The use of probiotics is increasingly popular in preterm neonates, as they may prevent necrotizing enterocolitis sepsis and improve growth and feeding tolerance. There is only limited literature on Saccharomyces boulardii CNCM I-745 (S. boulardii) in preterm infants.
MethodA prospective, randomized, case-controlled trial with the probiotic S. boulardii (50mg/kg twice daily) was conducted in newborns with a gestational age of 30–37 weeks and a birth weight between 1500 and 2500g.
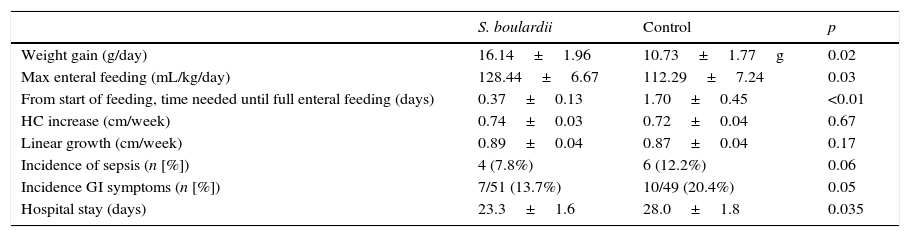
Results125 neonates were enrolled; 63 in the treatment and 62 in the control group. Weight gain (16.14±1.96 vs. 10.73±1.77g/kg/day, p<0.05) and formula intake at maximal enteral feeding (128.4±6.7 vs. 112.3±7.2mL/kg/day, p<0.05) were significantly higher in the intervention group. Once enteral feeding was started, the time needed to reach full enteral feeding was significantly shorter in the probiotic group (0.4±0.1 vs. 1.7±0.5 days, p<0.05). There was no significant difference in sepsis. Necrotizing enterocolitis did not occur. No adverse effects related to S. boulardii were observed.
ConclusionProphylactic supplementation of S. boulardii at a dose of 50mg/kg twice a day improved weight gain, improved feeding tolerance, and had no adverse effects in preterm infants >30 weeks old.
O uso de probióticos está cada vez mais popular em neonatos prematuros, já que podem prevenir a enterocolite necrosante (ECN) e a sepse e aumentar o crescimento e a tolerância de alimentação. Há apenas uma literatura limitada sobre a Saccharomyces boulardii CNCM I-745 (S. boulardii) em neonatos prematuros.
MétodoUm ensaio de caso-controle prospectivo randomizado com o probiótico S. boulardii (50mg/kg duas vezes por dia) foi realizado com recém-nascidos com idade gestacional de 30 a 37 semanas e peso ao nascer entre 1500 e 2500g.
ResultadosForam incluídos 125 neonatos, 63 no grupo de tratamento e 62 no de controle. O ganho de peso (16,14±1,96 em comparação a 10,73±1,77g/kg/dia, p<0,05) e a ingestão de fórmula com nutrição enteral máxima (128,4±6,7 em comparação a 112,3±7,2mL/kg/dia, p<0,05) foram significativamente maiores no grupo de intervenção. Assim que a nutrição enteral foi iniciada, o tempo necessário para atingir a nutrição enteral completa foi significativamente menor no grupo probiótico (0,4±0,1 em comparação a 1,7±0,5 dia, p<0,05). Não houve nenhuma diferença significativa em sepse. Não ocorreu ECN. Não foi observado nenhum efeito colateral relacionado à S. boulardii.
ConclusãoA suplementação profilática de S. boulardii a uma dose de 50mg/kg duas vezes por dia melhorou o ganho de peso, aumentou a tolerância de alimentação e não teve nenhum efeito colateral em neonatos prematuros >30 semanas de idade.
The gastrointestinal (GI) barrier function, gut motility, mucosal immunity, and digestive/absorptive capacity are all significantly underdeveloped in the preterm neonate.1 Preterm infants have an increased risk of poor growth, nosocomial infections, and necrotizing enterocolitis (NEC), and of developing a different intestinal microbiota than healthy breast fed infants.1,2 The latter is related to a higher incidence of delivery through cesarean section, decreased exposure to maternal microbiota, increased exposure to organisms that colonize neonatal intensive care units (NICUs), antibiotics (multiple courses), and delay in enteral feeding.3
The role for probiotics in the care of preterm newborns is debated. Probiotics are defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit to the host”.4 While reports of improved growth and a decreased incidence of NEC are enticing, many aspects on the mechanisms of action are still unclear.5,6 Studies have used different strains and dosages, making it difficult to draw evidence-based conclusions.5–7
Until now, researchers often selected strains belonging to bacterial species naturally present in the intestinal flora, such as lactobacilli and bifidobacteria.8Saccharomyces boulardii CNCM I-745 (S. boulardii) is a probiotic yeast isolated from the peel of fruits such as lychees, growing in Indochina.9S. boulardii has been poorly studied in preterm and low birth weight infants. The objective of the present study was to asses if S. boulardii administered to formula-fed preterm newborns >30 weeks of gestational age would improve weight gain and clinical outcome.
