To analyze the association between age at menarche and variables of body composition in girls from the Northern region of Brazil, the Brazilian Legal Amazon.
MethodThe sample was composed of 926 school girls, aged between 8 and 18 years, divided into two groups, those who had (G1; n=727; 72.5%) and had not undergone menarche (G2; n=199; 21.5%), from public and private schools, using the stratified random proportional sampling technique. Weight, fat weight, muscle weight, and body mass index were measured using bioimpedance analysis. Body height was measured using a stadiometer. Age at menarche was obtained using the conventional method. For the evaluation of sexual maturation, self-assessment was performed according to criteria described by Tanner.
ResultsThe highest cumulative distribution of menarche was found at age 11, and presented significant differences between G1 and G2 at ages 11 and 12 years in all variables of body composition, except body mass index Z-score. Fat and muscle mass were associated with age at menarche.
ConclusionThe present results support the notion of menarche anticipation in girls from Brazilian Amazon and its association with body composition. Further studies are needed to investigate the influence of other possible factors that may interfere with the time of growth spurt, thus determining the timing of puberty in these girls in comparison to other regions of Brazil.
Analisar a associação entre idade da menarca e variáveis da composição corporal em meninas na região norte do Brasil, a Amazônia brasileira.
MétodoA amostra foi composta por 926 meninas em idade escolar, entre 8 e 18 anos, divididas em dois grupos, com presença de menarca (G1), n=727 (72,5%) e ausência (G2) n=199 (21,5%), provenientes de escolas públicas e privadas, com a técnica de amostragem aleatória estratificada proporcional. Peso, massa de gordura, massa muscular e índice de massa corporal foram medidos através da análise de bioimpedância. A altura foi medida com um estadiômetro. A idade da menarca foi obtida pelo método status quo. Para a avaliação da maturação sexual, a autoavaliação foi realizada de acordo com os critérios descritos por Tanner.
ResultadosA maior distribuição cumulativa da menarca foi encontrada aos 11 anos, e apresentou diferenças significativas entre a presença e ausência de menarca aos 11 e 12 anos em todas as variáveis de composição corporal, exceto o escore-z do índice de massa corporal. Massa de gordura e massa muscular foram associadas com a idade da menarca.
ConclusãoOs resultados apresentados corroboram a antecipação da menarca em meninas da Amazônia brasileira e sua associação com a composição corporal. Mais estudos são necessários para investigar a influência de outros possíveis fatores que podem interferir na época do estirão de crescimento e determinar, assim, a ocorrência da puberdade em meninas amazônicas em comparação com as de outras regiões do Brasil.
Puberty is defined as the period when secondary sexual characteristics and sexual reproduction capacity develop; menarche is an event identified as a marker of reproductive maturity, but this does not occur simultaneously with psychosocial maturity. While genetic factors are the main determinants for puberty onset, other factors appear to be involved, such as nutritional status, general health, and psychological status.1 Age at menarche varies considerably in different countries, but different studies report that this event has been occurring earlierfor the last three decades in different parts of the world.2–5 In this sense, a tendency has been shown for an earlier age of onset of breast development, as well as an association with a higher BMI.6 Evidence shows an association between excess weight and age at menarche; indeed, there appears to be a coincidence between the worldwide epidemic of obesity and this anticipation on sexual maturation.3–5
Earlier puberty may lead to negative consequences and health outcomes, such as low self-esteem, adolescent body dissatisfaction, eating disorders, and early sexual initiation, as well as increased risk for diabetes mellitus and breast cancer in adulthood.2 As a consequence of profound biopsychosocial alterations and proximity to sexual maturation, adolescents are the target of worldwide studies to provide opportunities for the prevention of health problems. New studies tend to deepen the associations already reported with an approach in relation to menarche and body variables.
Therefore, the aim of the present study was to analyze the association between age at menarche and variables of body composition in girls in the northern region of Brazil, the Brazilian Legal Amazon.
MethodsThis was a school-based cross-sectional study carried out in the city of Porto Velho, Rondônia, with 428,527 inhabitants, located in the southwest of the Brazilian Legal Amazon. The estimated total population of enrolled students, attending primary and secondary schools in Porto Velho was 26,546 students, distributed in the 8- to 16-year age range, in the period from November 2014 to December 2015 period, according to the State Secretariat of Education (Rondônia). The schools were stratified according to the number of students using the simple randomization technique of pairing-proportionality with students from public, 479 (44.48%) and private schools, 598 (55.52%).
The equation, no=1/Eo2n=N.n/N+no,7 was used to calculate the sample size, based on a prevalence of 50% for excess weight, 3% margin of error, 95% confidence interval, which led to a total of 1026 participants. Considering the loss and refusal of 100 girls (9.75%), the study was conducted with 926 participants. After data collection for analysis, the girls were divided into two groups: those who had undergone menarche (G1; n=727; 72.5%) and those who had not (G2; n=199; 21.5%).
The selection process was conducted in three stages: first, a stratified sample proportional to the number of schools in each stratum (north, south, east, and west areas) of the city was made; in the second stage, schools were selected through a proportional random selection in each stratum; in the third stage, the classes were randomly selected, from which all female students were invited to participate in the study. This sampling process ensured that each student had the same chance of being selected. Inclusion criteria were all students and parents who had signed the informed consent form and agreed to participate in this two-stage study: stage 1 questionnaire for the identification of the presence and absence of menarche; and stage 2 anthropometric measurements (body weight, body height, and BMI).
Mean age at menarche was determined using the conventional method by questioning the girl if she had had her first menstruation. For positive responses, a retrospective questionnaire was used seeking to identify the day, month, and year of the event. Sexual maturity was assessed through self-assessment with comparison to pictures of the five breast development classification stages (B1–B5), according to criteria described by Marshall and Tanner (1969).8 Girls who had attained B2 or higher with menstruation were considered at menarche, and age at menarche was calculated.
Body height was measured using a portable stadiometer according to protocol recommended by Petroski,9 and body weight and BMI using a tetrapolar bioelectrical impedance analyzer (InBody, RJ, Brazil), according to protocol recommended by the National Institutes of Health.10 The team, which was previously trained to apply the questionnaire and take measurements, was made up of a physician-researcher and medical school students; all work was conducted in the school environment.
In the descriptive analysis, cumulative frequency of age was calculated. The Kolmogorov and Shapiro–Wilk normality test was used to verify whether the following data were normally distributed: body weight (kg), body height (cm), and BMI z-score (kg/(E[m])2, fat mass (kg) and muscle mass (kg), according to the groups, using as comparison between the two groups the mean, standard deviation, and Student's t-test for independent samples when the distribution was considered normal (p>0.05), and the median, interquartile range, and Mann–Whitney's U test for two independent samples for non-normal distribution (p<0.05), with significance level of p<0.05.
Multivariate logistic regression was also performed, estimating the effect of fat and muscle mass on adjusted age at menarche. From logistic regression, odds ratios and 95% confidence intervals (95% CI) were obtained. A p-value <0.05 was considered significant. All statistical analyses were performed using the statistical software package SPSS, version 17.0.
The study was approved by the Research Ethics Committee of the Federal University of Rondônia on 07/16/2014, CAAE:335422014.0.0000.5300. Regulatory norms for research involving human beings were followed in accordance with Resolution No. 466/2012, certified by the National Health Council on December 12, 2012.
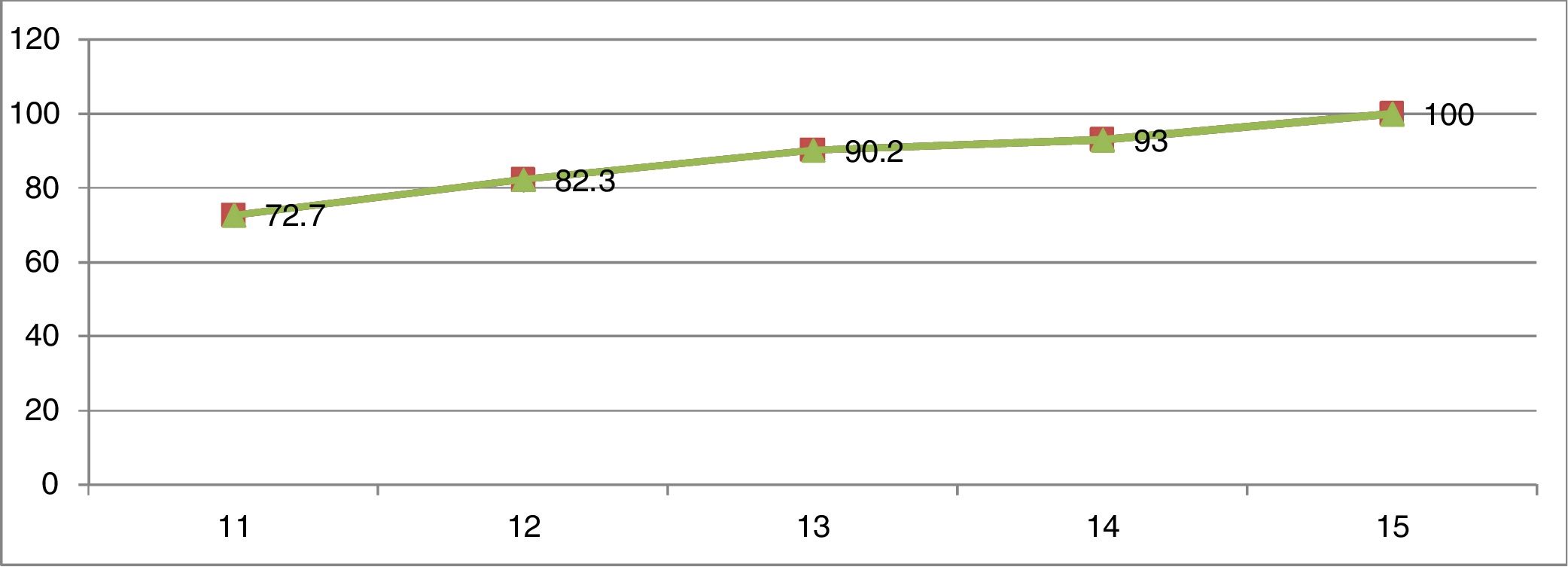
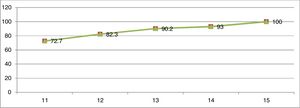
ResultsThe final sample consisted of 926 female schoolchildren, with 727 (72.5%) reporting the presence of menarche and 199 (21.5%) the absence of menarche. Fig. 1 shows the cumulative distribution of the proportion of age at menarche. Of the 524 girls aged ≤11 years, 381 had undergone menarche, corresponding to 72.70% of the total for age. At 12 years of age, this percentage increased by 10%, at 13 years by 7.9%, at 14 years by 2.8%, and finally at 15 years it increased by 7%, totaling 100% of girls who had undergone menarche (Fig. 1).
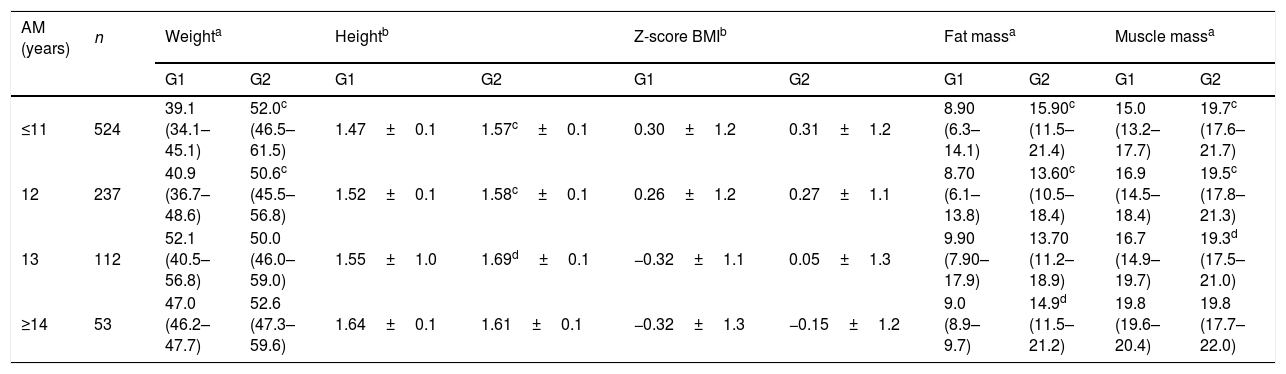
Table 1 shows that at the age groups of ≤11 and 12 years, girls in the G1 were significantly heavier than those in the G2 (p≤0.001). Concerning body height, girls were taller in the G1, at age groups of ≤11, 12 (p≤0.005) and 13 years (≤0.001). When BMI was assessed according to Z-score, using a cutoff point greater than or equal to Z-score +1 (percentile 85) for overweight, no statistical differences were observed between the groups. Fat mass was higher in G1 vs. G2 at all ages, with statistical significance at ≤11, 12 (p≤0.005), and ≥14 years (p≤0.001). Higher muscle mass was also observed in the G1 when compared with G2 at ages ≤11, 12 (p≤0.005), and 13 years (p≤0.001).
Mean, standard deviation, median, and interquartile of weight (kg), body height (m), Z-score BMI, fat mass (kg), and muscle mass (kg) according to age and absence (G1) and presence (G2) of menarche in schoolgirls in the city of Porto Velho, Rondônia, Brazil, 2014–2015.
| AM (years) | n | Weighta | Heightb | Z-score BMIb | Fat massa | Muscle massa | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | G1 | G2 | ||
| ≤11 | 524 | 39.1 (34.1–45.1) | 52.0c (46.5–61.5) | 1.47±0.1 | 1.57c±0.1 | 0.30±1.2 | 0.31±1.2 | 8.90 (6.3–14.1) | 15.90c (11.5–21.4) | 15.0 (13.2–17.7) | 19.7c (17.6–21.7) |
| 12 | 237 | 40.9 (36.7–48.6) | 50.6c (45.5–56.8) | 1.52±0.1 | 1.58c±0.1 | 0.26±1.2 | 0.27±1.1 | 8.70 (6.1–13.8) | 13.60c (10.5–18.4) | 16.9 (14.5–18.4) | 19.5c (17.8–21.3) |
| 13 | 112 | 52.1 (40.5–56.8) | 50.0 (46.0–59.0) | 1.55±1.0 | 1.69d±0.1 | −0.32±1.1 | 0.05±1.3 | 9.90 (7.90–17.9) | 13.70 (11.2–18.9) | 16.7 (14.9–19.7) | 19.3d (17.5–21.0) |
| ≥14 | 53 | 47.0 (46.2–47.7) | 52.6 (47.3–59.6) | 1.64±0.1 | 1.61±0.1 | −0.32±1.3 | −0.15±1.2 | 9.0 (8.9–9.7) | 14.9d (11.5–21.2) | 19.8 (19.6–20.4) | 19.8 (17.7–22.0) |
AM, age at menarche; BM, body mass.
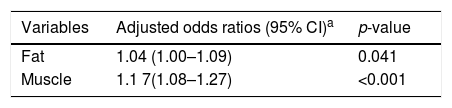
The logistic regression analysis for the chance of having undergone menarche, adjusted for chronological age, indicated an odds ratio of 1.04 (95% CI 1.00–1.09, p=0.041) for each 1kg increment in fat mass and an odds ratio of 1.17 (95% CI 1.08–1.27, p<0.001) for each 1kg increment in muscle mass.
DiscussionIn the present study, the occurrence of menarche was associated with changes in body composition, more strongly with gain in body fat and muscle mass. When comparing G1 with G2, the variables of body composition presented significant differences, and the ages of 11 and 12 years showed to be the point of greatest interest.
Based on this study, 72.7% of the girls at 11 years of age had already undergone menarche. Data published with the same group of girls found the mean age of menarche was 11.52 years.11 Similar studies performed in two cities in southeastern Brazil in 2012 and 2014 found that the mean age of menarche occurred between 12 and 12.2 years.12–14 In this manner, the present results reveal that in the northern region of Brazil, the Brazilian Legal Amazon, not only menarche occurs at an earlier age when comparing with data from the southeast region, but also that when reaching the age of 13 years, 90.2% of the girls had already undergone menarche, of whom only 17.5% had had menarche between 12 and 13 years of age, confirming that the percentage with the greatest impact was in the age group of up to 11 years.
In 1969, Marshall and Tanner,8 studying a group of British girls, found a mean age at menarche of 13.47±0.10, and emphasized the importance of the nutritional status on the age of menarche. Since then, a decrease in age at menarche has been observed, both in developed and developing countries, and studies from around the world have sought to clarify the relationship between the shift in age at menarche and its possible driving factors, among them being genetics, environment, seasonality, nutritional status, and physical activity; menarche was also used as an indicator of socio-economic change, as it suffers interference from the environment.13,15,16 Using this approach, a Brazilian study in the city of Jundiaí found that the mean age at menarche in 2010 was 12.08 years; in the same study in 2001, the mean age was 12.35 years.13 Similarly, a decreasing trend in age at menarche has been observed for the past 20 years in countries in the Americas, Europe, and Asia.16,17
In the United States, Anderson et al.18 investigated changes in age at menarche, showing that the mean age for this event in American girls decreased from 12.75 to 12.54 over a 20-year period. In addition, mean age at menarche has decreased over the last 40 years, attributing such reduction to an increase in girls’ obesity.19 Other factors have also been proposed as impacting on the decline of the age of menarche, including social, economic, health, and nutrition improvement.12
For the northern region of Brazil, only comparative data from previous decades are available, referring to the rural Caboclo population, resident on the banks of the Amazon River, on the Island of Ituqui (Pará-Brazil), in 1996 and 1997, in which girls aged 8–19 years and women from 20 to 92 years were analyzed. This study found a mean age at menarche of 12.29 years and considered that the advance in the age at menarche in that population reflected the changes in healthcare and nutrition that occurred in Brazil at the late 1960s.20
Regarding the body composition of the adolescents in the study groups (G1 and G2), significant differences were observed for all variables for those aged less than or equal to 11 and 12 years; only the Z-score for BMI did not present a significant difference for such ages. At 13 years the body height and muscle mass remained significant and, at 14 years, only body fat (Table 2). These findings are in agreement with recent studies, even though most of them did not address all the variables reported in the present study and in which the groups were divided regarding menarche, since this event corresponds to the final phase of pubertal evolution. Wang,21 in a data analysis from NHANESIII (The Third National Health and Nutrition Examination Survey), observed that the onset of sexual maturation (SM) is positively associated with the risk of overweight and obesity. These findings were consistent with a more recent longitudinal study that assessed the association between SM, excess weight, and central adiposity in girls and boys aged 8–18 years and found that weight is negatively associated with SM in boys, but in girls this association is positive and they present greater central adiposity.22
Multiple analysis to associate the presence of age at menarche (AM) in schoolgirls with the variables included in the model.
| Variables | Adjusted odds ratios (95% CI)a | p-value |
|---|---|---|
| Fat | 1.04 (1.00–1.09) | 0.041 |
| Muscle | 1.1 7(1.08–1.27) | <0.001 |
Regarding the logistic regression findings, using chronological age as an adjustment factor, the adjusted odds showed that the risk of occurrence of menarche for each 1kg of fat was 1.04 times higher; for each 1kg of muscle, 1.17 times higher. Similarly to the findings of Oliveira et al.,22 who concluded that both genders are taller and heavier in cases of early SM, a positive association with body fat in girls was observed in that study.
Regarding the relationship between weight gain and SM, some studies report that, in order to initiate menstruation, girls need to reach an estimated minimum weight of around 47.8kg,13 data consistent with the present study. However, the association between BMI and age at menarche is still not consensual; it has been observed in some studies,18 while others reported no association.19 Moreover, studies addressing the nutritional transition conducted in the 1980s, coincident with the increase in overweight and obesity rates in children and adolescents worldwide, have also shown that age at menarche has been decreasing each decade,23 and this observation was associated with the weight excess rates.11,12
Comparing an international18 and a Brazilian13 study with the results of the present study, it was observed that in the northern Brazilian Legal Amazon region, menarche not only occurs at an earlier age, but also that the girls who had undergone menarche presented higher levels of body fat and excess weight, findings consistent with a study conducted in Kuwait that found an association between age at menarche and excess weight in schoolchildren.24
In this regard, research has shown that puberty begins earlier in obese girls (20% to 30% higher than normal) than in girls with normal weight; in contrast, girls with malnutrition experience a delay in menstruation.25 Indeed, evidence indicates that childhood obesity is a predictor of early puberty.12,23 An increase in body fat mass may be a significant signal to cause leptin secretion, stimulating the hypothalamus, and consequently an excessive secretion of GnRH, stimulating the pituitary–ovary axis and starting the pubertal development.25
In turn, girls who reported menarche (G1) at age 11 gained more body fat when compared to those reporting menarche at age 14, despite the fact that body fat did not present any difference between the ages in the group that did not report menarche (G2). This fact may suggest that the precocity of menarche follows from a greater gain of fat, which is a consequence of menarche and not a determinant, a fact already reported by the Fels Longitudinal Study,19 which suggested that early menarche is a marker of body fat.
Moreover, when analyzing Table 1, 11-year-old girls presented a 10-cm (p<0.001) height difference between G1 and G2, and at 14 years the difference in absolute values significantly reduced showing a slight upward inversion for the G2 (1.61m for G1 vs. 1.64m for G2). This finding is in agreement with the most recent studies regarding the influence of menarche on body height, showing that, regardless of age at menarche, the peak of growth spurt occurs naturally without difference between the final height in the two groups. Table 1 showed no significant difference between the two groups in final height, which occurred around 16 years of age. The study by Marshall and Tanner8 stated that girls who mature earlier reach the same final height than those who reach maturity later. Other authors found a difference of 1cm between the girls, those who menstruate later being taller, but this difference was not considered statistically significant.26
In girls, growth spurt most often marks the onset of puberty, and accounts for 82.5% of their adult stature; therefore, pubertal gain represents approximately 20% of their final height,26 which maintains a correlation of 0.81 (considered moderately high) with the girl's height at the beginning of the spurt.
There are differences regarding the timing of the growth spurt. The final height of an individual is the height reached when growth has ceased, that is, their height in adult age. Prader,27 referring to the First Zurich Longitudinal Growth Study, considered that 99% of the final height was reached at 15.2 years in girls and 16.8 years in boys.
In addressing the outcome of early sexual maturation, a recent study concluded that girls who reach menarche earlier show a greater number of sexually transmitted diseases and gestation up to the age of 18 years.28 In turn, subfertility appears to be related, at least partially, to the reduction of ovulation frequency, and body weight seems to be a regulator of hypothalamic-pituitary-gonadal axis activity.
Finally, besides the outcomes of anticipated age at menarche and its association with excess weight, this issue brings about a worldwide health concern. In fact, in addition to the association of fat with the occurrence of menarche, the results from the present study also point to an association of muscle weight with age of menarche. This finding may bring new insight to questions on the causes of pubertal precocity, making it possible to analyze other aspects of modifiable factors, not just body fat, even in the presence of a high prevalence of excess weight. This is confirmed by the fact that the adjusted odds ratio for the risk of occurrence of menarche was associated with greater muscle mass; and considering that the variation in height and muscle was present, and albeit smaller, it was more stable for all ages. Such findings may be associated with growth, suggesting the need for a greater emphasis in this area. Low levels of estradiol inhibit IgF1, but higher levels act as a stimulus, and sources of estrogen of external origin may be an interesting path to be explored in this context; especially because this was a group of adolescents from the Brazilian Legal Amazon, where lifestyle characteristics and regional particularities may represent a point of interference in the hormonal axis which determines the timing of puberty.
Consideration should be given to the fact that the present study did not evaluate the occurrence of menarche; therefore, the data were only from the date of collection, other studies found have the same bias.14 The scarcity of Brazilian studies and the absence of previous comparative studies in the northern region of Brazil limit the comparative and evolutionary pattern of this association, valuing the percentage of risk.
Despite the impact of the association presented here, other studies that address body composition and age at menarche are necessary to better clarify the relevance of such findings for the future health of these adolescents.
Informed consentThe study was approved by the Research Ethics Committee of the Federal University of Rondônia on 07/16/2014, CAAE:335422014.0.0000.5300. Regulatory norms for research involving human beings were followed in accordance with Resolution number 466/2012, certified by the National Health Council on December 12, 2012, with necessary prior written consent from school directors and parents/guardians.
Conflicts of interestThe authors declare no conflicts of interest.
The authors wish to thank the medical school students who assisted in the collection of data, and the schoolteachers for their patience and valuable assistance.
Please cite this article as: Gemelli IF, Farias ES, Spritzer PM. Association of body composition and age at menarche in girls and adolescents in the Brazilian Legal Amazon. J Pediatr (Rio J). 2020;96:240–6.