To describe the use of nutritional supplements and blood status (hemogram, lipidogram, hepatic function, inflammatory markers, minerals, and homocysteine) in a sample of Brazilian T21 children with private health support before their first consultation with a T21 expert.
MethodThis descriptive cross-sectional study enrolled 102 participants. Brazilian families with a T21 member under 18 years old were contacted and those that consented answered a survey regarding socio-demographics and the use of nutritional supplements and shared the blood tests that their T21 members have collected for the first consultation with a T21 expert.
ResultsFrequencies and percentages were used to describe the variables. The most used supplements included vitamins (A, C and D), minerals (zinc and iron), omega-3, and antioxidants (curcumin). Hypothyroidism was observed in 56.9% of the participants. Hemogram alterations (increased hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin and red cell distribution width, leukopenia, and lymphocytopenia), dyslipidemia, altered hepatic and inflammatory blood markers were frequently found.
ConclusionsNutritional supplements (mainly vitamins, minerals, omega-3 and antioxidants) are frequently used by Brazilian T21 children independently of professional counseling and/or supervision and should be a question to be raised during the clinical anamnesis since some of them may impact medical conduct. Moreover, many blood tests are altered in this population and clinicians should be aware of them in order to warrant an appropriate screening and the implementation of risk management measures as soon as possible and improve the general health of these persons.
Down syndrome or trisomy 21 (T21) is the most common genetic disorder with a prevalence ranging from 1:600 to 1:800 live births in Brazil.1 The decreased life expectancy is attributed to associated risk factors but it has greatly increased with improved health care in the past decades. Despite this, many Brazilian families report that primary care clinicians usually do not follow the basic recommendations regarding health assistance for persons with T21 published by the Brazilian Ministry of Health.1 To avoid inappropriate health monitoring, many families that have resources consult with one of the few specialized clinicians with knowledge in T21, and those who do not, seek information on social networks.
In the last 10 years, social networks have become popular for caregivers to exchange information. On the one hand, these networks increase awareness about educational, health, and social issues related to the T21 population. On the other hand, the exchange of information on medication, nutritional supplements, and dietetic interventions may lead to decision-making without professional counseling and monitoring, bringing risks to the health of these persons.
To have data regarding the use of supplements, adoption of special feeding habits, and altered blood tests for Brazilian T21 children and teenagers the authors invited Brazilian families that consult with a pediatrician or nutritionist with expertise in T21 to participate in a research project. In the present study, the authors are reporting on the use of nutritional supplements and results from blood tests (hemogram, lipid profile, hepatic function, inflammatory markers, minerals, and homocysteine) obtained for the participant's first consultation with the expert. In this way, data being presented reflect habits adopted by the family without expert counseling and/or supervision.
MethodsThis descriptive cross-sectional study conducted with a sample of convenience was approved by the Ethics Committee on Human Research from UEL (3.637.165/2019) and data was collected from January 2020 to January 2022. Brazilian families were invited to participate through Whatsapp groups by one of the researchers that is a member of different Whatsapp T21 groups nationwide. The inclusion criteria were having a T21 member under 18 years old and having blood exams collected for their first consultation with a pediatrician or nutritionist with expertise in T21. This later inclusion criterion stands because, at least in Brazil, experts usually investigate a greater number of blood tests than do primary care clinicians. Families that fulfilled the inclusion criteria and that were willing to participate were contacted in private and received a link to the informed consent. After consenting, the families received a link to a survey including queries regarding socio-demographics and the use of nutritional supplements and were also asked to share the results of blood tests, which were compiled in an Excel worksheet.
Blood exams were from different analytical laboratories, so the authors have used the cut-off values from each laboratory to categorize the results as decreased (i.e., below normal range), normal, or increased (i.e., above normal range). Frequencies and percentages were used to describe the variables.
ResultsAfter the invitation, 189 families demonstrated interest to participate in the study and were contacted privately. Of those, 109 signed the informed consent. However, 7 families were excluded because they did not answer the survey (n = 1) or did not submit the blood exams (n = 6). The results reported represent 102 participants from 13 out of 26 Brazilian states.
Participants aged from 3 months to 16 years and data are presented in the Tables by age category, i.e., infants (≤ 24 months, n = 47), children (from 25 months to ≤12 years, n = 50), and teenagers (>12 years, n = 5).
