To evaluate the fatty acid composition of mature human milk of women living far from the coastal area of Brazil.
MethodsMature breast milk samples were obtained from 47 lactating women aged between 18 and 35 years, who delivered their babies at term and who exclusively or predominantly breastfed. Milk collection took place after the fifth week postpartum by hand expression. The fatty acid composition of the milk was determined by gas chromatography.
ResultsIt was observed that the concentration of eicosapentaenoic acid (0.08%) was higher than that observed in previous studies in Brazil. However, the content of docosahexaenoic acid (0.09%) found in human milk was one of the lowest verified in the world. The content of trans fatty acids (2.05%) was similar to that reported in national studies previous to the mandatory declaration of this fatty acid content in food labels, suggesting that this measure had no effect on reducing the content of this fatty acid in the usual diet of women.
ConclusionsLow levels of docosahexaenoic acid and high concentrations of trans fatty acids were observed in mature breast milk of women living far from the coastal area in Brazil.
Avaliar a composição de ácidos graxos do leite humano maduro de mulheres residentes em área distante da costa litorânea brasileira.
MétodosAmostras de leite materno maduro foram obtidas de 47 mulheres lactantes com idade entre 18 e 35 anos, que tiveram partos a termo e em aleitamento exclusivo ou predominante. A coleta de leite se deu a partir da 5ª semana pós-parto, por meio de ordenha manual. A composição de ácidos graxos do leite foi determinada por cromatografia gasosa.
ResultadosVerificou-se que a concentração de eicosapentaenoico (0,08%) foi superior ao observado em estudos brasileiros prévios. Entretanto, o teor de docosa-hexaenoico (0,09%) encontrado no leite humano foi um dos menores já verificados no mundo. O teor de ácidos graxos trans (2,05%) foi similar ao relatado em estudos nacionais prévios à obrigatoriedade de declaração do teor deste em rótulos de alimentos, sugerindo que esta medida não surtiu efeito na redução de seu teor na dieta habitual das mulheres.
Breast milk is the best food for the child in the first months of life, exerting a protective effect against the development of diseases in childhood.1 Its fatty acid composition,2–6 which has an important role in child growth and development, varies widely.
Docosahexaenoic fatty acid (DHA, C22:6-n-3) directly influences neuronal development, visual acuity7 and, together with the fatty acid eicosapentaenoic (EPA), affects the infant's immune system.8 Oily fish, such as salmon and sardines, are the main dietary sources of these fatty acids.
In contrast, the high content of trans fatty acids in human milk, resulting from maternal consumption of processed foods, can affect child growth and development in several ways, mainly by interfering in the process of desaturation of essential fatty acids (EFA) to long-chain polyunsaturated fatty acids (LC-PUFA) and as result of the conversion into unusual long chain isomeric fatty acids n-6 and n-3, impairing the synthesis of eicosanoids and membrane function.9,10
Conjugated linoleic fatty acid (CLA), also present in breast milk, is synthesized by biohydrogenation in the rumen of animals and endogenous conversion of trans-vaccenic acid, whose food sources are dairy products and meats.11 Evidence suggests a positive effect of CLA on body composition and anti-carcinogenic activity,12,13 although its effect on child development is unknown.
Studies that determined that the content of trans fatty acids,4,5 long-chain polyunsaturated fatty acids (LC-PUFA),4–14 and the content of CLA6,15 in breast milk of Brazilian women are scarce. As the fatty acid composition of breast milk is directly influenced by the eating habits of the breastfeeding woman and her body supplies,16 it has been suggested that the breast milk of women living in different geographic regions of the country, with access to different food sources of EPA and DHA, can vary regarding the content of these fatty acids.
Additionally, studies that evaluated the content of trans fatty acids in the milk of Brazilian women were performed prior to Decree #360/2003 by the National Agency for Sanitary Surveillance in 2006, which resulted in the mandatory declaration of this fatty acid content in food labels. This legislative measure may have influenced food industries to produce foods with lower levels of trans fatty acids, which might have resulted in its lower maternal consumption and concentration in human milk.
The hypothesis of this study is that the content of EPA and DHA in breast milk of women living far from the coastal area is lower than that verified in studies performed with women living in the coastal region,5,6,15 due to the distinct access to fish consumption. Additionally, it was expected that the content of trans fatty acids in the women's milk would be lower than that observed in studies performed prior to Decree # 360/2003, since after this legislative measure the labels of most industrialized products in the country declare the absence of trans fatty acids in foods.
