To correlate the nasal anatomical characteristics of newborns with the dimensions of short binasal prongs.
MethodObservational, cross-sectional study carried out in two hospitals in southern Brazil. The authors evaluated 1620 newborns with neonatal data and nasal dimensions. To measure the dimensions of the nasal region, the authors considered the width of the medial columella, the right nostril diameter, and the left nostril diameter. These data were correlated with the dimensions of two models of short binasal prongs.
ResultsOf the total newborns evaluated, 807 were female (49.8%), and 813 were male (50.2%). The majority were white (96.2%). The mean gestational age was 37.4 ± 2.9 weeks, ranging from 22 to 42 weeks. The birth weight was 2946.8 ± 699.3 g, ranging from 490.0 to 4740.0 g. Most of the nasal measures were significantly larger than both prong model measurements.
ConclusionThe sizes of short binasal prongs available on the Brazilian market do not match the nasal anatomical characteristics of newborns.
The newborns have anatomical and physiological characteristics that make them more vulnerable to respiratory failure.1 Because of this, they usually require ventilatory support from the first hours of life.2 Although invasive mechanical ventilation is important to improve the survival of these newborns, its use increases the risk of infection and lung injury.3,4 Furthermore, the long time on this ventilatory support is associated with increased mortality, neurological impairment, bronchopulmonary dysplasia, and retinopathy of prematurity in the postnatal period.5 For this reason, non-invasive ventilation (NIV) has become a widely used alternative to avoid intubation or prevent it from occurring early, thus contributing to the reduction of pulmonary complications associated with invasive mechanical ventilation.6,7
The NIV most used in neonatology is Continuous Positive Airway Pressure (CPAP).8 CPAP has been widely used in Neonatal Intensive Care Units (NICU) as a standard mode of NIV since it was introduced by Gregory et al. in 1971.9 It is necessary to use an interface that connects the circuit to the upper airways of the newborn to provide positive pressure.10 The latter is essential for delivering ideal positive pressure to the newborn.11
The short binasal CPAP prong is the most used device for providing NIV in neonatology,12 so it offers less airflow resistance and is more effective in preventing reintubation than nasopharyngeal prongs.13-16
The small size of the nostrils and facial structures, especially in premature infants, makes choosing the appropriate size for the interface challenging.17 Therefore, selecting the prong is based on the correlation between size and weight at NIV installation.12 However, only sometimes choosing the prong based on the correlation between size and weight will guarantee that the newborn will receive an adequate size prong because the characteristics and anatomical features vary between newborns.18
Some manufacturers offer devices that measure the diameter of the nostrils and indicate their respective sizes.17 However, in clinical practice, using these devices does not guarantee that the chosen prong is ideal for the newborn, as there is little variety of sizes available in the market, and the existing sizes often need to suit the anatomical characteristics of the noses of newborns. Furthermore, the distance between the insertion catheters and their diameter is fixed and does not allow them to be adjusted according to the anatomy of each newborn.
To investigate the difficulties related to short binasal CPAP prongs, this study aims to correlate the anatomical characteristics of newborns with the dimensions of commercial short binasal prongs.
MethodsThe present research was approved by the Ethics Committee on Research Involving Human Beings of the Pontifícia Universidade Católica do Paraná (number: 3.546.615). It is a cross-sectional study with prospective data collection on the anatomical dimensions of the nasal region of newborns and their correlation with the sizes of short binasal prongs used in NIV. Data were collected at Hospital Infantil Waldemar Monastier (HIWM) NICU, and Rocio Hospital NICU maternity from December 2018 to March 2020. All newborns admitted up to 28 days of life were included in the study. The newborns with facial malformations, choanal atresia, hemodynamic instability, or who used NIV before the assessment were excluded. Of the 1,620 newborns included in the sample, 73 were evaluated in the HIWM NICU, 1,343 were in the rooming-in care of Rocio Hospital, and 204 were in the Rocio Hospital NICU.