Most participants (80.4%) listed their ethnicity as Caucasian followed by mixed Caucasian/African (14.7%). Four participants declared themselves as Asiatic (3.9%) and 1 as African. Regarding mother scholarity, 60.8% reported having concluded a post-graduation course, 27.5% graduation, 7.8% high school and 3.9% did not conclude the fundamental cycle (i.e., primary and middle school). Family income was reported to be lower than 2 Brazilian minimum wages by 6.9% of the families; between 2 and 4 by 14.7% of the families; between 4 and 10 by 37.3% of the families and higher than 10 by 40.7% of the families. The monthly Brazilian minimum wage is approximately $242 US dollars (conversion rate 5.0 BRL:1 USD).
A question regarding prenatal diagnosis of T21 was inserted in the questionnaire and 71% reported a post-partum diagnosis (Table 1).
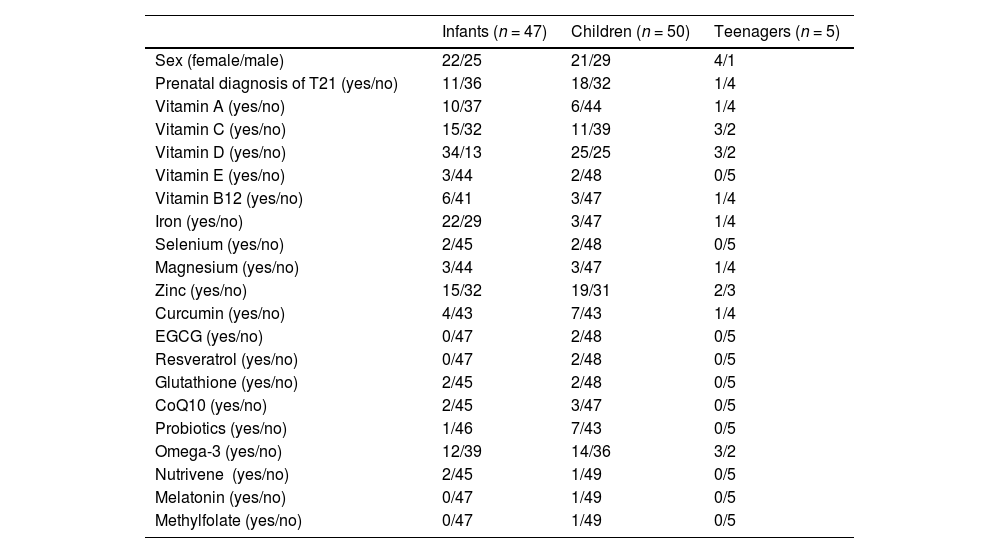
Sociodemographic data and reported use of supplements by Brazilian families before their first consultation with a T21 expert.
Infants: ≤2 years (n = 47); children: >2 and ≤12 years (n = 50); teenagers: >12 and ≤16 years (n = 5); EGCG, epigallocatechin gallate; CoQ10, coenzyme Q10.
As presented in Table 1, vitamin use was frequent among participants: 16.7% used vitamin A, 28.4% vitamin C, 60.8% vitamin D, 4.9% E and 9.8% B12. Vitamin D (72.3%) and iron supplementation (46.8%) were the most frequently used among infants. Zinc was the most supplemented mineral (35.3%) and curcumin was the most supplemented antioxidant (11.8%). Probiotics were reported by 7.8% and omega-3 by 28.4%.
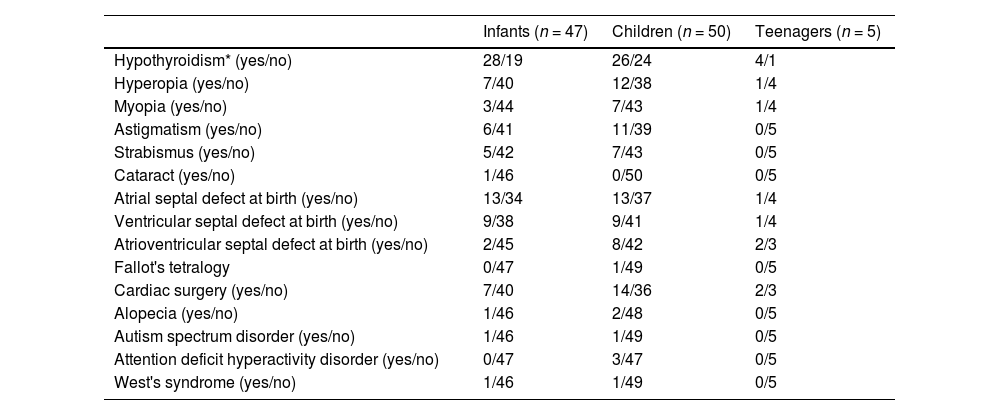
The reported comorbidities are shown in Table 2. Hypothyroidism was computed if the caregiver informed this condition or the use of levothyroxine. This condition was present in 56.9% of the participants and, noteworthy, it was already present in 59.6% of the infants.