The present study aimed to evaluate the fatty acid composition of mature human milk of women living in the city of Ribeirão Preto, state of São Paulo, Brazil.
MethodsStudy populationA prospective study was conducted among 103 pregnant women patients at Basic Health Units (BHU) in Ribeirão Preto, in order to test the accuracy of a quantitative food frequency questionnaire for pregnant women. Data collection was carried out in five BHUs, located in the central, south, east, and west regions of the city. Inclusion criteria were age between 18 and 35 years; normal weight before the pregnancy; and absence of conditions that could change the usual food intake. The study used a convenience sample; sample size determination was based on the recommendation that 100 individuals are needed for assessment of agreement between methods of dietary intake evaluation.17 The first evaluation of the prospective study was conducted between September of 2009 and May of 2010.
The present study included 47 lactating women that completed the prospective study, who delivered their babies at term; breastfed them exclusively (breast milk intake only, with no other liquids or solid food), or predominantly (intake of maternal milk and other water-based liquids), after the fifth week postpartum; and who gave birth at term (after 37 weeks of gestation). The collection of human milk samples was performed between May of 2010 and January of 2011.
The subjects agreed to participate by signing an informed consent. The implementation of this study was approved by the Municipal Health Secretariat of Ribeirão Preto, and approved by the Research Ethics Committee of the Centro de Saúde-Escola da FMRP, USP (Protocol No. 378/CEP-CSE/FMRP-USP).
Fatty acid composition of human milkSamples (5-10mL) of mature breast milk (between the 5th and 14th week postpartum) were obtained from the same breast offered to the baby by hand expression by the woman herself, in the morning immediately after the first feeding of the baby, before the mother's breakfast. The milk was stored at -80°C until the analysis.
The fatty acid content of the milk was determined in the Nutrition and Metabolism Laboratory of the Faculdade de Medicina de Ribeirão Preto. For this analysis, aliquots of 0.8mL were used for the extraction of fat by the Bligh and Dyer method, and methylated with potassium hydroxide in methanol at 0.5M.18
The methyl esters of the fatty acids in human milk were determined by gas chromatography using a Shimadzu GC-2014 gas chromatograph (Shimadzu Europe - Duisburg, Germany) equipped with an AOC-20i auto-injector (Shimadzu Europe -Duisburg, Germany) with a capillary column of polyethylene glycol - Supelcowax 10 (30 feet long, 0.25mm internal diameter, 0.25mm thick film; Supelco Inc. - Bellefonte, PA). Helium was used as carrier gas at a flow rate of 1.0mL/min. Synthetic air was used for flame ionization with detection at 280°C.
Separation of fatty acids was performed with a temperature gradient in a capillary column of polyethylene glycol. The initial column temperature was 100°C, which was maintained for 1minute; after that, the temperature was increased at a rate of 13°C per minute up to 195°C, and maintained for 5minutes. It was then elevated to 240°C at a rate of 15°C per minute, and maintained at that temperature for 30minutes. The injections of 1μL samples were performed in split mode. The temperature of the injector and detector was 250°C.
The pattern used in the identification consisted of a mix of methyl esters of fatty acids from Supelco (Supelco 37 Component FAME mix; Supelco Inc. – Bellefonte, PA) with addition of patterns of c9,t11-CLA and t10,c12-CLA. Quantitation was performed by area normalization, and the results were presented as percentages by weight.
Statistical analysisMean and standard deviation (SD) for continuous variables, and frequency for categorical variables, were obtained through the Statistical Package for the Social Sciences (SPSS), release 17 (SPSS Inc. – Chicago, USA).
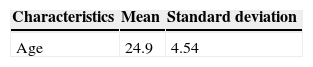
ResultsMost participants belonged to socioeconomic class C, whose educational level averaged more than 8 years of study, and the mean age was 25 years, as shown in Table 1.
