Clinical-laboratory comparison of a population of children and adolescents with DM1 followed at a Brazilian outpatient university clinic, at two different periods (2014 and 2020), regarding changes made both to the insulin therapy scheme and to the nutritional approach to carbohydrate counting.
MethodsThe data of patients with DM1 aged 0–19 years enrolled in the service in 2014 and 2020 were collected. Student's t-test was performed to compare the means of HbA1c and the variables of interest.
ResultsNPH + regular insulin was predominantly used in 2014 (49.1%), while in 2020, the predominance shifted to insulin analogs (48.4%). Pump use tripled from 1.3% in 2014 to 4.4% in 2020, and the percentage of patients performing carbohydrate counting reduced from 28.3% to 17.8%. Regarding HbA1c, the 2014 group of patients had a mean of 9.8%, while the 2020 group had a mean of 9.6% (p = 0.49).
ConclusionThe change in treatments between 2014 and 2020 did not result in a significant improvement in HbA1c levels. However, it was identified the importance of carbohydrate counting and the use of insulin analogs to improve metabolic control in this population at both times.
Diabetes mellitus (DM) is a complex metabolic disorder characterized by chronic hyperglycemia resulting from defects in insulin secretion, insulin action, or both.1 Type 1 DM (DM1) treatment involves insulin therapy, monitoring, and education.2 Insulin therapy regimens should mimic the physiological pancreatic secretion of insulin. The strategy of choice is basal-bolus therapy with multiple daily insulin applications (MDI) or an insulin infusion system (IBS). Glycemic control is monitored by measuring glycated hemoglobin (HbA1c) levels, associated with self-monitoring of capillary blood glucose (AMGC) and continuous device-based interstitial fluid glucose monitoring systems (SMCG).3
For children and adolescents, the recommendation is to keep the percentage of HbA1c below 7.0%. The target of 7.5% is restricted to some cases: inability to recognize and manage hypoglycemia, history or unawareness of severe hypoglycemia, and lack of access to insulin analogs. The Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS) have shown that keeping HbA1c below 7% reduces microvascular complications in DM1.1
The pediatric endocrinology division of the Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto (HC-FMRP-USP) has a specific service for the follow-up of children and adolescents with diabetes. It is a reference center for diabetes treatment in the public health system in a region with nearly 3 million inhabitants.
Due to the high prevalence of laboratory tests showing a lack of metabolic control in people with diabetes in follow-up in the year 2014, the following were implemented: a complete multidisciplinary team, trained and specific for the care of patients with diabetes; change of the place of care with an increase in the number of rooms for individual care, including the non-medical team; specific protocol for nutritional guidance and follow-up, including with specific group for carbohydrate counting guidance; activities with parents, such as cooking workshops; training of pediatricians of the municipal network and the Regional Health Directorate for early diagnosis and conduct in pediatric intercurrences in patients with diabetes; telephone access to the medical team available full time; and implementation of a specific outpatient clinic for new technologies in diabetes. These changes have been implemented, but their effectiveness has not been measured. It seems important to evaluate whether there has been a change in metabolic control parameters and anthropometric parameters.
This study made a comparison using an evolutionary description of the clinical and laboratory profile of patients with DM1 being followed in the service in 2014 and 2020 to measure the effectiveness of these changes.
MethodsThis was a historical and, subsequently, descriptive clinical trial. Study population: children and adolescents with DM1, from 0 to 19 years of age, followed for at least 12 months before data collection, before and after incorporation of nutritional guidance based on carbohydrate counting, long and fast-acting insulin analogs, and continuous subcutaneous insulin infusion systems, composing the following subgroups: a) Group 2014 (n = 225/323); b) Group 2020 (n = 225/334). Excluded were: patients with comorbidities and those with other forms of diabetes (MODY, type 2 DM, and neonatal DM).
The following data were obtained from the medical records: age, sex, self-reported ethnicity, weight and height for body mass index (BMI) calculation, type of insulin used, use of carbohydrate counting, use of IBS, presence of diabetic ketoacidosis at diagnosis, and the average of the last 3 HbA1c values. The percentage of HbA1c was measured via an enzymatic method using high-performance liquid chromatography (HPLC). Values below 5.7% were considered normal, and those above 6.1% were considered high.
