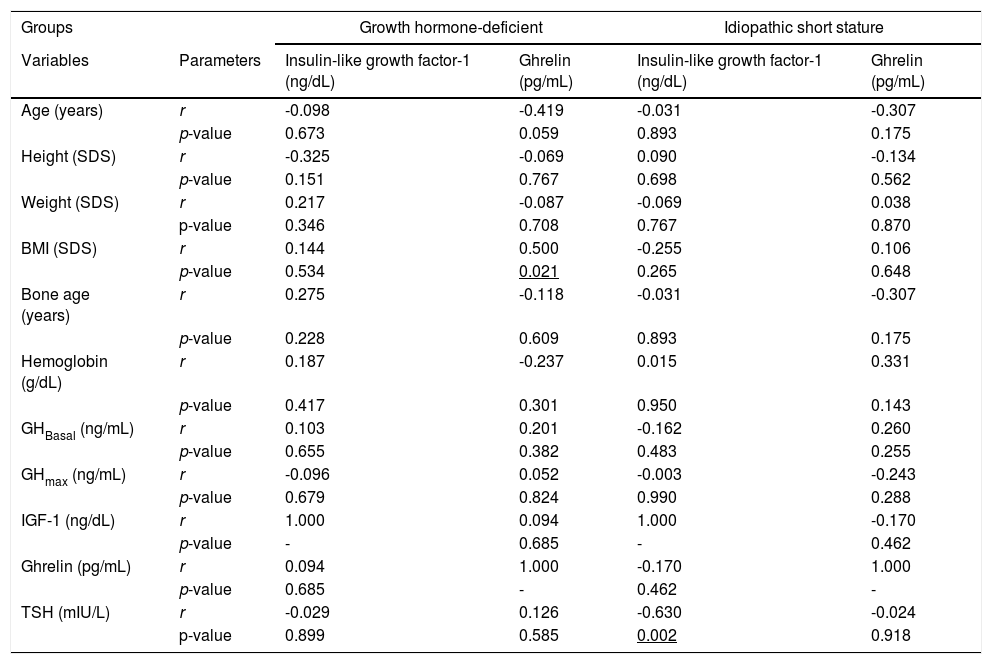
There was no significant correlation with the other different variables of the study in the separate study groups except TSH which showed a negative significant correlation with the IGF-1 in the ISS group only (r = -0.630, p = 0.002) (Table 1).
Correlations between Insulin-like growth factor-1 and ghrelin, and other study variables in each study group.
SDS, standard deviation score. GHBasal, growth hormone measurement at time “0”. GHmax, Peak GH concentration. TSH, thyroid-stimulating hormone. BMI, Body mass index. IGF-1, Insulin-like growth factor-1.
The role of endogenous ghrelin in the growth process of children is unclear. Ghrelin is one of the key hormones that influence the secretion of growth hormone with a significant orexigenic effect and accordingly metabolic effect.6
This study was conducted to determine the role of endogenous ghrelin as a pleiotropic hormone that may influence the growth in children with idiopathic short stature, versus those who are growth hormone deficiency. In the current study, it was obvious that the height is more affected in GHD group as expected, but the BMI is less affected in comparison to the ISS group.
Old and recent studies have shown that the BMI in children with GHD was higher than in children without at the time of diagnosis.7,8 Some studies stated different effects of GH therapy on BMI; one stated that BMI increased with long-term GH therapy,9 another study revealed that no changes in BMI were detected along with the treatment.10
In 2020, Al Shaikh et al. found that BMI decreased significantly in ISS on GH therapy.11 Despite the significant growth rate improvement,12 the controversy between studies revealed that GH alone could not be relied upon to improve the BMI, suggesting the involvement of other factors.
Ghrelin is believed to have a significant role in adjusting the body energy status through stimulation of appetite. It was found to be compelled by neuro-endocrine mechanism, depressed with positive energy balance and increased with the negative balance status.13,14
Yin et al. also commented in the context of lean and short children, that ghrelin may have reflected the nutritional status of those children rather than causing it.13
According to the present study, Ghrelin showed a significant negative correlation with IGF-1 in the studied population, but no significant correlation was found among the GHD group or ISS group separately.
Ghrelin and IGF-1 were found to have a negative correlation in both constitutional and familial short stature in a study conducted by Camurdan et al., which was explained by the possible stimulatory effects of low IGF-1 on ghrelin.15
On the contrary, Stawerska et al. observed that there was no correlation between ghrelin concentration and IGF-I SDS in prepubertal children known to be small-for-gestational-age (SGA) versus appropriate-for-gestational-age (AGA), despite assessing both with normal height and with short stature.16
In the context of nutritional status reflected by the BMI, the present study revealed that ghrelin was negatively correlated with height, weight and BMI within the studied children. There is a moderate positive statistically significant correlation between ghrelin and BMI (SDS) in the GHD group, while a weak negative non-significant correlation in the ISS group.
Wilasco et al. found that in healthy children, total ghrelin and des-acyl ghrelin values did not correlate with any anthropometric parameter and only acyl ghrelin had a weak negative correlation with SDS of the skin fold.17 However, Camurdan et al. study on the constitutional delay of growth and puberty (CDGP) and familial short stature (FSS) reported that Ghrelin was negatively correlated with chronological age, bone age, weight, height, height SDS, and BMI in short stature patients and controls.15 The same results were also reported among children with CDPG18 and ISS.6,19
It is worth mentioning that, serum IGF-1 concentrations were positively correlated with age in prepubertal and early pubertal children while negatively correlated in late puberty.20
In the current study, all the study's patients were prepubertal; and despite being statistically insignificant, the correlation between IGF-1 and age was negative in the ISS group. Moreover, height SDS had an almost blunt response with IGF-1 while negatively correlated with Ghrelin in the same group.
This may be explained by the suboptimal nutritional status in ISS patients that affects both IGF-1 and Ghrelin. IGF-1 is influenced by a wide variety of factors; one of them is the nutritional status.21 Additionally, a compensatory elevation of ghrelin levels might represent an orexigenic stimulator6,8,13,15,22,23 and energy balance controller.24–28 However, Wudy et al. in 2005 didn't find any differences between poor and good eaters in relation to the total ghrelin.22
Interestingly, the authors observed that thyroid-stimulating hormone (TSH) levels in the euthyroid ISS group had only a negative correlation with IGF-1 levels. The same was reported recently in the study done by Adamczewska et al., in 2020.29 They found that ghrelin levels were closely related to TSH but the authors couldn't find such a correlation in the present study's sample.
On the contrary, Smyczynska et al., in 2010, found no correlation between the thyroid profile and IGF-1 before administration of rGH to GHD patients, and the response change after around 6 months of replacement therapy where there was a decrease in thyroid function without significant IGF-1 elevation.30
“The contribution of the findings in deterioration of growth processes requires further studies”.29
In conclusion; there was negative feedback between ghrelin and IGF-1 as well as GH, while positive feedback with BMI.
In the ISS group, BMI was more affected, with only a negative non-significant correlation with ghrelin. The results reflected the nutritional affection of the ISS patient. Additionally, the role of Ghrelin may contribute to idiopathic short stature pathogenesis.
Only TSH levels showed a negative significant correlation with IGF-1 levels in the euthyroid ISS.
A larger cohort is recommended to evaluate the contributing factors of short stature in ISS children and to check the role of ghrelin administration as a therapy in cases of ISS to improve their nutritional status and hence anthropometric measurements.