To compare the efficacy of intravenous ibuprofen at high (20-10-10mg/kg/dose) and low doses (10-5-5mg/kg/dose) the closure of patent ductus arteriosus in preterm newborns.
MethodsA cohort study with historical control of newborns that received high- and low-dose intravenous ibuprofen, from 2010 to 2013 in a neonatal intensive care unit, for closure of the patent ductus arteriosus, documented by echocardiography. Secondary outcomes included the number of ibuprofen cycles, incidence of bronchopulmonary dysplasia, necrotizing enterocolitis, changes in renal function, and death.
ResultsSeventy-seven patients received three doses of ibuprofen for the treatment of patent ductus arteriosus, with 33 receiving high-dose and 44 low-dose therapy. The ductus closed after the first cycle in 25 (56.8%) low-dose patients and in 17 (51.5%) high-dose patients (p>0.99). Sixteen patients received a second cycle of ibuprofen, and the ductus closed in 50% after low-dose and in 60% after high-dose therapy (p>0.99). Seven patients required surgery for ductus closure, 13.6% in the low-dose group and 3% in the high-dose group (p=0.22). Thirty-nine patients developed bronchopulmonary dysplasia, 50% in the low-dose group and 51.5% in the high-dose group (p>0.99). Twenty-two (50%) low-dose patients died vs. 15 (45.5%) high-dose patients (p=0.86).
ConclusionsThere was no difference in closure of the ductus arteriosus or occurrence of adverse effects between the two dose regimens.
Comparar a eficácia do ibuprofeno endovenoso em doses altas (20, 10 e 10mg/kg/dose) e em doses baixas (10, 5 e 5mg/kg/dose) para o fechamento do canal arterial em recém-nascidos pré-termo.
MétodosEstudo de coorte com controle histórico pesquisando recém-nascidos que receberam ibuprofeno endovenoso, no período de 2010 à 2013 na unidade de internação neonatal, em doses altas e baixas para o fechamento do canal arterial, documentado por ecocardiograma. Como desfechos secundários foram avaliados o número de ciclos de ibuprofeno realizados, a incidência de displasia broncopulmonar, enterocolite necrosante, alteração de função renal e óbito.
Resultados77 pacientes receberam 3 doses de ibuprofeno para tratamento do canal arterial, sendo que 33 dose alta e 44 dose baixa. 25 (56.8%) dos que receberam dose baixa fecharam o canal após o 1° ciclo e 17 (51,5%) fecharam após receberem dose alta (p>0.99). 16 pacientes receberam o 2° ciclo, destes 50% fecharam o canal após uso de dose baixa e 60% após o uso de dose alta (p>0.99). 7 pacientes foram à cirurgia para fechamento do canal, sendo 13,6% do grupo que recebeu dose baixa e 3% dose alta (p=0.22). 39 pacientes desenvolveram displasia broncopulmonar, 50% do grupo de dose baixa e 51,5% do grupo de dose alta (p>0.99). 22 (50%) dos pacientes do grupo dose baixa evoluiu a óbito versus 15 (45,5%) dos pacientes do grupo de dose alta (p=0.86).
ConclusãoNão encontramos diferença em relação ao fechamento do canal arterial, assim como ocorrência de efeitos adversos, quando comparamos os dois esquemas posológicos.
Patent ductus arteriosus (PDA) is the most common cardiac abnormality in preterm newborns, especially those under 28 weeks old of gestational age.1 This alteration causes a left-right shunt through the ductus arteriosus and can become clinically evident. When symptomatic, it increases the need for ventilatory support, increases the risk of peri-intraventricular hemorrhage,2 of bronchopulmonary dysplasia (BPD)3,4 and necrotizing enterocolitis,5 and reduces survival rates.6 However, there is no clear evidence of the long-term results of PDA treatment.7 Several randomized controlled trials have failed to show any benefit from treatment on survival and long-term outcomes.1,8,9
A more conservative approach has been increasingly suggested, especially in patients with good clinical evolution. This is due to the high chance of spontaneous closure, more common in newborns weighing >800g, without the need for mechanical ventilation and with no signs of heart failure or pulmonary congestion.10,11
In patients whose patent ductus arteriosus becomes clinically relevant and symptomatic, its constriction can be induced with cyclooxygenase inhibitors, such as indomethacin and ibuprofen, with permanent closure rates of around 60–80%.12–14 Both have side effects, but ibuprofen seems to have fewer negative effects on the cerebral and renal blood flow and also possibly fewer gastrointestinal side effects.15–17
The recommended standard dose of intravenous ibuprofen for patent ductus arteriosus closure is 10mg/kg/dose on the first day and 5mg/kg/dose on the second and third days.18,19 However, Dani et al.16 demonstrated that the use of ibuprofen at high doses (20-10-10mg/kg/day) is more effective than the standard regimen for PDA in preterm newborns with a gestational age <29 weeks, without increasing the rate of adverse effects.