Sociodemographic characteristics of infants living in Ribeirão Preto, SP, Brazil, 2010 (n = 47).
| Characteristics | Mean | Standard deviation |
|---|---|---|
| Age | 24.9 | 4.54 |
| Number | Frequency | |
|---|---|---|
| Ethnicity | ||
| White | 27 | 57.44 |
| Mixed-race | 13 | 27.65 |
| Black | 6 | 12.77 |
| Asian | 1 | 2.12 |
| Socioeconomic classa | ||
| B2 | 5 | 10.63 |
| C | 34 | 72.34 |
| D | 8 | 17.02 |
| Educational level (years of study) | ||
| < four years | 1 | 2.12 |
| Four to eight years | 13 | 27.65 |
| > eight years | 33 | 70.23 |
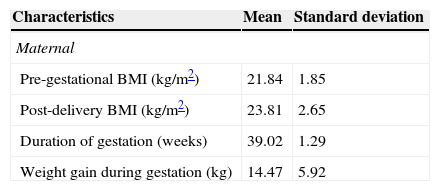
Maternal and infant anthropometric characteristics, as well as duration and type of delivery, are described in Table 2. It is noteworthy that 60% of women had inadequate weight gain during pregnancy.
Maternal and infant anthropometric characteristics and type of delivery. Ribeirão Preto, SP, Brazil, 2010 (n = 47).
| Characteristics | Mean | Standard deviation |
|---|---|---|
| Maternal | ||
| Pre-gestational BMI (kg/m2) | 21.84 | 1.85 |
| Post-delivery BMI (kg/m2) | 23.81 | 2.65 |
| Duration of gestation (weeks) | 39.02 | 1.29 |
| Weight gain during gestation (kg) | 14.47 | 5.92 |
| Number | Frequency | |
|---|---|---|
| Adequacy of weight gain during gestation | ||
| Adequate | 19 | 40.43 |
| Insufficient | 16 | 34.04 |
| Excessive | 11 | 23.4 |
| Type of delivery | ||
| Normal | 31 | 65.96 |
| C-section | 16 | 34.04 |
| Mean | Standard deviation | |
|---|---|---|
| Infants | ||
| Birth weight (kg) | 3.26 | 0.38 |
| Birth length (cm) | 49.05 | 2.39 |
BMI, body mass index.
Table 3 describes the fatty acid composition of mature breast milk of nursing mothers living in the city of Ribeirão Preto, SP, Brazil. Among the saturated and monounsaturated fatty acids, higher values were observed for palmitic (C16: 0) and oleic (C18: 1n-9) fatty acids, respectively. Among the trans fatty acids and LC-PUFAs, there was a higher contribution of vaccenic (C18: 1 11t) and arachidonic acid (ARA) (C20: 4 n-6) fatty acids, respectively. The total essential fatty acids (linoleic and α-linolenic acid) was 22.42%, that of LC-PUFAs (ARA, EPA and DHA) was 0.66% (0.08% of EPA and 0.09% of DHA), and that of CLAs was 0.05%.
Fatty acid composition (%) of mature human milk of women living in Ribeirão Preto, SP, Brazil, 2010-2011 (n = 47).
| Fatty acids | Mean | SD |
|---|---|---|
| Saturated | 42.67 | 5.5 |
| C6:0 (caproic acid) | 0.09 | 0.0 |
| C8:0 (caprylic acid) | 0.29 | 0.1 |
| C10:0 (capric acid) | 1.95 | 0.5 |
| C11:0 (undecylenic acid) | 0.012 | 0.0 |
| C12:0 (lauric acid) | 7.46 | 2.6 |
| C13:0 (tridecanoic acid) | 0.03 | 0.0 |
| C14:0 (myristic acid) | 6.81 | 2.3 |
| C15:0 (pentadecanoic acid) | 0.23 | 0.1 |
| C16:0 (palmitic acid) | 19.50 | 2.0 |
| C17:0 (margaric acid) | 0.29 | 0.1 |
| C18:0 (stearic acid) | 5.82 | 1.0 |
| C20:0 (arachidic acid) | 0.009 | 0.0 |
| C21:0 (heneicosanoic acid) | 0.024 | 0.1 |
| C22:0 (behenic acid) | 0.03 | 0.0 |
| C24:0 (lignoceric acid) | 0.12 | 0.1 |
| Monounsaturated | 29.15 | 3.7 |
| C14:1 (myristoleic acid) | 0.19 | 0.1 |
| C15:1 (pentadecenoic acid) | 0.02 | 0.0 |
| C16:1 (palmitoleic acid) | 2.11 | 0.7 |
| C17:1 (10-heptadecenoic acid) | 0.18 | 0.0 |
| C18:1n9c (oleic acid) | 26.46 | 2.8 |
| C20:1n9 (gadoleic acid) | 0.12 | 0.0 |
| C22:1n9 (erucic acid) | 0.04 | 0.0 |
| C24:1n9 (lignoceric acid) | 0.03 | 0.1 |
| Trans | 2.05 | 0.5 |
| C18:1 trans 11 (vaccenic acid) | 1.68 | 0.3 |
| C18:1 trans 9 (elaidic acid) | 0.28 | 0.1 |
| C18:2n-6tt (linolelaidic acid) | 0.09 | 0.1 |
| Conjugated | 0.49 | 0.1 |
| CLA C18:2 c,t (cis, trans octadienoic acid) | 0.47 | 0.1 |
| CLA C18:2 t,c (trans, cis octadienoic acid)* This result represents the sum of CLA C18:2 t,c with CLA C18:2 7t9c | 0.02 | 0.0 |
| n-3 Polyunsaturated | 2.11 | 0.4 |
| C18:3 n-3 (α- linolenic acid) | 1.54 | 0.4 |
| C20:3 n-3 (eicosatrienoic acid) | 0.40 | 0.1 |
| C20:5 n-3 (eicosapentaenoic acid) | 0.08 | 0.1 |
| C22:6 n-3 (docosahexaenoic acid) | 0.09 | 0.1 |
| n-6 Polyunsaturated | 21.87 | 4.6 |
| C18:2 n-6 (linoleic acid) | 20.96 | 4.4 |
| C18:3 n-6 (γ-linoleic acid) | 0.09 | 0.1 |
| C20:3 n-6 (dihomo-γ-linoleic acid) | 0.34 | 0.1 |
| C20:4 n-6 (arachidonic acid) | 0.48 | 0.1 |