The variables collected were neonatal data and dimensions of the nasal region. The neonatal data collected were gender, gestational age, birth weight, weight on the date of the assessment, and race (considering the classification of the Brazilian Institute of Geography and Statistics as yellow, white, indigenous, brown, or black). To define race, the researcher and the evaluator considered the information in the medical records and the skin color of the newborn. To measure the dimensions of the nasal region, the width of the medial columella (WMC), right nostril diameter (RND), and left nostril diameter (LND) were considered, all measured in millimeters. Two healthcare professionals were assigned to collect the data to ensure the measurements' accuracy and consistency. The team comprised a physiotherapist, the principal researcher, and a nursing technician trained in properly using measuring instruments.
For healthy newborns, data collection occurred during their stay in the maternity ward's unit on the first or second day of life, during nursing care and evaluation by the Pediatrician. For newborns requiring NICU assistance, data was collected during the NICU stay when the newborn was hemodynamically stable. To assess the dimensions of the nasal region, the newborn was positioned in dorsal decubitus in the heated cradle, simple cradle, or incubator, and the evaluator used a previously calibrated Kingtools® digital caliper 150 mm, making three consecutive measurements for each evaluated item. The scale was calibrated before the measuring of the newborn's weight (Welmy® brand, model 109 E - 15 kg). Then, after removing the clothes, the newborns were positioned in dorsal decubitus on the scale shell to weigh the newborn.
The statistical program Statistica® v.10 – Statsoft was used. A minimum significance level of 5% was considered for all tests, and the sample gives a test power of 95%. Central tendency and dispersion were expressed as means and standard deviation (SD) for continuous variables with a symmetric distribution. In medians, minimum and maximum values (median, minimum–maximum) for those with the asymmetric distribution. The categorical variables were expressed in absolute and relative frequency.
The authors considered three measurements to analyze the nose taken from the WMC, RND, and LND. The most adjusted average of the three measurements was obtained by: evaluation of their differences by the Bland-Altman Method, estimation of the degree of difference by the t-test for a sample, verification if the differences between the measures are different from zero and the degree of agreement by Lin's coefficient of agreement. All these evaluations were made according to the weight of the evaluation date according to the classifications of the GMI® and Fanem® prongs. Finally, the decision of which measures to calculate the mean was taken based on the t-test for one sample and Lin's coefficient of agreement.
Pearson's concordance coefficients were calculated to estimate the degree of association between birth weight and nose measurements, considering coefficients between 0.00 and 0.19 as very weak correlation, between 0.20-0.39 as weak, between 0.40-0.69 as moderate, between 0.70-0.89 as strong, and between 0.90-1.00 as very strong.19
The WMC, RND, and LND measurements were compared using the t-test for one sample, considering the sizes and classifications determined by the manufacturer.
The sample was estimated according to the sample calculation formula for comparing two groups according to unpaired quantitative variables, considering a mean of 5.0 mm in one group, 3 mm in the other group, a standard deviation of 0.60 and 0.50, respectively, magnitude of the effect of 2 mm, significance level of 1%, type II error of 5%, test power of 95%, with the estimate pointing to a sample size of 150 cases, with a minimum number of 15 cases in the lowest gestational age ranges.
ResultsThe sample comprised 1620 newborns, 807 female (49.8%) and 813 male (50.2%), with a mean gestational age of 37.4 ± 2.9 weeks, ranging from 22 to 42 weeks. The newborns had an average birth weight of 2946.8 ± 699.3 g, ranging from 490.0 to 4740.0 g. It was found that 3% of the NBs weighed less than 1250 g, 7% weighed between 1250 g and 2000 g, 36% between 2000 g and 3000 g, and 54% of the NBs weighed more than 3000 g. In addition, most of the newborns were white (96.2%).