From the year 2012, aiming to achieve higher rates of PDA closure in preterm newborns, ibuprofen dose regimens were changed for the drug treatment of PDA in the Neonatal Intensive Care Unit of Hospital de Clínicas de Porto Alegre (HCPA). Higher doses of ibuprofen started to be utilized, rather than the previously used standard dose. Therefore, the doses changed to 20mg/kg of ibuprofen for the first intravenous infusion on day 1, followed by 10mg/kg on days 2 and 3, when newborns showed a hemodynamically significant ductus arteriosus confirmed by the echocardiography.
This study aims to compare the therapeutic effectiveness and the impact of the two ibuprofen doses on PDA closure rates in preterm newborns, as well as other morbidities such as BPD, renal impairment, need for surgery for PDA closure, and death.
MethodsA cohort study with historical control was performed. It included all preterm newborns who received intravenous ibuprofen in the period of January 2010 to December 2013 for the treatment of patent ductus arteriosus in the Neonatology Service of HCPA. The HCPA is a university tertiary referral hospital with the capacity to admit up to 50 newborns. It has 20 neonatal intensive care unit (NICU) beds, which encompass less complex cases to extremely preterm newborns and those with rare diseases. The study was approved by the ethics committee of the institution.
The population was divided into two groups, one receiving low-dose ibuprofen (10-5-5mg/kg/day – single daily dose), treated before the change in dose regimens and those receiving high-dose ibuprofen (20-10-10mg/kg/day – single daily dose) for three days, treated after the change in the care protocol.
All patients underwent echocardiography for the diagnosis of PDA. Treatment was indicated for those with significant clinical signs and presence of respiratory symptoms, and with echocardiography findings showing left-right shunt through the ductus arteriosus with left atrium/aortic root ratio ≥1.5mm.
Newborns with congenital heart malformation, those without an echocardiogram to confirm the diagnosis, and those who did not complete the treatment due to clinical instability or death were excluded.
The collected demographic data included gestational age, birth weight, days of life at the beginning of treatment, need for other ibuprofen treatment cycles, need for surgical closure, death, and neonatal morbidities, such as bronchopulmonary dysplasia (defined as need for supplemental oxygen or ventilatory support at 28 days of life), necrotizing enterocolitis (presence of pneumatosis or intestinal perforation), need for ventilatory support (continuous positive airway pressure [CPAP], noninvasive mechanical ventilation [NIMV], mechanical ventilation [MV], and high-frequency ventilation [HFV]), presence of chorioamnionitis, sepsis with positive blood cultures, and peri-intraventricular hemorrhage grades III and IV. Serum creatinine and urea levels 48h before and after completion of each treatment cycle were also recorded.
The closure of the patent ductus arteriosus, the primary outcome, was defined as complete PDA closure observed on echocardiography.
This study compared the clinical outcome of patients receiving higher doses of ibuprofen with those previously treated with lower doses.
Statistical analysisSample size calculationTo detect a difference of 30 percentage points (from 40% to 10% of ductus arteriosus closure failure), considering power of 80% and α=0.05, 32 patients were necessary in each group.
Statistical testsData were stored in a database created with the software SPSS® version 18.0 (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0 IL, USA), which was also used for statistical analyses. Categorical variables were described as absolute and relative frequencies, continuous variables with symmetrical distribution as mean and standard deviation, and the asymmetrical variables as median and interquartile range. Categorical variables were compared using Fisher's exact test, the quantitative variables with symmetrical distribution by Student's t-test, and those with asymmetrical distribution by the Mann–Whitney test. The significance level was set at 0.05.
The study was approved by the institutional research committee, and all researchers signed a consent form in order to obtain access to patients’ medical records, in which they swore to maintain complete confidentiality.