The present study showed that the concentration of EPA in mature human breast milk was higher than that observed in Brazilian studies (between 11% and 38% higher).5,6,15 However, low levels of DHA were found, between 36% and 70% lower than those reported in Brazil.5,6,15 The content of trans fatty acids in human milk was similar to that reported in national studies prior to the implementation of mandatory declaration of this fatty acid content in food labels.5,6
The content of DHA found in the present study (0.09%) was much lower than the value reported in studies performed in coastal cities (Rio de Janeiro) by Tinoco et al. in mature milk of adult women (0.30%),5 by Meneses et al. in mature milk of adolescents (0.20%),6 and by Torres et al. in the mature milk of adult women (0.22%).15 The value is also lower than that observed in another study, conducted in a city far from the coastal area of Brazil in the mature milk of adult women (Viçosa-MG) (0.14%).4 The amount of DHA in the breast milk was lower than that observed in women living in the United States, Israel, Tanzania, the Netherlands, Australia, China, the Caribbean, Italy, the Philippines, and Japan;2 it was similar to that observed in women in India (0.09%), Malaysia (0.09%), and the rural region of South Africa, (0.10%), considered the lowest concentrations ever recorded in the world,3 which can result in impaired child development, as these fatty acids have low levels of endogenous synthesis in newborns.19
Torres and Trugo,19 in a literature review, identified four Brazilian studies that determined the content of DHA in the erythrocyte membrane of pregnant women and infants. The data demonstrated that Brazilian women have a deficiency of this fatty acid when compared with other countries, which can also be demonstrated by the results of studies evaluating the content of DHA in breast milk.4–6,15 The authors suggest that the Brazilian diet, characterized by low fish consumption and high consumption of vegetable oils (especially soybean oil, rich in n-6 PUFA), promote a higher n-6/n-3 ratio, affects the endogenous conversion of alpha-linoleic fatty acid to EPA and DHA, as the linoleic acid (n-6) competes with the alpha-linoleic acid (n-3) for the conversion of endogenous EPA and DHA.20
Recent data on household food availability in Brazil (POF 2008-2009)21 support the hypothesis of deficient intake of foods rich in DHA. The data suggest a mean consumption of fish per capita of 24.1g/day in women of childbearing age. It is noteworthy that in order to achieve the National Academy of Sciences’ recommendation of 0.2g/d of DHA for pregnant and lactating women, a daily intake of 43g of sardines or hake fish would be required.19
The EPA content of breast milk observed in the present study (0.08%) was higher than that observed in studies conducted in coastal regions by Torres et al. (0.071%),15 Meneses et al. (0.05%),6 and Tinoco et al. (0.05%).5 However, it was lower than that observed in a study conducted in the capital of Brazil (0.17%).14 In the latter study, comprising 77 mothers who delivered at term, transition milk, which tends to present higher concentrations of LC-PUFAs when compared to mature milk, was used for the determination of EPA.22,23
Arterburn et al.,2 in a review study on the content of EPA and DHA in milk of women from different countries (the United States, Canada, Italy, China, Japan, Australia, the Caribbean, Israel, the Philippines, and the Netherlands), concluded that the EPA content in breast milk is lower than DHA, but more constant, as DHA is highly sensitive to the maternal diet, varying mainly in relation to the consumption of seafood. In plasma, DHA supplementation showed a linear increase in the concentrations of EPA, probably due to the retroconversion. However, there was no increase in the concentrations of DHA after supplementation with EPA, possibly due to the low conversion of EPA to DHA. Data from several studies suggest that supplementation with α-linoleic acid affects the increase in EPA concentrations in plasma phospholipids, but does not result in an increase in DHA. These data suggest that the content of EPA in plasma and breast milk is influenced by other fatty acids, thus increasing its concentration.2