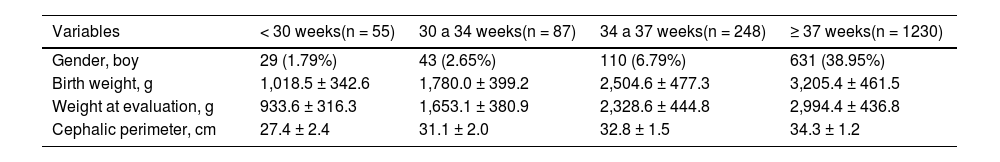
Table 1 presents the characteristics related to gender, birth weight, and weight measured on the day of evaluation of the newborns according to the classification of gestational age. The average weight on the assessment date was 2749.9 ± 659.9 g, ranging from 490 to 4740 g.
General characteristics of newborns according to gestational age classification.
Note: Gender – absolute and relative frequency; Birth weight; Weight at evaluation – mean and standard deviation.
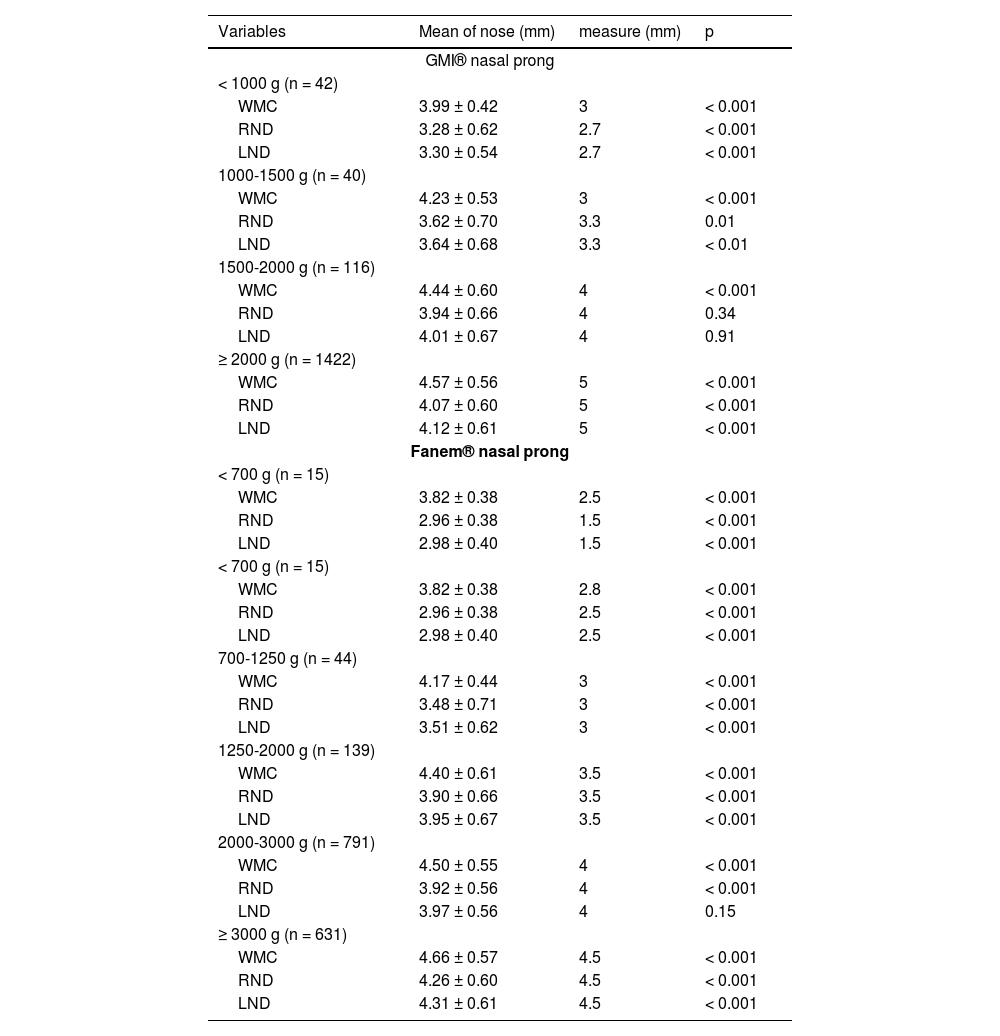
There was no strong correlation (r > 70) between any nose measurement and birth weight for both brands evaluated.