MethodsPatient inclusionStable formula fed preterm neonates admitted to the NICU of the Shengjing Hospital of the China Medical University in Shenyang (China) were included in this prospective randomized controlled double-blinded study, performed from April to July 2013. Informed consent was obtained from the infants’ parents/guardians. The study protocol was approved by the University Hospital Ethical Committee.
The sample size was calculated prior to the start of the study for a significance level of p<0.05 (two-sided), with a power of 80% (β=0.2) to estimate the needed sample size, and with a weight gain standard deviation of 9g/day in both groups and a weight gain difference between the two groups of 5g/day. This resulted in a sample size of 125 infants, considering a 20% drop out rate.
Inclusion and exclusion criteriaInclusion criteria were hospital-born formula-fed infants with a gestational age of 30–37 weeks and a birth weight between 1500 and 2500g.
Exclusion criteria were severe neonatal pathologies, such as severe birth complications, GI malformations, chromosomal abnormalities, known immunodeficiency, hydrops fetalis, central venous catheter, antifungal drugs, and probiotics. All included patients received parenteral nutrition and/or preterm formula. No neonates received mother's milk. Minimal enteral nutrition or trophic feeding was started as soon as possible at 1mL/kg/day. Minimal enteral feeding is the practice of feeding small volumes of enteral feed in order to stimulate the development of the immature GI tract of the preterm infant; it improves GI enzyme activity, hormone release, blood flow, motility, and microbial flora. Clinical benefits include improved milk tolerance, greater postnatal growth, reduced systemic sepsis, and shorter hospital stay.10 As soon as minimal enteral feeding was tolerated, the patient was randomly allocated to one of two groups at a 1/1 ratio (S. boulardii or control group). Randomization was conducted according to a random computer-determined allocation order considering birth weight. Feeding volume was increased when it was well tolerated according to the local protocol.
InterventionThe intervention group received S. boulardii CNCM I-745, administered two times per day as separate medication, not mixed with formula, at a dosage of 50mg/kg (Bioflor®; CMS Shenzhen Kangzhe Pharmaceutical Co. Ltd., Shenzhen, China; manufactured by Biocodex, Paris, France); 50mg is approximately 109 colony forming units (CFU). The dosage of the probiotic was derived from previous studies in neonates.11 Nothing was administered to the control group. The study period ended at the 28th day after birth or when the infant was discharged from the hospital, if this was possible earlier. However, the minimal duration of the intervention was at least 7 days. Observational and routine clinical data were collected from all infants. Blinding was possible because the nursing staff who administered S. boulardii to the infants was not involved in the daily care and the attending neonatal team was unaware of the randomization assignments.
OutcomePrimary outcomes were short-term growth parameters: weight gain (g/kg/day) and linear growth (cm/week). Secondary outcomes included: days of parenteral nutrition needed to reach full enteral feeding, maximal enteral feeding volume tolerated (mL/kg/day), and duration of hospitalization (days). Feeding intolerance was defined when vomiting and gastric residuals were considered too important. Complications were defined as incidence of NEC (defined as suspected or confirmed positive Bell stage II or more) and sepsis (defined as positive blood culture).9
Statistics-registrationThe data were collected and entered into a statistical database (SPSS, version 16.0; IBM, Armonk, USA). The data are presented as mean±standard deviation. The demographic data and procedure variables were analyzed using the t-test or the chi-squared test. A p-value of <0.05 was considered to indicate a statistically significant difference. This study did not receive external funds, and was registered at the website https://clinicaltrials.gov under the number NCT02310425.