The 2006–2007 WHO curves for individuals aged 0–19 years were used to calculate BMI/age percentiles. All data were stored in a spreadsheet. Descriptive statistical analysis was performed, and the mean and standard deviation were calculated. Comparisons were made between the means of the data at both time points using the Student's t-test; p < 5% was considered statistically significant. R software (The R Project for Statistical Computing, Vienna, Austria) was used for statistical analysis.
The project was approved by the local Ethics Committee of HC-FMRP-USP (approval no: 4.191.296).
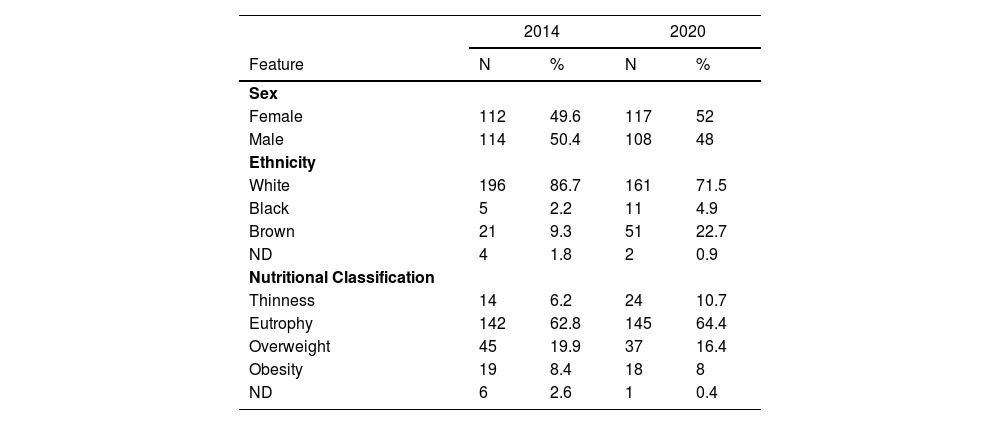
ResultsThis study included 225 patients with DM1 in 2014 and 225 patients in 2020. Only two patients in follow-up in 2014 remained in the service until 2020. The age of the patients with diabetes ranged from 2 to 19 incomplete years (mean: 12 ± 3). Table 1 presents the distribution by sex, ethnicity, and nutritional status. The diagnosis was made during an episode of ketoacidosis in 2014 in 46.5% of patients with diabetes and 50.7% in 2020.
Patient characteristics.
N, number;% percentage of total; ND, not described in the medical record.
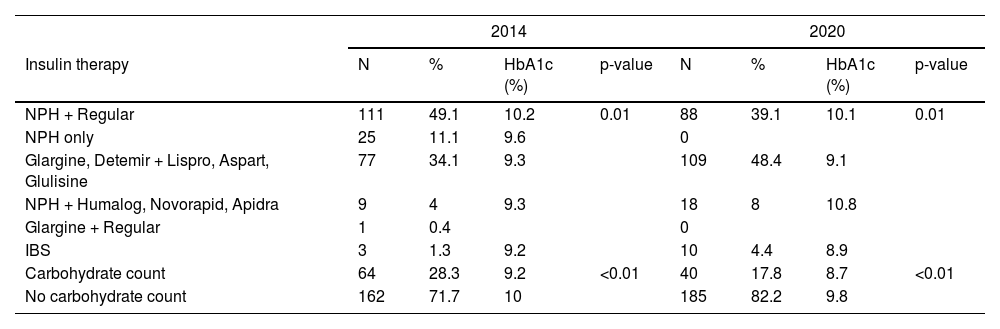
Table 2 presents the insulins used and their combinations. There was a predominance of the use of NPH + regular in 2014 (49.1%) and insulin analogs in 2020 (48.4%). IBS was used in 1.3% of patients with diabetes in 2014 and 4.4% in 2020. The percentage of patients counting carbohydrates decreased from 28.3% to 17.8%. Mean HbA1c levels were compared using Student's t-test in the following groups of patients: those using NPH + regular and NPH alone versus patients using glargine or determir analogs with lispro, aspart, or glulisine, and patients who did carbohydrate counting versus patients who did not.
Types of insulin used and mean HbA1c values.
HbA1c, glycated hemoglobin.