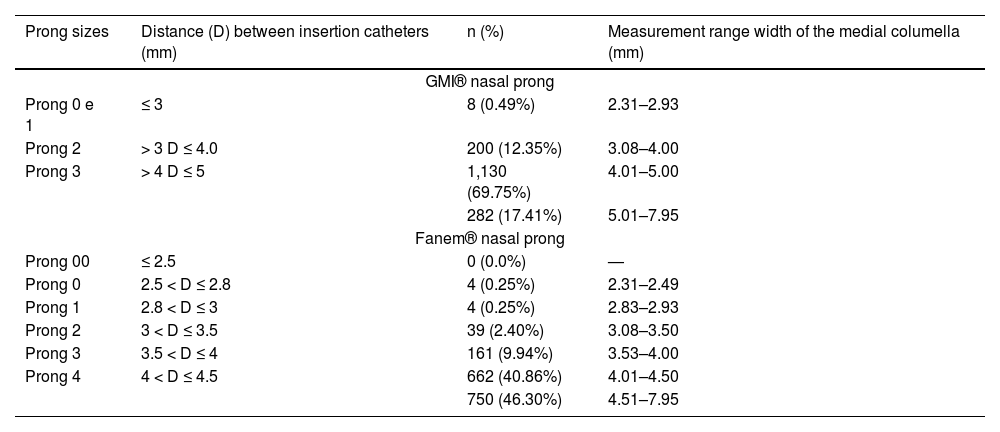
The anatomical characteristics of the newborns were correlated with the weight at evaluation according to the classification of the GMI® and Fanem® prongs. GMI® prongs are available in four sizes, and Fanem® prongs are in six sizes.
Table 2 shows the comparison between nose measurements and GMI® and Fanem® nasal prongs. The nose measurements were, for the most part, significantly larger than the prong measurements.
Comparison of nose with nasal prongs GMI®, and Fanem® measurements according to weight (n = 1620).
Note: t-test for one sample; WMC, width of medial columella; RND, right nostril diameter; LND, left nostril diameter.
Some manufacturers offer devices that measure the diameter of the nostrils and indicate their respective sizes, the width of the medial portion of the nasal columella of newborns and the diameter of the right and left nostrils were compared to the dimensions of both prongs evaluated, without considering the weight of the newborn.
Table 3 shows the measurements of frequency distribution of newborn's medial portion of the nasal columella according to the GMI® and Fanem® prong dimensions classification. For GMI® prong sizes, in 282 newborns (17.41%), the measurement of the medial portion of the nasal columella was larger than the largest available prong size, being inadequate for the use. In 1338 newborns, the dimensions of the prongs, theoretically, would meet the measurements observed in the newborns. For Fanem® prong sizes, in 750 newborns (46.30%), the width of the nasal columella was greater than the largest prong size available. That is, they would be inadequate for the use. On the other hand, in 870 newborns, the dimensions of the prongs would meet the measurements observed in the newborns.
Frequency distribution of measurements of the medial portion of the nasal columella of newborns according to the GMI® and Fanem® prong dimensions classification (n = 1620).
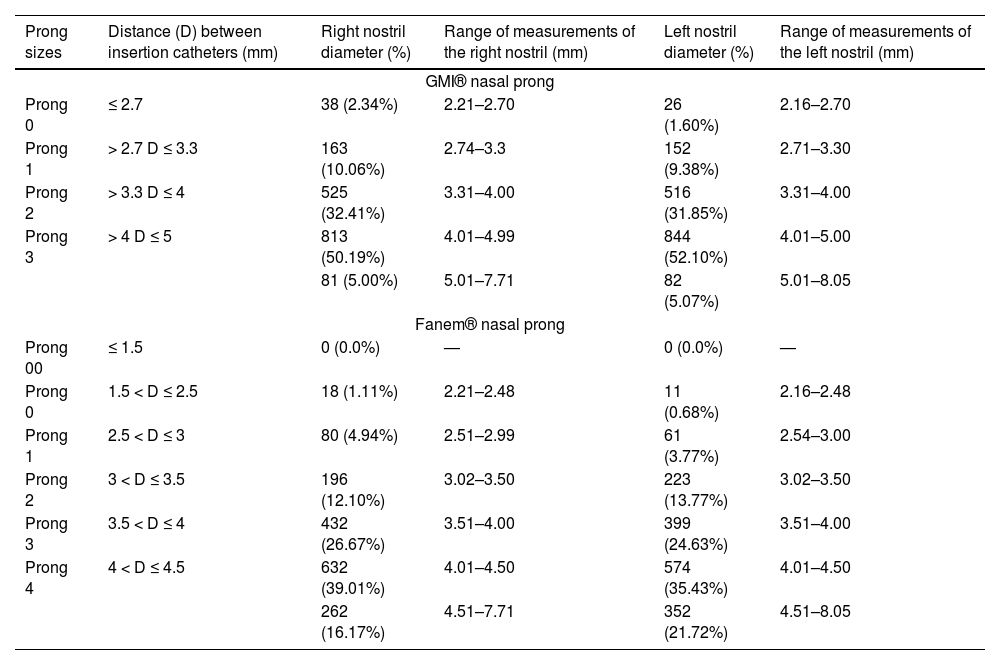
Table 4 shows the frequency distribution of newborns' right and left nostril measurements according to GMI® and Fanem® prong dimensions. For GMI® prongs, the authors observed that in 81 newborns (5.00%), the diameter of the right nostril was greater than that of the insertion catheters. As for the left nostril, this occurred in 82 newborns (5.07%), indicating that the largest prong (size 3) would be too small for these newborns. For Fanem® prongs, it was observed that in 262 newborns (16.17%), the diameter of the right nostril was greater than the diameter of the insertion catheters. Regarding the left nostril, this occurred in 352 (21.72%), indicating that the largest prong (size 4) would be small for these newborns, allowing loss of positive pressure during NIV application.
Frequency distribution of newborns' right and left nostril measurements according to GMI® and Fanem® prong dimensions (n = 1620).
The present study evaluated the dimensions of the nasal region of newborns, aiming to compare them to the commercial existing sizes of two models of prongs used in the reported NICU concerning newborn weight. In clinical practice, it is common to observe that the prong chosen by the health professional does not meet the anatomical characteristics of both premature and term newborns, thus favoring the occurrence of nasal injury.
It was found that there is no strong correlation between the measurements of the newborn's nose and birth weight, both for the GMI® and Fanem® prongs. In this way, the choice of prong size according to the newborn's weight becomes unfeasible since the chosen prong will not reflect the real needs of the newborn, forcing the care team to choose a new prong size. In addition, this fact increases health expenses due to material waste and increases the handling time, causing stress in the newborn.
Besides the option of choosing the prong size by weight, Fanem® now offers a specific ruler with the demarcations of the diameters of the prongs and the distances between the insertion catheters so that the professional can choose the size of the prong that is most appropriate for the newborn. However, for 46.30% of the newborns evaluated in this study, the largest prong available by Fanem® would be too small for the newborn, making it difficult to adapt and compromising the effectiveness of the treatment.