Frequency of comorbidities reported to be present in a sample of Brazilian T21 children and teenagers.
| Infants (n = 47) | Children (n = 50) | Teenagers (n = 5) | |
|---|---|---|---|
| Hypothyroidism* (yes/no) | 28/19 | 26/24 | 4/1 |
| Hyperopia (yes/no) | 7/40 | 12/38 | 1/4 |
| Myopia (yes/no) | 3/44 | 7/43 | 1/4 |
| Astigmatism (yes/no) | 6/41 | 11/39 | 0/5 |
| Strabismus (yes/no) | 5/42 | 7/43 | 0/5 |
| Cataract (yes/no) | 1/46 | 0/50 | 0/5 |
| Atrial septal defect at birth (yes/no) | 13/34 | 13/37 | 1/4 |
| Ventricular septal defect at birth (yes/no) | 9/38 | 9/41 | 1/4 |
| Atrioventricular septal defect at birth (yes/no) | 2/45 | 8/42 | 2/3 |
| Fallot's tetralogy | 0/47 | 1/49 | 0/5 |
| Cardiac surgery (yes/no) | 7/40 | 14/36 | 2/3 |
| Alopecia (yes/no) | 1/46 | 2/48 | 0/5 |
| Autism spectrum disorder (yes/no) | 1/46 | 1/49 | 0/5 |
| Attention deficit hyperactivity disorder (yes/no) | 0/47 | 3/47 | 0/5 |
| West's syndrome (yes/no) | 1/46 | 1/49 | 0/5 |
Infants: ≤2 years (n = 47); children: >2 and ≤12 years (n = 50); teenagers: >12 and ≤16 years (n = 5); F: female; M: male.
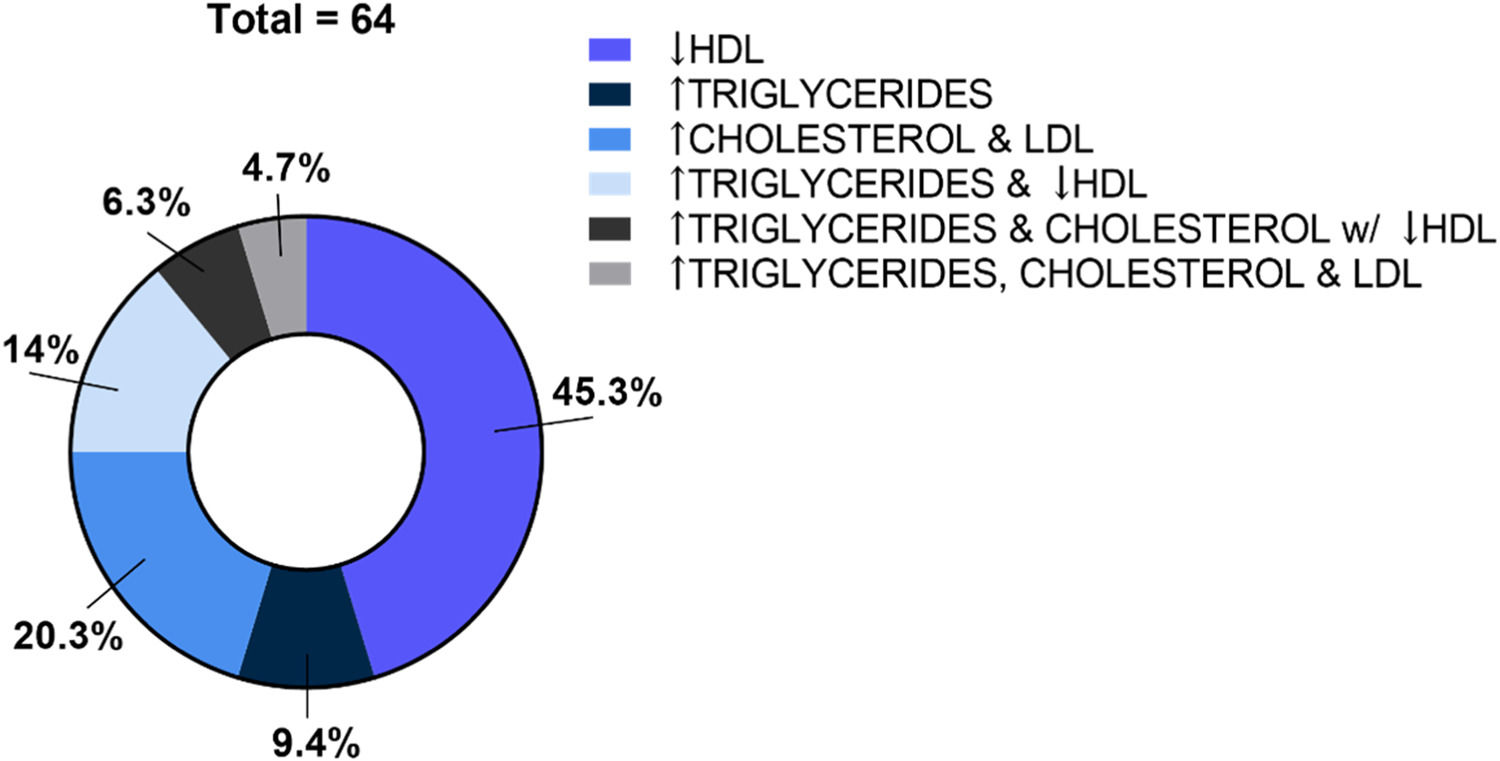