ResultsA total of 84 patients received ibuprofen for PDA closure in the abovementioned period; seven were excluded, three because they received five ibuprofen doses rather than one or two cycles of three doses, and four patients due to the incomplete treatment because of death or major clinical instability. There were no losses due to cardiac malformations or lack of diagnosis on echocardiography. The percentage of losses was 8.3%. The study population consisted of 77 patients, of whom 33 (42.8%) received high-dose and 44 (57.1%) low-dose ibuprofen.
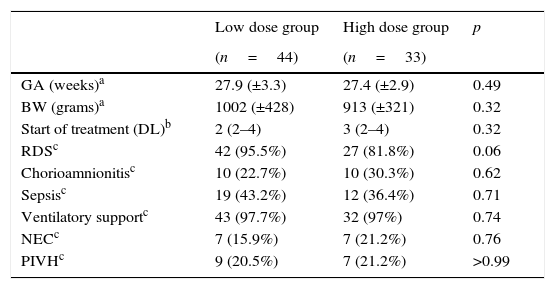
There was no significant difference between the two groups regarding birth weight (p=0.32), gestational age (p=0.49), days of life at the beginning of the treatment with ibuprofen (p=0.32), chorioamnionitis (p=0.62), need for mechanical ventilation (p=0.74), necrotizing enterocolitis (p=0.76), peri-intraventricular hemorrhage (p>0.99), hyaline membrane disease (p=0.06), and infection with a positive culture (p=0.71) (Table 1).
Characteristics of the studied patients.
| Low dose group | High dose group | p | |
|---|---|---|---|
| (n=44) | (n=33) | ||
| GA (weeks)a | 27.9 (±3.3) | 27.4 (±2.9) | 0.49 |
| BW (grams)a | 1002 (±428) | 913 (±321) | 0.32 |
| Start of treatment (DL)b | 2 (2–4) | 3 (2–4) | 0.32 |
| RDSc | 42 (95.5%) | 27 (81.8%) | 0.06 |
| Chorioamnionitisc | 10 (22.7%) | 10 (30.3%) | 0.62 |
| Sepsisc | 19 (43.2%) | 12 (36.4%) | 0.71 |
| Ventilatory supportc | 43 (97.7%) | 32 (97%) | 0.74 |
| NECc | 7 (15.9%) | 7 (21.2%) | 0.76 |
| PIVHc | 9 (20.5%) | 7 (21.2%) | >0.99 |
GA, gestational age; BW, birth weight; DL, days of life; RDS, respiratory distress syndrome; sepsis, presence of positive blood culture; NEC, necrotizing enterocolitis; PIVH, peri-intraventricular hemorrhage.
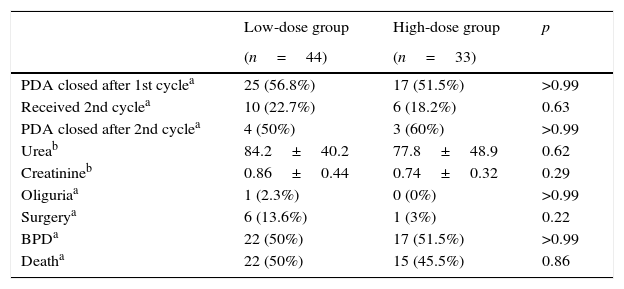
Among those who received low-dose treatment, 25 (56.8%) had PDA closure after the first cycle of ibuprofen and 17 (51.5%) after receiving high-dose treatment (p>0.99). A total of 22.7% of the patients in the low-dose group received a second cycle vs. 18.2% of patients in the high-dose group (p=0.63).
Sixteen patients received a second ibuprofen cycle, but only 13 were submitted to an echocardiogram at the end of treatment; eight patients from the low-dose group and five from the high-dose group. Four patients (50%) had PDA closure after the low-dose and three (60%) after the high-dose treatment (p>0.99).
Regarding the secondary outcomes, seven patients underwent surgery for PDA closure; 13.6% in the low-dose and 3% in the high-dose group (p=0.22). A total of 53 patients (68.8%) were assessed on the 28th day of life for bronchopulmonary dysplasia; 30 from the low-dose and 23 from the high-dose group. Twenty-two patients from the low-dose and 17 from the high-dose group developed BPD (p>0.99); 22 (50%) patients from the low-dose group died vs. 15 (45.5%) patients from the high-dose group (p=0.86). Urea and creatinine levels after the first cycle showed no statistically significant differences between the groups (p=0.62 for urea and p=0.29 for creatinine) and only one patient had oliguria, which was from the low-dose group (Table 2).