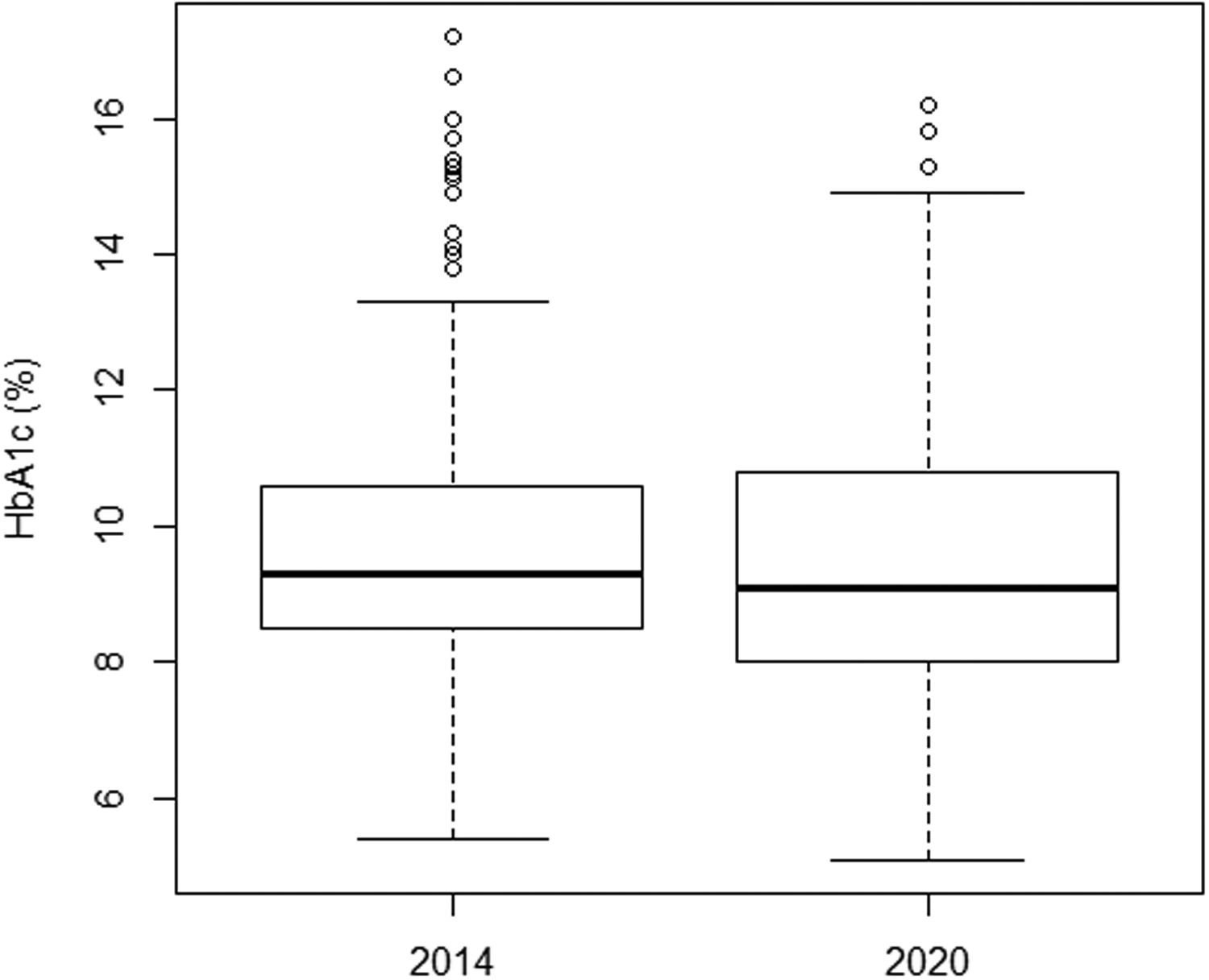
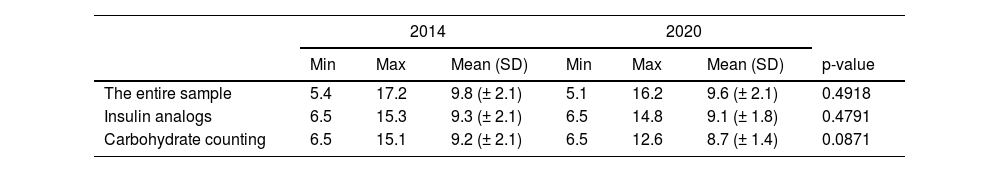
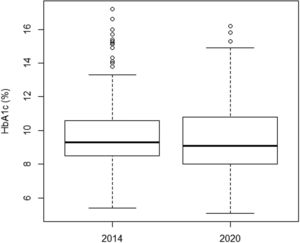
There was no significant improvement in the mean HbA1c value when comparing both time points, from 2014 to 2020, from 9.8 to 9.6% (Table 3). There was also no significant improvement in analog users (9.3% and 9.1%) and in the group doing carbohydrate counting (9.2% and 8.7%). Figure 1 illustrates the variation in mean HbA1c levels at both time points. However, when a subclassification of the 2014 and 2020 groups was made according to the type of insulin therapy received, an improvement in HbA1c was observed in the subgroups that used insulin analogs (association between rapid-acting with long-acting analogs), as well as in those who performed the carbohydrate count (Table 2).
Comparison of mean HbA1c values (%).
Values are given for the whole sample, in patients with diabetes taking insulin analogs, and in those who performed carbohydrate counting.
The present study showed the clinical and laboratory profile of patients being followed up at the HCFMRP-USP, a reference center for the 25 municipalities in its coverage area. In both periods, there was an equal distribution between the sexes (Table 1) and a predominance of self-reported white ethnicity (86.7% in 2014 and 71.5% in 2020). Nutritionally, 6.2% of the sample in 2014 and 10.7% in 2020 had a low weight, and 8.4% in 2014 and 8% in 2020 were obese. Most patients were eutrophic: 62.8% in 2014 and 64.4% in 2020. Thus, there was no significant difference between the two groups regarding nutritional classification.
The distribution of data by sex and ethnicity is consistent with the literature. The highest prevalence of DM1 in the United States was found in young white men. Most autoimmune disorders disproportionately affect women. However, for DM1, this difference is not observed in all populations.1,4 These results are similar to those of a Brazilian study by Felício et al.5 involving both adults and children (mean age 23.9 ± 10.8), where a prevalence of 56% was found in women and 57.2% in whites. Also, the mean BMI was 22.6 kg/m², indicating eutrophy.
For 46.5% of patients with diabetes in 2014 and 50.7% in 2020, the diagnosis was only made in the presence of diabetic ketoacidosis (DKA). The incidence of DM1 diagnosis in the presence of DKA with an adjusted global rate between 2006 and 2016 in 13 countries was 29.9%.6 However, it varied by region, between 15% and 70% in Europe and North America.7
In a multicenter study from 2012, patients from 20 cities in the five Brazilian regions were evaluated; an incidence of 42.3% was observed.7 More recently, in 2019, Souza et al.8 showed a diagnosis of diabetes in the presence of DKA in the southern region of Brazil in 58.8% of cases. In the present study, the data are like that described in the literature.
Like other countries and other regions in Brazil, this data does not indicate a comfortable situation. However, training physicians to diagnose diabetes early was insufficient to change these numbers. Perhaps, increasing the knowledge of the population could help reduce the diagnosis only in the presence of DKA.
Concerning insulinization (Table 2), in 2014, 49.1% of patients with diabetes used NPH + regular insulin, compared to 39.1% in 2020. In parallel, there was an increase in the use of insulin analogs (48.4%), reflecting the team's effort to change the insulinization scheme. The percentage of IBS users changed from 1.3% to 4.4%, and the total number of patients with diabetes using carbohydrate counting dropped from 28.3% in 2014 to 17.8% in 2020.
The mean HbA1c in both time points was very similar (9.8% versus 9.6%) (Figure 1). These values are higher than the recommended ≤7.0%. The high values reflect poor diabetes control in both groups. In the present study, the mean HbA1c level was lower in patients using insulin analogs [mean: 9.3% (2014) and 9.1% (2020)] (Table 2) than in patients using NPH and regular [mean: 10.2% (2014) and 10.1% (2020)]. This difference was significant and showed that insulin analogs reduced HbA1c values. Furthermore, in 2014, an improvement in HbA1c was observed in the subgroups that used fast-acting insulin analogs, whether associated with NPH insulin or long-acting analogs (Detemir or Glargine) [mean: 9.3% (2014)] when compared to the NPH and regular [mean: 10.2% (2014)]. This same observation was not repeated in 2020.
Hermansen et al.9 compared the basal/bolus insulin regimen using insulin analogs with the NPH + regular insulin regimen. They found that patients using the insulin analogs had better glycemic control, lower risk of general and nocturnal hypoglycemia, and less chance of weight gain than those using NPH + regular. The improved glycemic control in patients using insulin analogs can be because this regimen promotes less glycemic variability and less risk of hypoglycemia than NPH + regular insulins.10,11 Furthermore, when comparing regimens using insulin glargine versus NPH as basal insulin in a multiple-daily regimen, in adolescents, insulin glargine was at least as effective, or superior, in glycemic control, especially in patients with higher HbA1c values at the start of treatment.12
Despite a significant improvement in glycemic control in patients using insulin analogs, access to this product is still limited in Brazilian public services. Fast-acting analog insulins were incorporated into the public service in 2017 and, subsequently, long-acting analogs in 2019. The incorporation into the public service was a gain, however, their supply is carried out through requesting specialized medicines and not as a basic component distributed in primary health care. Furthermore, to access analogs, the patient must meet the pre-established criteria for their dispensation. Those facts, added to the frequent lack of inputs in some regions, make access and adequate treatment difficult.
The mean HbA1c levels were lower in patients who did carbohydrate counting (9.2% in 2014 and 8.7% in 2020) than in those who did not (10% in 2014 and 9.6% in 2020). Like using insulin analogs, carbohydrate counting affected HbA1c levels positively. However, most patients using insulin analogs also did carbohydrate counting. This result may be indicative of a combined effect of the two strategies.
Albuquerque et al.13 evaluated the effect of carbohydrate counting in children and adolescents with DM1 using NPH + regular insulin. The counting group had lower HbA1c levels than the non-counting group. Carbohydrate counting facilitates better glycemic control, aids in better lipid control and weight maintenance, meets the patient's nutritional needs, and improves the patient's acceptance of the diet and the disease.14 Also, it improves several other aspects of the lives of children and adolescents with diabetes that are usually restricted due to the disease, such as eating out, choice of foods and quantities, and social participation.15
In a Brazilian study conducted in three reference centers (FAMERP, UNICAMP, and Conjunto Hospitalar do Mandaqui) in 2009, the mean HbA1c of 239 patients with DM1 was 10% ± 2.3.16 Another national multicenter study, published in 2020, included 367 adolescent patients with DM1 from 14 public clinics in 10 Brazilian cities. It found a mean HbA1c of 9.6% ± 2.4.17 In the international scope, in a study conducted in California in 2020 involving 264 young people with DM1, the adjusted mean HbA1c was 9.7%.18 These results are similar to those found in this study.
With the advent of new technologies for glucose monitoring, such as continuous glucose monitoring devices in interstitial fluid and the concept of time in range, HbA1c is no longer the gold standard measure of glycemic control since it does not reflect glycemic variability.19 However, for the patients in this study, managed in a public hospital, HbA1c is still extremely important.
This study showed that the changes implemented between 2014 and 2020 in the service did not facilitate significant improvement in mean HbA1c levels: 9.8% in 2014 to 9.6% in 2020, which is considered high. When comparing only the average HbA1c levels of patients who used analogs, no evolution was observed either, from 9.3% to 9.1%.
In patients with diabetes who had carbohydrate counting, a non-significant reduction in mean HbA1c was observed between the two periods (9.2% to 8.7%). This study identified a significant improvement in HbA1c in patients who used analogs compared to patients who used NPH + regular and patients who had carbohydrate counts.
In addition to access to insulin analogs and personalized treatment with carbohydrate counting, another fundamental factor for adequate treatment and control of the disease is the presence of a cohesive multidisciplinary team, made up of nutritionists, psychologists, nurses, physical educators; all specialized in type 1 diabetes care, given the peculiarities of the disease. Unfortunately, this is not the reality of many Brazilian centers.
The strength of the present study was to compare two periods of the same service under the responsibility of the same medical supervision team and to measure the result of several changes in the treatment systematics. The nursing, nutritionist, and psychologist teams were the same in both periods. To our knowledge, this was the first study of its kind. It involved the only university service in a large population coverage area. A limitation of the present study is that the patients in 2014 were not the same as those in 2020. Also, the resident physicians responsible for the care varied.
In conclusion, the present study showed that despite changes in the outpatient clinic between 2014 and 2020, the metabolic control of patients with DM1 overall did not significantly improve. However, it is worth highlighting, that it was identified a significant improvement in HbA1c in patients who used analogs compared to patients who used NPH + regular and patients who had carbohydrate counts at both times. Further studies will be needed to assess whether other treatment modifications could have better effects.