As expected, the trans fatty acid content in breast milk observed in the present study (2.05%) was lower than that in lactating women from the United States (7%),24 and Canada (7.1%), 25 where the consumption of processed foods, fast foods, and bakery products is high. The content of trans fatty acids observed in the present study was similar to those found in European countries such as Germany (3.81%)26 and France (1.9%).27
One hypothesis of the study was that the level of trans fatty acids would be lower than that reported in studies previous to the Brazilian resolution that made the statement of this fatty acid content on food labels mandatory. However, it was observed that the content of trans fatty acids found in the present study (2.05%) was similar to that observed in these previous studies. Tinoco et al., in a study performed in Rio de Janeiro, whose data collection occurred between 2001 and 2003, verified a trans fatty acids content of 2.19% in mature breast milk.5 Silva et al., in a study performed prior to the resolution in the city of Viçosa, found a concentration of 2.36% in maternal milk.4 Thus, the data suggest that this regulatory measure did not have an impact on the trans fatty acid content of lactating women's diet. One possible explanation would be that the amount of trans stated on food labels corresponds to the amount per food serving. Manufacturers are not required to declare the nutrient levels when foods contain less than 0.2 grams of trans per serving and the content can therefore be declared as “zero” or “does not contain trans fatty acids” in the nutritional composition label, thus creating a misconception that the food is free of trans fatty acids.
The high content of trans fatty acids is associated with the low content of LC-PUFAs in milk; possible explanations point to the fact the trans fatty acids interfere with the metabolism of EFAs, by inhibiting the desaturation of linoleic and alpha-linoleic acids in LC-PUFAs; due to low intake of EFAs, as foods high in trans fatty acids have lower amounts of EFAs; and by affecting membrane metabolism and structures,10 thus resulting in a possible impairment in child growth and development.
Few studies have investigated the concentration of CLA in human breast milk. The content observed in the present study (0.49%) was similar to that observed by Torres et al. (0.54%) in the milk of women from the city of Rio de Janeiro,15 as well as by Mosley et al. in the milk of North-American women (0.52%).24 The main dietary sources of these fatty acids are dairy products, but the content of these fatty acids may vary according to cattle-raising characteristics.28
The cis9, 11trans(c9,t11) and trans10, cis12 (t10,c12) isomers of CLA are associated with the possible beneficial effects on human health, such as cancer prevention29 and body fat reduction.13 There are no recommendations for the intake of CLA and its effect on children.
One limitation of this study was the use of a convenience sample, precluding the extrapolation of data to the general population. Additionally, only one milk sample was collected from each woman, not considering the variability of the content of fatty acid composition.
The study showed low levels of DHA in the breast milk in women living in the city of Ribeirão Preto. However, the concentration of EPA was higher than that found in previous Brazilian studies. The trans fatty acid content in the mature milk was similar to that observed in studies conducted prior to the mandatory declaration of this fatty acid content in food labels, suggesting that this measure did not alter the levels of this fatty acid in the usual maternal diet.
FundingThe study received financial support from the Fundação de Apoio ao Ensino, Pesquisa e Assistência do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FAEPA). RYN is the recipient of a Master's degree grant from FAPESP (2010/12320-1), and GSFC is the recipient of a doctoral grant from FAPESP (2010/00408-1).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Nishimura RY, de Castro GS, Jordão Junior AA, Sartorelli DS. Breast milk fatty acid composition of women living far from the coastal area in Brazil. J Pediatr (Rio J). 2013;89:263–8.