The authors found that in newborns weighing less than 2000 g, the width of the medial portion of the newborn's nasal columella was significantly greater than the distance between the insertion catheters of the corresponding GMI® prongs. This aspect could cause nasal injury since the septum would be clamped due to the inadequate size of the prong.20
For newborns weighing 2000 g or more, the distance between the GMI® prong insertion catheters would be adequate. However, the nostril diameter was significantly smaller than the prong diameter, and the insertion catheters would not enter the nostrils. According to Alessi,21 prongs with large insertion catheters can cause blanching, compression, or necrosis in the nostrils, resulting in permanent nasal disfigurement.
When comparing the width of the medial portion of the nasal columella of newborns with the distances of the insertion catheters of the Fanem® prongs, it was found that for all groups of studied weights, the septum measurement was significantly greater, indicating that the prong would pinch the columella, which can cause nasal damage.
When the dimensions of the GMI® prong insertion catheters were considered intended for premature infants weighing less than 1000 g, and those of the Fanem® for premature infants weighing less than 2000 g and the diameter of the nostrils of those newborns, the authors observed that the prong would be small for the referred newborns. The prong with an insertion catheter smaller than the nostril diameter allows increased mobility of the prongs, which may result in nasal injury.20 In addition, when the insertion catheters do not adjust appropriately to the opening of the nostrils, there is a loss of positive pressure, compromising the effectiveness of the treatment. Finally, professionals may mistakenly squeeze the prong over the nasal structures to compensate for the leak, increasing the chances of nasal injury.17
The results of this study indicate that the existing sizes do not meet the characteristics of newborns and that in clinical practice, this aspect justifies the difficulties encountered by health professionals in adapting the prong to the newborn. Therefore, medical equipment manufacturers should consider offering a wider variety of standard sizes of CPAP nasal prongs based on the anatomical characteristics of newborns. This would allow healthcare professionals to choose the most appropriate size based on the newborn's characteristics, helping to improve the treatment adjustment and effectiveness. Furthermore, the development of a prong size selection guide based on anthropometric measurements, such as distance between nostrils, nostril width, and other relevant craniofacial characteristics, would help healthcare professionals choose appropriate nasal prongs based on NB measurements. So, the professionals' training is essential for therapy's success. They must be aware of individual differences in the anatomical characteristics of newborns and be able to evaluate and select appropriate nasal prongs based on these characteristics and maintain their proper positioning.
An interface with an adequate size is essential to ensure that the newborn receives the necessary pressures and enjoys the benefits of NIV. In addition, it reduces the risk of nasal injury, optimizes the professional's work since he spends less time trying to adapt the prong to the nostrils, and reduces public spending on health, both by preventing waste with materials and by reducing the expenses allocated to the treatment of comorbidities associated with nasal injury.
A limitation of this study was the small sample number of premature infants, mainly those weighing less than 700 g. The limitation occurred because the inclusion criteria comprised only hemodynamically stable newborns without NIV before the assessment. Currently, the use of CPAP in the delivery room is a reality in many Brazilian NICUs, and it has been widely used in premature infants to avoid intubation and its adverse consequences on newborns. The present sample consisted predominantly of newborns with a birth weight of 3,000 grams or more. This sample composition may have influenced the findings and should be considered when interpreting the results. However, the authors emphasize that the present research was based on the available sample and the demographic characteristics of the newborns.
The findings of this study highlight a significant mismatch between the nasal anatomical characteristics of newborns and the dimensions of available short binasal CPAP prongs in Brazil. This disparity challenges healthcare professionals, hindering adequate NIV provision to newborns. This study offers important insights into relationships between anatomical characteristics and dimensions of short nasal prongs in a diverse population of newborns, which may have relevant clinical implications for using these prongs in neonatology.
Future research and collaboration between medical device manufacturers, clinicians, and researchers should focus on developing appropriate prong sizes that cater to newborns' specific nasal anatomical characteristics, ensuring optimal NIV delivery and reducing the associated challenges and complications. Furthermore, future research investigating the effects of prong dimensions on gas escape, positive pressure loss, and device mobility is fundamental to understanding the effectiveness and safety of this equipment.