Treatment outcomes.
| Low-dose group | High-dose group | p | |
|---|---|---|---|
| (n=44) | (n=33) | ||
| PDA closed after 1st cyclea | 25 (56.8%) | 17 (51.5%) | >0.99 |
| Received 2nd cyclea | 10 (22.7%) | 6 (18.2%) | 0.63 |
| PDA closed after 2nd cyclea | 4 (50%) | 3 (60%) | >0.99 |
| Ureab | 84.2±40.2 | 77.8±48.9 | 0.62 |
| Creatinineb | 0.86±0.44 | 0.74±0.32 | 0.29 |
| Oliguriaa | 1 (2.3%) | 0 (0%) | >0.99 |
| Surgerya | 6 (13.6%) | 1 (3%) | 0.22 |
| BPDa | 22 (50%) | 17 (51.5%) | >0.99 |
| Deatha | 22 (50%) | 15 (45.5%) | 0.86 |
PDA, patent ductus arteriosus; BPD, bronchopulmonary dysplasia.
This study did not show greater efficiency of higher doses of intravenous ibuprofen over the standard dose for PDA closure in preterm newborns. In addition, no differences were found regarding the occurrence of short-term adverse effects or neonatal morbidities when the two regimens were compared.
The optimal dose of ibuprofen for PDA treatment remains controversial. The standard regimen is based on limited and scarce pharmacokinetic data.19–21 Desfrere et al.22 suggested that a higher-dose regimen, with 20, 10, 10mg/kg/day for three days, could attain higher PDA closure rates in newborns with less than 27 weeks of gestational age; however, the tolerability and safety should be carefully assessed in further studies before being routinely recommended.
Meißner et al.10 found a clear trend toward higher rates of PDA closure with higher doses with no increase in adverse effects, but without statistical significance.
Hirt et al. demonstrated that, with the increase in postnatal age, plasma ibuprofen clearance also increased, resulting in a reduction of half-life (42.2h at three days vs. 17.7h at five days),19 probably due to the maturation of cytochrome P450, which metabolizes ibuprofen.23 These authors concluded that the ibuprofen dose regimen should be prospectively tested.
Considering the hypothesis that the increase in ibuprofen doses for PDA closure would be more effective than the standard dose, with no increase in adverse events, Dani et al. designed a randomized controlled study, which assessed 70 newborns, of whom 35 received the standard dose, and 35, the double dose. The authors observed that the high-dose regimen was more effective for PDA closure in preterm newborns younger than 27 weeks of gestational age, with no increase of adverse events rates.16
In disagreement with the results of the study by Dani et al.,16 the use of high doses of ibuprofen in the present analysis showed no significant difference regarding the increase in PDA closure rates. Additionally, no difference regarding the effectiveness of the second cycle of treatment between the two groups was found. Although not statistically significant, the need for PDA surgical closure was higher in the group that received low doses of ibuprofen. However, the number of assessed patients was not sufficient to definitely analyze this outcome.
This study showed no statistically significant differences regarding neonatal morbimortality, such as the presence of bronchopulmonary dysplasia, peri-intraventricular hemorrhage, necrotizing enterocolitis, and death between the groups that received different doses of ibuprofen.
Renal failure or oliguria has been reported in 5–7% of patients treated with ibuprofen for PDA closure, but it is reversible.1 Among the patients in the present study, only one had oliguria and this patient belonged to the low-dose group. The administration of higher doses was well tolerated and patients showed no adverse effects. There was no significant difference related to serum levels of urea and creatinine after treatment with ibuprofen between the two groups. These findings are in agreement with the data previously published in the literature, which reported only mild adverse effects.22
To guarantee the safety and efficacy of different ibuprofen dose regimens, the authors emphasize the small number of assessed patients as a limitation of this study, in addition to its retrospective design. However, although it appears reasonable to consider higher doses when treatment is started after the 5th day of life, due to the drug pharmacokinetics, it was not possible to analyze this in the present study due to the small number of assessed patients. Randomized prospective studies could further elucidate this issue.
This study showed no benefit in changing the ibuprofen dose regimen to double doses for the treatment of symptomatic PDA in preterm newborns. There was no significant difference when comparing the two treatment dose regimens.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Dornelles LV, Corso AL, Silveira RC, Procianoy RS. Comparison of two dose regimens of ibuprofen for the closure of patent ductus arteriosus in preterm newborns. J Pediatr (Rio J). 2016;92:314–8.
The study is associated with the Neonatology Service, Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil.