To perform a systematic review with meta-analysis and meta-regression to correlate the total scores of asthma control with the increase in the total scores of health-related quality of life levels of parents of asthmatic children.
SourcesThe search was carried out in the following databases: PubMed (MEDLINE); Embase and ScienceDirect (Elsevier); SciELO and LILACs (Bireme) in June 2017. The included studies assessed asthma control through the Asthma Control Questionnaire (ACQ), Asthma Control Test (C-ACT/ACT), and Global Initiative for Asthma (GINA) questionnaires, whereas the Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ) was applied to assess the HRQoL of parents and family members.
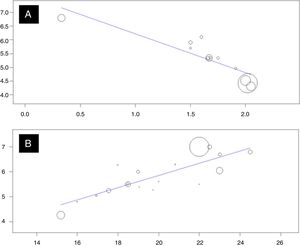
Summary of the findings294 articles were evaluated in the selected databases, of which (n=38) were excluded for duplicity; (n=239) after the reading of the titles and abstracts and (n=5) after reading the studies in full, totaling 12 studies eligible for the meta-analysis. Of the 12 eligible articles, 11 (92%) were published in the last five years, and evaluated children and adolescents aged 1–20 years, totaling 2804 samples. In the evaluation of the correlation between the disease control scores by ACQ and C-ACT/ACT, the results were satisfactory for both ACQ analyses [R2: −0.88; p<0.001], and for C-ACT/ACT [R2: 0.82; p<0.001].
ConclusionsThe results show that asthma control levels can influence the total HRQoL scores of parents or relatives of children and adolescents with asthma.
Realizar uma revisão sistemática, com meta-análise e meta-regressão para relacionar os escores totais do controle da asma com o aumento dos escores totais dos níveis de qualidade de vida relacionada à saúde de pais de crianças asmáticas.
FontesAs buscas foram aplicadas nas bases de dados: PubMed (Medline); Embase e ScienceDirect (Elsevier); SciELO e LILACs (Bireme). A busca foi realizada nas bases de dados em saúde em junho de 2017. Os estudos incluídos precisavam ter avaliados o controle da asma pelos questionários Asthma Control Questionnaire (ACQ), Asthma Control Test (C-ACT/ACT) e Global Initiative for Asthma (GINA) e para a QVRS dos pais e familiares o Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ).
Resumo dos achadosForam avaliados 294 artigos nas bases de dados escolhidas, quanto aos níveis de duplicidade nas bases de dados (n=38), excluídos por falta de enquadramento na leitura dos títulos e resumos (n=239) e após leitura integral dos estudos (n=05), restaram 12 estudos elegíveis à meta-análise. Dos 12 artigos elegíveis, 11 (92%) foram publicados nos últimos cinco anos, avaliaram crianças e adolescentes de 1 a 20 anos, total de 2.804 amostras. Na avaliação da correlação entre a pontuação dos escores de controle da doença por ACQ e C-ACT/ACT, os resultados se mostraram satisfatórios tanto para as análises de ACQ [R2: -0.88; p <0,001], quanto para C-ACT/ACT [R2: 0,82; p <0,001].
ConclusõesOs resultados demonstram que os níveis de controle da asma podem influenciar nos escores totais da QVRS de pais ou familiares de crianças e adolescentes com asma.
Asthma is one of the most common chronic diseases in childhood and is characterized by airway inflammation, airflow limitation and bronchial hyperresponsiveness, resulting in recurrent episodes of wheezing, dyspnea, chest tightness, and coughing.1,2 Such disease morbidities can have substantial effects on the health-related quality of life (HRQoL)3 of the children and their immediate family members.4 At the same time, the degree to which asthma influences the HRQoL depends on multiple factors, among which disease control is the main limitation for the quality of life of this population.5
Currently, the guidelines and consensuses for asthma management1,6 recommend that the interventions for asthma be intentionally applied aiming at disease control, resulting in a condition close to normal for the patients, increasing the physical, psychological, social, and affective levels, with the purpose of normalizing the HRQoL standards of the pediatric population.7 At the same time, for clinical interventions to be effective in controlling the disease, there are limitations related to several circumstances of genetic, environmental, cultural, or personal nature that must be precisely controlled for the proper management of asthma, resulting in a decrease in the asthma burden.8
Nevertheless, studies indicate that the health status of asthmatic children may not only directly or indirectly affect the health-related quality of life (HRQoL) of the patients, but also that of their relatives.9,10 That is, when there is no effective disease control, asthma can cause a reduction in the HRQoL levels of the children, and also of their parents, associated with worse clinical results of the disease.11 Moreover, other studies indicate that asthma control is proportional to the domains of parental health-related quality of life, especially when there is not good treatment adherence, leading to an exponential increase in asthma attacks, as well as recurrent visits to the emergency units or hospitalizations, directly affecting the disease burden, with the increase in work and school absenteeism.12,13
Considering these facts, the present study aimed to perform a systematic review, with a meta-analysis and a meta-regression to correlate the total scores of asthma control with the increase in the total scores of health-related quality of life of asthmatic children's parents.
Materials and methodsThe study is characterized by a systematic review, with a meta-analysis and meta-regression, based on the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),13 by applying a search strategy to five databases on health, to identify the main studies that assessed total disease control scores, as well as those related to the health-related quality of life of parents or caregivers of children diagnosed with asthma.
Aiming to evaluate disease control, both the subjective assessment criteria were used, through the Asthma Control Questionnaire (ACQ),14,15 the Asthma Control Test (C-ACT16/ACT17), and the Global Initiative for Asthma (GINA),1 as well as the objective evaluation criteria (pulmonary function and exhaled nitric oxide), and the primary outcome was studies that evaluated disease control through one of the questionnaires (ACQ, C-ACT/ACT, or GINA) and performed the assessment of the health-related quality of life (HRQoL) of parents or caregivers of children diagnosed with asthma, using the Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ)6 score, as well as the modules of limitations in activities and emotional functions. Thus, the primary endpoint correlated the increase in children's asthma control scores with the increase in their parents’ total HRQoL scores.
Search strategyAs the search strategy, the logic based on specific descriptors was used (language: English-USA), associated with the Boolean operators (AND and OR), using parentheses () to define intercalations within the same logic and quotation marks (”) for the identification of compound words, being applied as follows: (Asthma AND (“Control Disease” OR “Asthma Control”) AND (PACQLQ OR “Pediatric Asthma Caregiver Quality of Life Questionnaire”). The searches were carried out in December 2017 in the following databases: PubMed (MEDLINE); Embase and ScienceDirect (Elsevier); SciELO and LILACs (Bireme), and revised in March 2018, for the purpose of checking new articles during the period of the meta-analysis construction.
Aiming to prevent the excessive inclusion of articles, the searches were limited to the following fields: Title, Keywords, and Abstract. Thus, the descriptors should be mandatorily included in at least one of the three search fields, hence as example the search performed in the PUBMED database: (Asthma[Title/Abstract] OR Asthma[MeshTerms]) AND (“Control Disease”[Title/Abstract] OR “Control Disease”[MeshTerms] OR “Asthma Control”[Title/Abstract] OR “Asthma Control”[MeshTerms]) AND (PACQLQ[Title/Abstract] OR PACQLQ[MeshTerms] OR “Pediatric Asthma Caregiver Quality of Life Questionnaire”[Title/Abstract] OR “Pediatric Asthma Caregiver Quality of Life Questionnaire”[MeshTerms]).
Additionally, no other filters were added for limitations, such as: article language, target audience, or date of publication. After selecting the potentially eligible articles for the systematic review in the databases, the files were exported in the following formats (extensions): *.txt (MEDLINE), *.bib (Bibitex), or *.ris (Ris), containing the following information: authors, article title, keywords, journal, year, type of article, and abstract.
Recruitment and selection biasFor the purpose of recruiting potentially eligible articles for the systematic review, the specific software for systematic review StArt (State of the Art through Systematic Review)17 was used after the exportation of the studies selected from the databases, which was the basis for the identification of articles that were previously selected for the study flowchart, which included four phases: (a) identification – study recruitment; (b) selection – duplicates and exclusion based on the reading of titles and abstracts; (c) eligibility – exclusion after reading the full-text studies and (d) inclusion – eligible studies, according to pre-established inclusion criteria.
Each phase was performed separately by two researchers (CM; CR) and analyzed by a third reviewer (PMP), following three criteria for inclusion or exclusion of articles: (a) articles equally selected by the two researchers were included; (b) articles not selected were excluded; (c) articles included by only one researcher were analyzed by the reviewer, which after being considered adequate, were included.
For the purpose of study inclusion through other methods (gray literature), the evaluation/inclusion criterion was adopted after reading the references (citations) of the studies included during the eligibility phase (article full-text reading).
Inclusion criteria and study characteristicsFor inclusion in the systematic review, the articles were required to be cross-sectional studies or early stages of longitudinal case–control studies, or randomized clinical trials that addressed the total disease control scores as primary or secondary data, as well as the total scores of health-related quality of life levels of asthmatic children's caregivers, through the PACQLQ.
Studies that did not use the disease control criteria through the ACQ, C-ACT, ACT, or GINA were excluded, in addition to those that did not use PACQLQ to measure the levels of HRQoL of parents/caregivers.
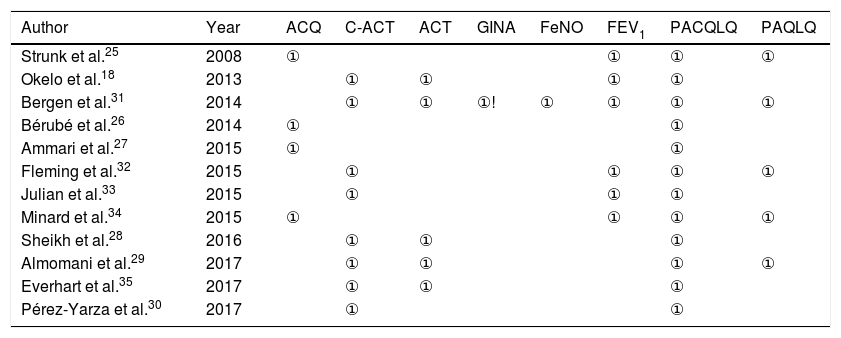
Data extraction and presentationFor data extraction and identification of the studies eligible for the systematic review, pre-formatted tables were used to record the main author, year of publication, study site, study design, number of participants, age group, and journal. Additionally, as data extraction eligibility criteria, the study comparative analysis data were extracted for the following tests: ACQ, ACT, C-ACT, GINA, fractional exhaled nitric oxide (FeNO), Pulmonary function (FEV1), PACQLQ, and PAQLQ, with the score of ① being assigned to variables available for extraction in the studies and ⓪ for variables absent in the studies.
Data presentation was structured to demonstrate the values regarding study selection design, general characteristics by year of publication, the eligibility and extraction of the outcome variables, as well as the mean and standard deviation of the variables, in addition to the primary outcome correlations.
Statistical analysisTo perform the meta-analysis, after the eligibility of the articles and identification of the outcome variables, the software Open Meta-Analysis18 was used, and the randomized mean-rate statistics (univariate) applied, with a confidence interval of 95 (95% CI), heterogeneity (I2), with a significance level of p<0.05, to evaluate the mean score between the asthma control scores (ACQ and ACT/C-ACT), as well as the PACQLQ and PAQLQ.
To evaluate the PACQLQ between groups of children with and without asthma control and for the comparison between the PACQLQ and PAQLQ, randomized mean-rate statistics (bivariate) were used, with a 95% confidence interval (95% CI), heterogeneity (I2), with a significance value of p<0.05. To evaluate the estimate of the mean values of total asthma control score variation and total PACQLQ scores, a meta-regression analysis was applied with randomized proportional metrics (R2) the correlation between the total scores was estimated, with a significance value of p<0.05.
Systematic review registryFor the purpose of the systematic review registry, the study was previously registered on the website of the Center for Reviews and Dissemination – PROSPERO (http://www.crd.york.ac.uk/PROSPERO), identified by the registration number: CRD42017071120.
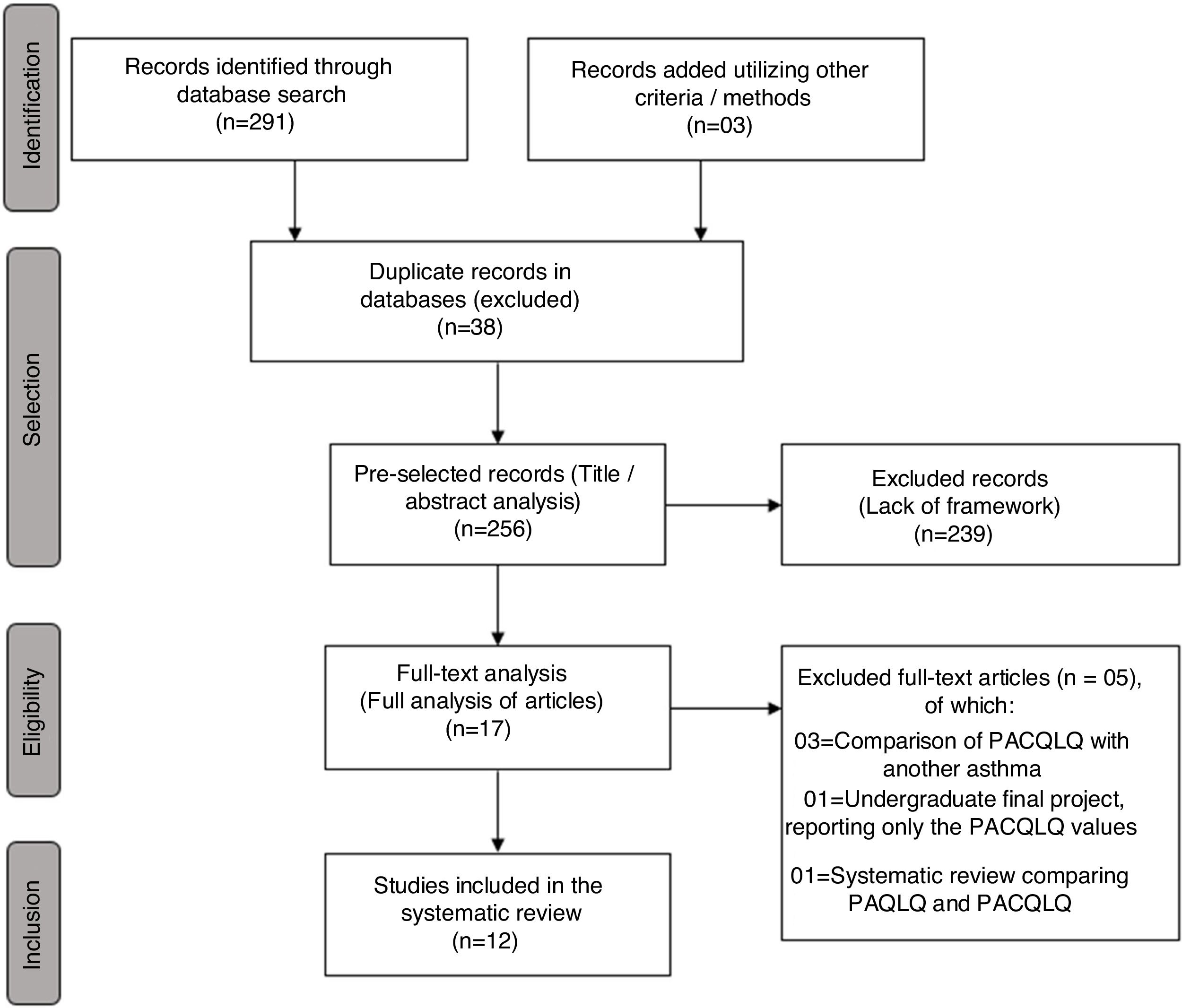
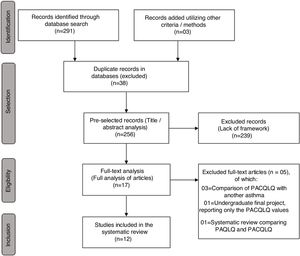
ResultsInitially, 291 articles were retrieved through Health Databases (PubMed: 98, Embase 103, ScienceDirect: 79, SciELO: 5, LILACS: 6) and three articles through records found in the gray literature, totaling 294 articles evaluated regarding the number of duplicates in the databases (n=38), those excluded due to lack of appropriateness after reading the titles and abstracts (n=239) and after reading the full-text articles (n=5), thus yielding a total of 12 studies eligible for the meta-analysis, as shown in the flowchart (Fig. 1).
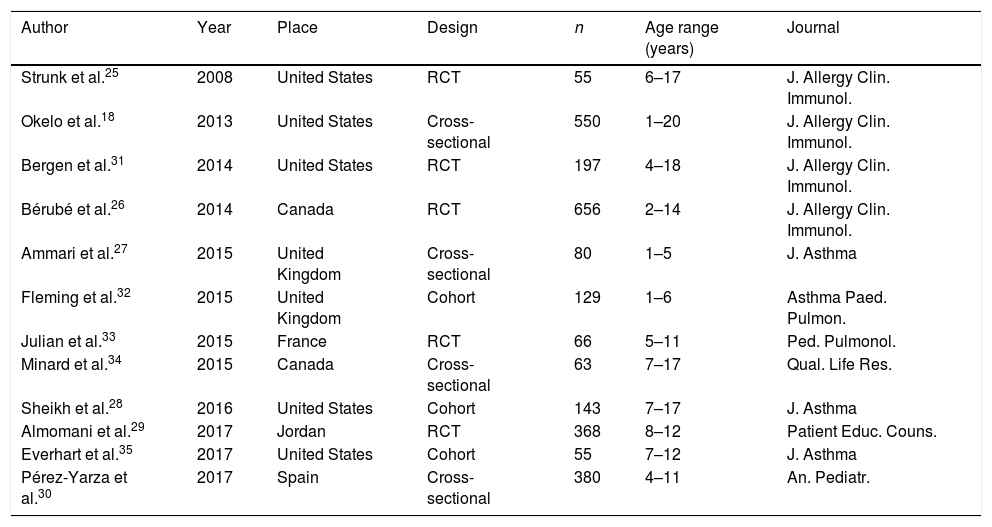
Of the 12 eligible articles, 11 (92%) were published in the last five years, evaluating children and adolescents aged 1 to 20 years, totaling 2804 samples. Seven (58%) studies were carried out in the North American continent, four (33%) in the European continent, and one (9%) in the Asian continent, having been published in the last ten years (2008–2017), as shown in Table 1.
General identification of studies eligible for the meta-analysis.
| Author | Year | Place | Design | n | Age range (years) | Journal |
|---|---|---|---|---|---|---|
| Strunk et al.25 | 2008 | United States | RCT | 55 | 6–17 | J. Allergy Clin. Immunol. |
| Okelo et al.18 | 2013 | United States | Cross-sectional | 550 | 1–20 | J. Allergy Clin. Immunol. |
| Bergen et al.31 | 2014 | United States | RCT | 197 | 4–18 | J. Allergy Clin. Immunol. |
| Bérubé et al.26 | 2014 | Canada | RCT | 656 | 2–14 | J. Allergy Clin. Immunol. |
| Ammari et al.27 | 2015 | United Kingdom | Cross-sectional | 80 | 1–5 | J. Asthma |
| Fleming et al.32 | 2015 | United Kingdom | Cohort | 129 | 1–6 | Asthma Paed. Pulmon. |
| Julian et al.33 | 2015 | France | RCT | 66 | 5–11 | Ped. Pulmonol. |
| Minard et al.34 | 2015 | Canada | Cross-sectional | 63 | 7–17 | Qual. Life Res. |
| Sheikh et al.28 | 2016 | United States | Cohort | 143 | 7–17 | J. Asthma |
| Almomani et al.29 | 2017 | Jordan | RCT | 368 | 8–12 | Patient Educ. Couns. |
| Everhart et al.35 | 2017 | United States | Cohort | 55 | 7–12 | J. Asthma |
| Pérez-Yarza et al.30 | 2017 | Spain | Cross-sectional | 380 | 4–11 | An. Pediatr. |
RCT, randomized clinical trial.
Table 2 shows the main study evaluation variables, both for disease control and health-related quality of life assessment of children with a clinical diagnosis of asthma and their parents; ① was assigned to variables available for extraction and ⓪ for variables not disclosed or evaluated by the studies.
Outcome variables assessed in the meta-analysis.
| Author | Year | ACQ | C-ACT | ACT | GINA | FeNO | FEV1 | PACQLQ | PAQLQ |
|---|---|---|---|---|---|---|---|---|---|
| Strunk et al.25 | 2008 | ① | ⓪ | ⓪ | ⓪ | ⓪ | ① | ① | ① |
| Okelo et al.18 | 2013 | ⓪ | ① | ① | ⓪ | ⓪ | ① | ① | ⓪ |
| Bergen et al.31 | 2014 | ⓪ | ① | ① | ①! | ① | ① | ① | ① |
| Bérubé et al.26 | 2014 | ① | ⓪ | ⓪ | ⓪ | ⓪ | ⓪ | ① | ⓪ |
| Ammari et al.27 | 2015 | ① | ⓪ | ⓪ | ⓪ | ⓪ | ⓪ | ① | ⓪ |
| Fleming et al.32 | 2015 | ⓪ | ① | ⓪ | ⓪ | ⓪ | ① | ① | ① |
| Julian et al.33 | 2015 | ⓪ | ① | ⓪ | ⓪ | ⓪ | ① | ① | ⓪ |
| Minard et al.34 | 2015 | ① | ⓪ | ⓪ | ⓪ | ⓪ | ① | ① | ① |
| Sheikh et al.28 | 2016 | ⓪ | ① | ① | ⓪ | ⓪ | ⓪ | ① | ⓪ |
| Almomani et al.29 | 2017 | ⓪ | ① | ① | ⓪ | ⓪ | ⓪ | ① | ① |
| Everhart et al.35 | 2017 | ⓪ | ① | ① | ⓪ | ⓪ | ⓪ | ① | ⓪ |
| Pérez-Yarza et al.30 | 2017 | ⓪ | ① | ⓪ | ⓪ | ⓪ | ⓪ | ① | ⓪ |
ACQ, asthma control questionnaire; ACT, asthma control test; C-ACT, asthma control test in children; GINA, Global Initiative for Asthma; FEV1, forced expiratory volume in the first second; FeNO, fractional exhaled nitric oxide; PACQLQ, Pediatric Asthma Caregivers’ Quality of Life Questionnaire; PAQLQ, Pediatric Asthma Quality of Life Questionnaire; NA, not available; ①, criterion adopted by the study, ⓪, criterion not adopted or not declared in the studies. ①!, criterion adopted in the study, but only for the distinction between controlled, partially controlled and uncontrolled asthma groups.
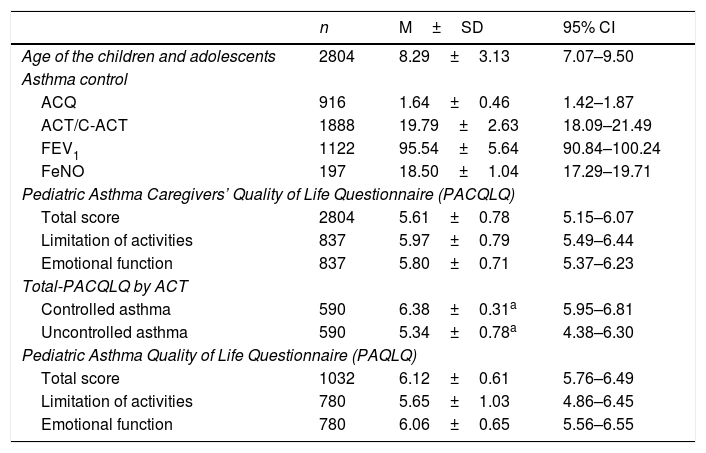
Table 3 shows the means, standard deviations, and confidence intervals of the variables studied in the meta-analysis. Additionally, the correlation values between the asthma control tests (objective and subjective) and the total scores of the PACQLQ are presented, showing that there are important correlations between increased asthma control and increased quality of life scores of parents of children with asthma. These values were confirmed in the comparison between the means of the total PACQLQ scores with a subsample of 590 patients with and without disease control (p=0.047), where the parents of children with controlled asthma showed a 1.04 fold-higher mean score (1–7), in comparison to parents of children who did not have disease control.
Overall assessment of data extracted from the 12 studies eligible for the meta-analysis.
| n | M±SD | 95% CI | |
|---|---|---|---|
| Age of the children and adolescents | 2804 | 8.29±3.13 | 7.07–9.50 |
| Asthma control | |||
| ACQ | 916 | 1.64±0.46 | 1.42–1.87 |
| ACT/C-ACT | 1888 | 19.79±2.63 | 18.09–21.49 |
| FEV1 | 1122 | 95.54±5.64 | 90.84–100.24 |
| FeNO | 197 | 18.50±1.04 | 17.29–19.71 |
| Pediatric Asthma Caregivers’ Quality of Life Questionnaire (PACQLQ) | |||
| Total score | 2804 | 5.61±0.78 | 5.15–6.07 |
| Limitation of activities | 837 | 5.97±0.79 | 5.49–6.44 |
| Emotional function | 837 | 5.80±0.71 | 5.37–6.23 |
| Total-PACQLQ by ACT | |||
| Controlled asthma | 590 | 6.38±0.31a | 5.95–6.81 |
| Uncontrolled asthma | 590 | 5.34±0.78a | 4.38–6.30 |
| Pediatric Asthma Quality of Life Questionnaire (PAQLQ) | |||
| Total score | 1032 | 6.12±0.61 | 5.76–6.49 |
| Limitation of activities | 780 | 5.65±1.03 | 4.86–6.45 |
| Emotional function | 780 | 6.06±0.65 | 5.56–6.55 |
| Correlation between PACQLQ (total score) | n/Studies | R2 | p-Value |
|---|---|---|---|
| ACQ | 12 | −0.88 | <0.001b |
| ACT/C-ACT | 18 | 0.82 | <0.001b |
| FEV1 | 15 | 0.26 | 0.356 |
| FeNO | 03 | 0.48 | 0.679 |
n, number of participants; M±SD, mean and standard deviation; 95% CI, 95% confidence interval; ACQ, asthma control questionnaire; ACT, asthma control test in children aged 12–17 years; C-ACT, asthma control test in children aged 4–11 years; FEV1, forced expiratory volume in the first second; FeNO, fractional exhaled nitric oxide; PACQLQ, Pediatric Asthma Caregivers’ Quality of Life Questionnaire; PAQLQ, Pediatric Asthma Quality of Life Questionnaire.
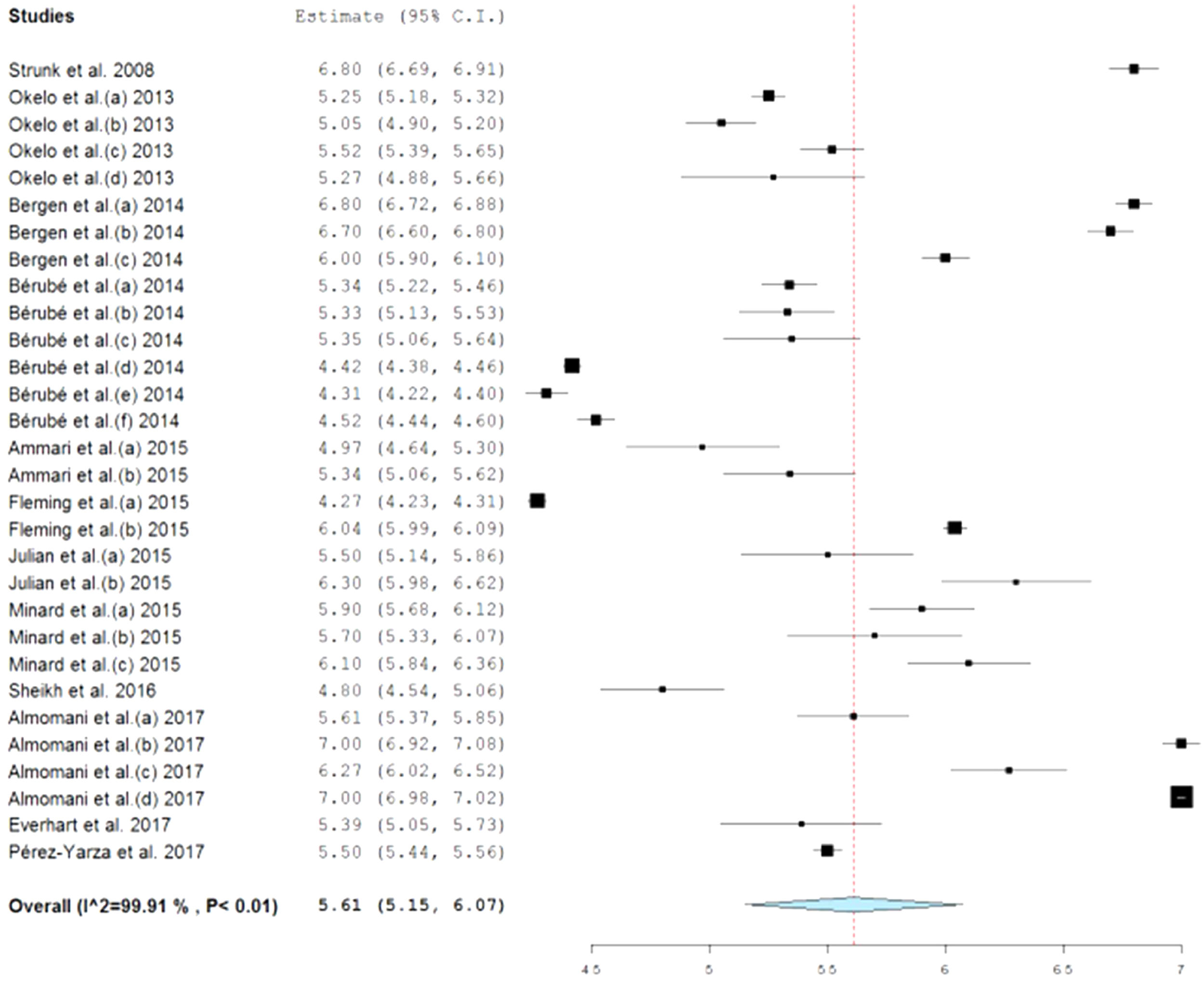
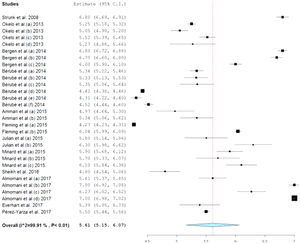
In Fig. 2, the forest plot of the mean score estimate of the PACQLQ total scores shows values within normality (5.61 points), but with high heterogeneity (I2: 99.9%, p<0.001) with a minimum score of 4.27 and maximum of 7.0 points, where the higher the score (1–7), the better the health-related quality of life of parents and relatives of children with asthma.
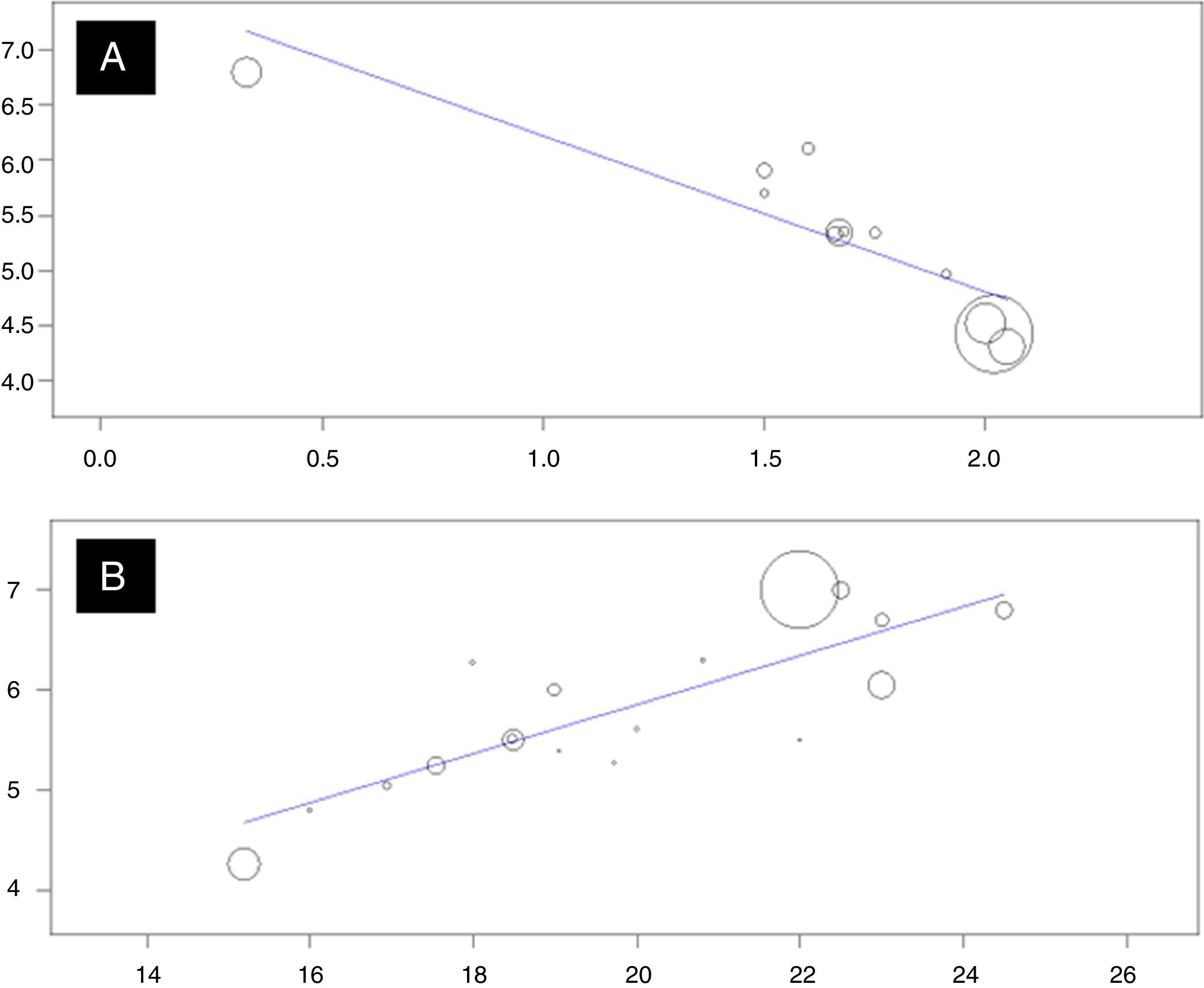
Finally, when evaluating the correlation between the disease control scores through the ACQ and C-ACT/ACT, the results were satisfactory for both ACQ analyses (R2: −0.88; p<0.001), and for C-ACT/ACT (R2: 0.82; p<0.001), indicating that the greater the asthma control, the higher the quality of life of parents and relatives of children and adolescents with a clinical diagnosis of asthma (Fig. 3).
DiscussionThe health-related quality of life (HRQoL) of people with chronic diseases has been the subject of discussions and research in the last two decades, aiming at assessing the control and leveling of the global burden of disease, not only of patients but also of the patients’ family members or caregivers. In the case of pediatric asthma, the disease impact on the HRQoL of parents and family members may be influenced by the lack of control of their children's disease. As asthma is a multifactorial disease, effective control depends on how parents manage treatment adherence, preventing symptom recurrence, which leads to an increased number of follow-up visits to doctors or emergency units and hospital admissions, as well as increased occupational absenteeism. Thus, one of the main possibilities for children and their parents to have HRQoL at acceptable levels of normality is to keep their child's asthma controlled, free of symptoms and exacerbations.
In the present meta-analysis, such possibilities are confirmed in two of the four asthma control tests evaluated (ACT and ACQ), demonstrating that, according to the increase in total scores of disease control in the pediatric population, together the total scores of HRQoL of the children's parents or caregivers are significantly increased by the total PACQLQ scores. At the same time, when considering objective variables such as the forced expiratory volume in the first second (FEV1) and fractional exhaled nitric oxide (FeNO), such hypotheses are considered to be non-existent, due to the correlation between increased pediatric asthma control and increased HRQoL scores of their parents or caregivers. These findings are explained by Okelo et al.,18 who concluded in their study that there is no direct association between the values of children's lung function and HRQoL levels. This is due to the fact that spirometry has limitations regarding its use in the pediatric population,19 in which children diagnosed with asymptomatic asthma usually have spirometry values within the normal limits, differently from the adult population,20 in whom lung function is the main marker for medical diagnosis and disease control assessment. As for the FeNO, studies indicate that the evaluation of exhaled nitric oxide is an important method of assessment, but to evaluate the lung inflammatory level, and is not equally effective in quantifying the degree of disease control in the pediatric population.11,20,21
For more than two decades, the HRQoL has been studied in the scientific area and in interventions for the management of chronic and acquired diseases, with the main focus being the assessment of HRQoL in the disease-affected population.3 However, throughout this time, new approaches have demonstrated the importance of evaluating HRQoL not only of this population, but also of their direct relatives, as in the case of parents and caregivers, which in some cases, as in studies associating asthma severity, or lack of disease control, have shown that pediatric asthma has an even greater impact on parents/guardians than on the pediatric population itself .4,21–24
However, in the present meta-analysis, the results evaluating the HRQoL of parents and caregivers of children and adolescents diagnosed with asthma show that the levels of the parents’ HRQoL are at satisfactory levels (Fig. 2), not only for the total scores of the PACQLQ (5.61±0.78), but also for the domains of activity limitations (5.97±0.79) and emotional function (5.80±0.71) on a 1–7 point Likert scale, showing that the higher the score, the better the HRQoL levels (Table 3). This result is due to the fact that many of the studies show in their results total PACQLQ scores close to the maximum score (seven points), showing acceptable levels of the parents’ HRQoL, as follows: Strunk et al.,25 Okelo et al.,18 Bérubé et al.,26 Ammari et al.,27 Sheikh et al.,28 Almomani et al.,29 and Pérez-Yarza et al.30
In the comparison between the HRQoL of parents and caregivers and their children's disease control, total scores of PACQLQ between two ACT groups (controlled and uncontrolled asthma, Table 3), significant differences were observed for the group with controlled asthma compared to the uncontrolled asthma group, with 1.04-fold higher mean total scores (p=0.047), demonstrating that the parents, even with acceptable levels of quality of life, show higher scores on the questionnaire compared to children who do not have effective disease control. In 2008, Strunk et al.25 evaluated the quality of life of asthmatic children and their respective parents and relatives, demonstrating that both parents and direct relatives of asthmatic children score higher (6.8) on the quality of life questionnaire than asthmatic children (6.6). Bergen et al. (2014)31 evaluated the quality of life of children and their families, and also identified that parents and family members had a better quality of life than the children (6.5 vs. 6.2). Children with well-controlled asthma had a quality of life score (PAQLQ and PACQLQ) that showed a significant difference between children with partially controlled or uncontrolled asthma (p<0.001).
When the health-related children's quality of life (PAQLQ) was evaluated, it showed that they had a good quality of life (6.1), with total scores close to 7, according to the Likert scale. Strunk et al.,25 Bergen et al.,31 Fleming et al.,32 Julian et al.,33 Minard et al.,34 and Everhart et al.,35 in their evaluations of HRQoL, found values similar to the findings in the present study, which identified that despite all the facets of asthma, children with the disease can still maintain a good HRQoL.
In the meta-regression, the present study showed that asthma control and HRQoL have a significant correlation between their total scores. When the correlation between PACQLQ and ACQ was performed, an R2 of −0.88 (p<0.001) was reached, and between PACQLQ and ACT, an R2 of 0.82 was obtained (p<0.001).
The findings of the studies by Strunk et al.,25 Okelo et al.,18 Bérubé et al.,26 Ammari et al.,27 Sheikh et al.,28 Almomani et al.,29 and Pérez-Yarza et al.,30 in their analyses, show there is a significant correlation between the PACQLQ and the asthma control parameters evaluated by the ACQ or ACT and C-ACT questionnaires.
Bérubé et al.,26 Sheikh et al.,28 and Pérez-Yarza et al.30 in their studies suggest that the lack of asthma control can significantly affect the physical, emotional, and social parameters of children and their parents/family members. As the main hypothesis for such statements, they associate the lack of disease control with loss of productivity (school and work absenteeism), affecting the levels of HRQoL of their parents/family members.
In the study by Sheikh et al.,28 the results provide statistical evidence that the quality of life in parents and relatives of asthmatic children improved simultaneously with improved levels of asthma control (PACQLQ at the start of the study: 4.7 points and at the end, 6.8 points; the value of asthma control by ACT at the beginning of the study was 16 points, and at the end, 21.1 points).
The findings of this study suggest that quality of life is a useful treatment adherence and disease control predictor. Parents’ or family members’ decision-making regarding the management and control of their children's asthma may be affected by reduced quality of life, thus it is important to include quality of life analysis as part of the clinical assessment, as well as the implementation of care aimed at increasing asthma control in children and, consequently, the increase in HRQoL, having as its main characteristics the integral care for both the children and their families.
Moreover, Pérez-Yarza et al.,30 regarding the results of the ACT-related analyses, demonstrated not only a good correlation with the clinical perception of asthma control, but also with some other clinical variables, such as the classification of asthma severity and the number of exacerbations and, finally, with HRQoL, evaluated by the PAQLQ and PACQLQ scores. With this conclusion, the authors associate the increase in the parents’ quality of life to the children's asthma control, associated to active education programs, effective medical care, and adherence to appropriate treatment.
The lack of studies with objective measures such as pulmonary function and exhaled nitric oxide comparing the HRQoL of parents and caregivers, or even other tools such as ACT or GINA, is a limiting factor, because it restricts the findings in the PACQLQ evaluation in the ACT and C-ACT tests. Moreover, even though PACQLQ is a validated and widely disseminated tool both in the clinical and scientific areas, the lack of other specific tools to evaluate the HRQoL of parents and caregivers of asthmatic children is another limiting factor for the comparative analysis, both of HRQoL and the outcomes evaluated in the present meta-analysis.
Finally, this meta-analysis clearly shows that the asthma control levels can influence the total quality of life scores of parents or relatives of children and adolescents with asthma. Thus, the authors emphasize the importance of adequate follow-up of this population, with emphasis on factors that can lead to an unfavorable outcome of their condition, such as lack of adherence to the treatment of pediatric asthma and lack of disease control.
Associated institutionPontifícia Universidade Católica do Rio Grande do Sul (PUCRS); Postgraduate Program in Pediatrics and Child Health; Núcleo de Educação em Saúde da Criança (NESC).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Costa DD, Pitrez PM, Barroso NF, Roncada C. Asthma control in the quality of life levels of asthmatic patients’ caregivers: a systematic review with meta-analysis and meta-regression. J Pediatr (Rio J). 2019;95:401–9.