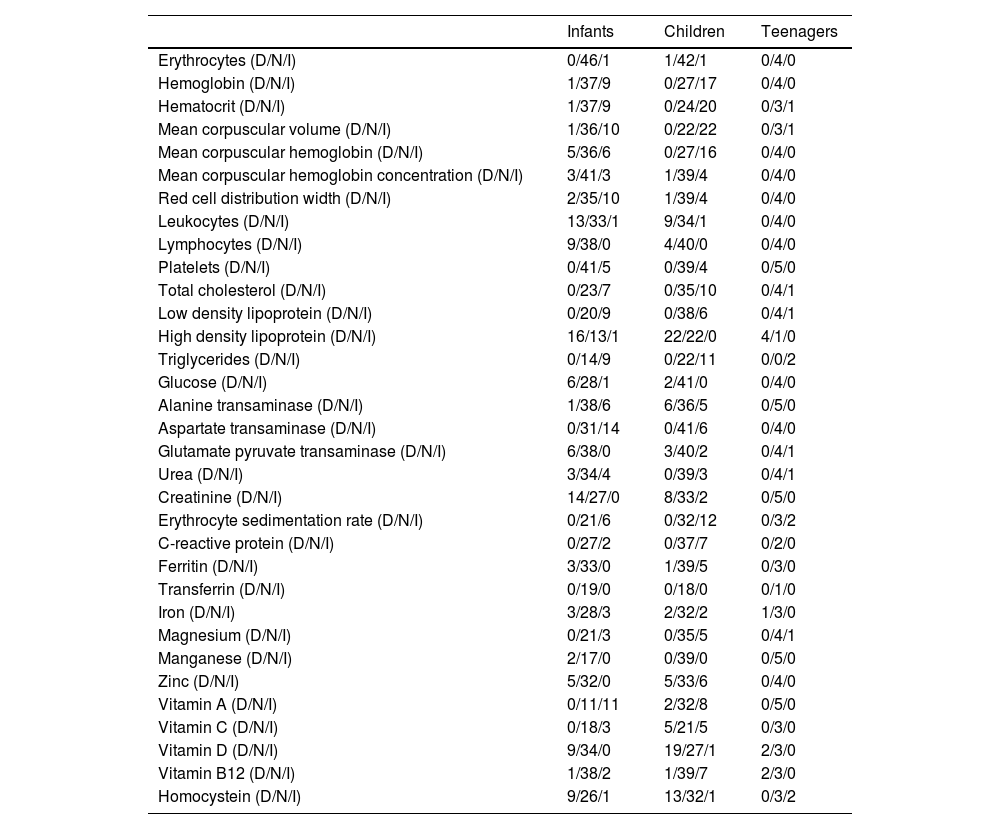
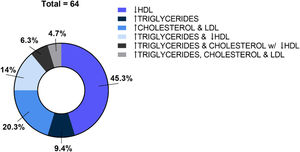
Not all the blood tests have been provided by all the participants, but the available data indicated several alterations in a considerable percentage of participants. In Table 3 the reader can find the distribution by categories (i.e., decreased, normal, increased) among age classes (i.e., infants, children, and teenagers). In infants and children, there were frequent and multiple hemogram alterations. Dyslipidemia was frequent in the three age categories and could be categorized as isolated or combined dyslipidemias as presented in Figure 1. Increased inflammatory markers such as erythrocyte sedimentation rate, C-reactive protein, and ferritin were present in some participants. Iron, magnesium, and manganese levels were not altered in most of the participants. Decreased zinc was observed in 10/85 participants. Increased concentration of vitamin A was present in 19/64 participants whereas decreased concentration of vitamin D in 30/95. Most of the participants presented normal or higher levels of vitamin B12 whereas homocysteine was decreased in 22 participants.
Blood tests in a sample of Brazilian T21 children and teenagers before their first consultation with a T21 expert.
Infants: ≤2 years (n = 47); children: >2 and ≤12 years (n = 50); teenagers: >12 and ≤16 years (n = 5). D, decreased values; N, normal values; I, increased values. This classification was based on each laboratory cut off/reference values.
In the present study, the supplements frequently used by the participants included vitamins (A, C, and D), minerals (zinc and iron), omega-3, and antioxidants (curcumin).
A survey, from which families mainly from the US participated,2 reported over 150 supplements used by T21 children/teenagers including antioxidants (25.8%), especially curcumin and green tea extract/epigallocatechin gallate; vitamins (18.9%); proprietary products (15.8%) and fatty acids (10.8%).
Dietary supplements are commonly used by the general population to promote health and wellness but in T21, supplements use is a point of intense debate possibly because the main focus of the researchers is the intellectual disability and supplements have failed to offer a significant effect on this so far.3 However, it should be taken into consideration that some supplements are used to improve general health by counteracting some deficits that may occur in T21 such as decreased absorption of vitamins,4,5 presence of a prooxidant status,6,7 altered one-carbon metabolism3,8 and decreased levels of some minerals.9
One point that should be highlighted is that Lewanda's study with T21 children and teenagers reported that most parents learned about supplements through a parent group or a friend and in 20% of the cases, the pediatrician was unaware of the supplements being used by their patients.2 In the present study, the authors do not have this data, but we can assume that many parents were using supplements without specialized supervision as well since the questionnaire reflects the moment before the first consultation with an expert. This may be of concern for three main reasons. Firstly, the supplement and its dose should be individualized. Secondly, some supplements may have adverse effects that should be monitored (e.g., hepatic monitoring for persons using epigallocatechin gallate) or avoided in some situations (e.g., increased risk of bleeding posed by curcumin or fish oil when a person will go through surgery).10,11 And thirdly, some supplements may interact with some drugs and both families and clinicians must be aware of these possibilities to prevent unnecessary risks. One example would be the interaction between metallic cations (zinc, magnesium, iron) and some antibiotics (quinolones and tetracyclines) leading to therapeutic failure of the antibiotics.12
Thyroid dysfunction is the most common endocrine disorder in T21 and abnormalities include subclinical hypothyroidism, congenital hypothyroidism, and autoimmune diseases. Prevalence reported in the literature is 4–8% for hypothyroidism in children13 and 25–60% for subclinical hypothyroidism.14 Congenital hypothyroidism is estimated to be 28–35 times higher than in the general population.13 Luton et al. studied the development of 13 human fetal T21 thyroid glands between 23 and 33 weeks of gestation and found thyroid glands to be smaller and with fewer follicles compared to controls,15 indicating thyroid dysfunction in T21 already in the prenatal period.
In the present study, hypothyroidism was computed if the caregiver informed this condition or the use of levothyroxine and this approach resulted in 56.9% of the participants being categorized as having hypothyroidism, including 60% of the infants. The present study's approach was not sensitive to discriminate between hypothyroidism and subclinical hypothyroidism but there is no consensus among clinicians if subclinical hypothyroidism should or should not be treated in T21.14,16
The American Academy of Pediatrics and the Brazilian Health Ministry recommend screening thyroid function at birth, 6 months, and then annually with an increased frequency in those with subclinical hypothyroidism.1,17 Despite these recommendations, up to 25% of patients over 1 year of age do not receive the recommended screening in the United States.18
Regarding blood tests, the present data indicated several alterations in a considerable percentage of the participants.
In hemogram, alterations included increased hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin and red cell distribution width, leukopenia, and lymphocytopenia corroborating previous studies from the literature.6,19 Increased expression of the enzyme cystathionine-β-synthase (CBS), encoded by a gene in chromosome 21, affects homocysteine metabolism, altering methionine resynthesis from folate and the availability of active folate (folate trap), increasing the size of the erythrocyte (macrocytosis). The mean corpuscular volume of erythrocytes in persons with T21 is larger than normal in the absence of anemia and red blood cell survival half-time seems to be shorter, suggesting that erythrocytes have a younger mean age in persons with T21. The increased red blood cell turnover may indicate an accelerated aging process of red blood cells and can increase redox-active iron whether not compensated by functioning transport or storage contributing to the impaired redox homeostasis observed in T21 persons.6
Lymphopenia in T21 is thought to be due to dysfunction of the trisomic thymus associated with severe dysregulation in cytokine production.19 There are many genes encoded in chromosome 21 that are implicated in hematopoiesis and their triplication seems to play a role in the hematological abnormalities observed in T21.19 Most of the studies on neonates/infants and children with T21 report macrocytosis, quantitative and qualitative anomalies of lymphocytes, thrombocytosis, and granulocytosis. Thrombocytopenia, leukocytosis, and neutrophilia are commonly associated with transient abnormal myelopoiesis. Moreover, hypothyroidism is associated with various degrees of anemia, eosinophilia, structural anomalies of neutrophils, monocytosis, and hypoplasia of all myeloid lineages.19
Summing up, altered hematopoiesis is frequently observed in T21 and there are many risk factors that may play a role in these alterations.
Regarding lipid profile, dyslipidemia occurred in the three age categories (64/79 participants, i.e., 81%) and it presented mainly as decreased HDL and/or elevated triglycerides. Even though dyslipidemia is often related to obesity, visceral adiposity, and insulin resistance, which are common in T21 teenagers and adults,20 these are not common in T21 children, who usually have low birth weight and slow weight gain and growth velocity. Despite normal or low weight, dyslipidemia is still reported to be frequent in T21 children. Adelekan et al. reported in T21 children aged 4–10 years abnormal lipid profiles independent of weight status when compared to siblings.21 Garcia de la Puente et al. evaluated the lipid profile of T21 children and teenagers (2–18 years) and, similarly to us, found an unfavorable lipid profile, especially low HDL cholesterol, and high triglycerides in 45.9% and 26.2% of the participants, respectively.22 A significant association was found between dyslipidemia and obesity but, in general, there was a low prevalence of obesity and a high prevalence of dyslipidemia. Interestingly, a study carried out on T21 fetuses found that they presented increased cholesterol and apolipoprotein compared to non-T21 fetuses.23 There are some other studies describing abnormal lipid profiles in T21 children and teenagers but, despite this, there is no recommendation for screening for dyslipidemia in this population in some countries.24 In Brazil, the Ministry of Health does recommend screening but just in case of obesity.1 Considering the recognized role of dyslipidemias as a risk factor for diseases, lipid screening should be included in routine health checkups in T21 children independently of overweight or obesity so preventive strategies could be implemented earlier. The mechanisms hypothesized to play a role in dyslipidemias in T21 children may involve hypothyroidism, leptin resistance, and the pro-inflammatory status observed in this population.
In the present study, increased levels of hepatic (AST, ALT), as well as inflammatory (erythrocyte sedimentation rate, C-reactive protein, and ferritin) blood markers, were observed in some participants. Studies have also reported elevated inflammatory biomarkers in apparently healthy T21 children, i.e., without acute febrile or diarrheic episodes.25 T21 people, even non-obese, tend to develop fatty liver. Valentini et al. observed that non-obese T21 children exhibited mean TNF-α levels greater than those reported in the general population and showed an inexplicable elevation of AST levels, thus suggesting an increased risk for systemic inflammation of unknown origin that could explain their susceptibility to non-alcoholic fatty liver disease.26 In addition, liver dysfunction can also be triggered by obstructive sleep apnea, which is commonly observed in T21 children. Chronic intermittent nocturnal hypoxia induces oxidative stress in the liver.27 Other factors commonly present in T21 that can pose a risk to hepatic function and inflammatory markers include myeloproliferative disorder/abnormal myelopoiesis, celiac disease and other autoimmune diseases, gallstone formation due to gallbladder hypomotility, pre-disposition to major hepatotropic viral infections and chronic diseases.27 In the databank, there were only 2 children confirmed for celiac disease and 1 was positive for IgA gliadin (unpublished results).
With respect to the blood concentration of minerals, most of the participants presented normal values for iron, magnesium, and manganese. Decreased zinc was observed in 10 out of 85 participants.
The Brazilian Pediatric Society recommends prophylactic iron supplementation in exclusively breastfed infants, without risk factors, from 180 days of life to the end of the second year.28 For premature or low-born weight newborns, the onset must occur at 30 days. This recommendation is because iron deficiency anemia is estimated to occur in 3% of infants and contributes to a range of neurological disorders. Possibly because of this guideline, 46.8% of the infants from the present study were using iron supplementation but T21 has some specificities that must be taken into consideration by the pediatrician assisting these infants when deciding to follow or not the mentioned recommendation.
T21 children have a similar risk for iron-deficiency anemia as the neurotypical population, but it may be missed because of macrocytosis, which is present in up to one-third of T21 patients. The American Academy of Pediatrics suggests obtaining a complete blood cell count with differential and either a combination of ferritin and C-reactive protein, or a combination of serum iron and total iron-binding capacity (TIBC), beginning at 1 year of age and annually thereafter to detect anemia or iron deficiency in this population.29 Empiric iron supplementation should be avoided in T21 in the absence of anemia because increased brain iron levels trigger the formation of oxidative species and increase the production of beta-amyloid proteins increasing the risk for neurodegeneration6 and T21 is already a risk factor for early-onset neurodegeneration. Moreover, it should be considered that infants who are feed infant formula do not need iron supplementation since formulas are excessively fortified and can, per se, result in undesirable iron intake.6
Studies have reported decreased zinc blood levels in T2130 which has been explained by an increased consumption due to the triplicated enzyme superoxide dismutase (SOD-1). In this study, only 11.7% of the participants presented zinc levels below the reference value and this may be partially explained by the fact that 35.7% of them were being supplemented with zinc.
The few studies that investigated vitamin blood concentration in T21 reported deficiencies when T21 kids/teenagers were compared with non-T21 groups.4,5 The results from the present study did not find vitamin concentration below the limit values except for vitamin D, which was decreased in 31.6% of the participants even though it was the most used supplement (60.8%). However, vitamin D deficiency/insufficiency is a global health issue that afflicts more than one billion children and adults worldwide, regardless of the trisomy presence.31 For vitamin A, only 1.4% of the participants were below the normal range values and for vitamin B12, 4.3%. This low frequency of deficiency was unrelated to supplementation because only 16.7% of the participants were supplementing vitamin A and 9.8% of vitamin B12.
Homocysteine is an amino acid formed from the demethylation of methionine and metabolized through two pathways: remethylation (dependent on vitamin B12 and folic acid) and transsulfuration (dependent on vitamin B6 and catalyzed by CBS). Most of the participants presented normal or higher levels of vitamin B12. Homocysteine was decreased in 26% of children and adolescents and apparently increased in teenagers. In earlier studies involving T21 patients, some investigators showed lower levels of plasmatic homocysteine32 which could be explained by the triplicated CBS gene.8
Even though the data being presented are important for understanding the use of supplements and blood exams altered in T21 Brazilian children and teenagers, this study has some limitations. Firstly, the participants were a sample of convenience from children with private health support which may have introduced selection bias. The authors do not know the differences between Brazilian children with public and private support. Moreover, considering the family income and the mother's scholarity, the participants do not express a sample of the Brazilian population. Another limiting factor was the very low number of teenagers which prevented broad conclusions for this age group. Moreover, blood exams were from different laboratories countrywide, and the authors relied on the report on their techniques and classifications without the authors’ scrutinization.
In conclusion, nutritional supplements (mainly vitamins, minerals, omega-3, and antioxidants) are frequently used by Brazilian T21 infants, children, and teenagers independently of professional counseling and/or supervision and should be a question to be raised during the clinical anamnesis since some of them may impact medical conduct. Moreover, many blood tests are altered in this population and clinicians should be aware of them in order to warrant an appropriate screening and the implementation of risk management measures as soon as possible and improve the general health of these persons.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors would like to thank all the families that kindly shared the information used in this study.