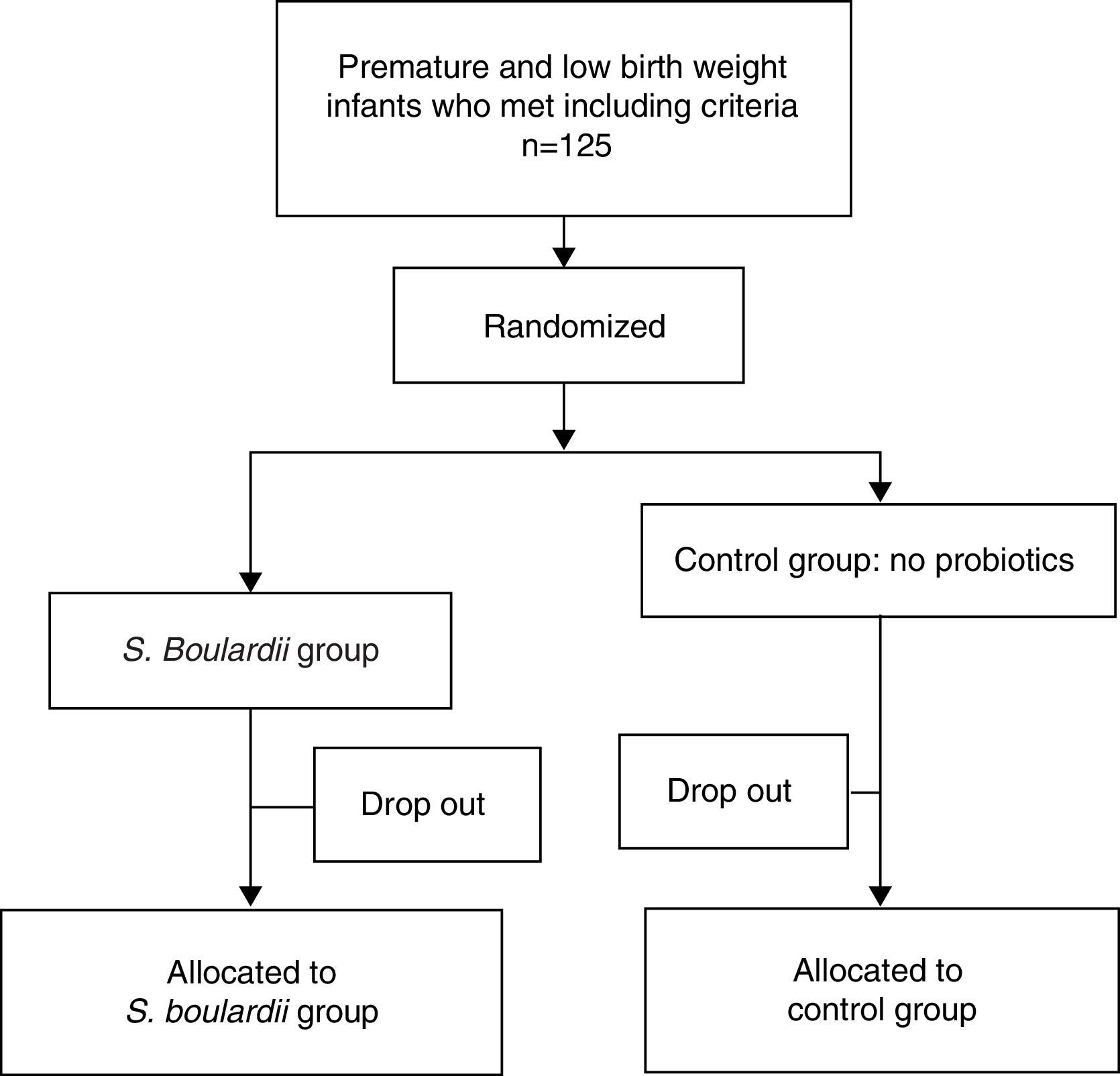
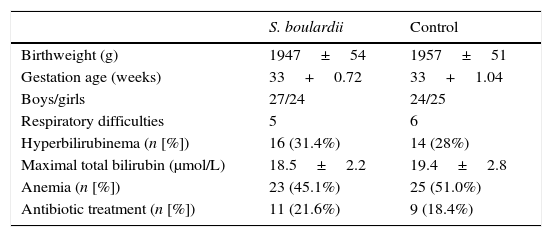
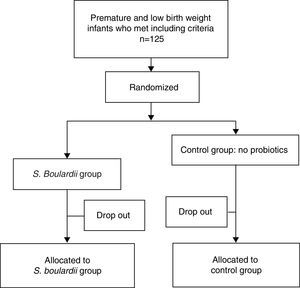
ResultsPatient descriptionA total of 125 formula-fed preterm neonates were enrolled and randomly allocated. Sixty-three patients received S. boulardii as soon as they could tolerate minimal enteral feeding and 62 neonates were included in the control group. In total, 25 (20%) patients were considered dropouts (12 [19.1%] in the S. boulardii group and 13 [20.1%] in the control group) (Fig. 1). Reasons for dropout were withdrawal of consent (n=9), loss to follow-up (n=11), central venous catheter (n=1), congenital syphilis (n=1), and inappropriate inclusions (congenital intestinal atresia [n=2], trisomy 21 [n=1]). Fifty-one subjects could be analyzed in the intervention group and 49 in the control group. The characteristics of all neonates at study entry are listed in Table 1, and did not show any statistically significant difference.
Characteristics (mean+1 SD) of the included infants.
| S. boulardii | Control | |
|---|---|---|
| Birthweight (g) | 1947±54 | 1957±51 |
| Gestation age (weeks) | 33+0.72 | 33+1.04 |
| Boys/girls | 27/24 | 24/25 |
| Respiratory difficulties | 5 | 6 |
| Hyperbilirubinema (n [%]) | 16 (31.4%) | 14 (28%) |
| Maximal total bilirubin (μmol/L) | 18.5±2.2 | 19.4±2.8 |
| Anemia (n [%]) | 23 (45.1%) | 25 (51.0%) |
| Antibiotic treatment (n [%]) | 11 (21.6%) | 9 (18.4%) |
Respiratory difficulties: includes respiratory distress syndrome and wet lung.
S, Saccharomyces; p>0.05 (all).
S. boulardii was administered for the first time at 2.63 days after birth (range: day 1 to day 6; in 46 infants within 3 days, and in only five infants between day 4 and 6). The total number of days of S. boulardii administration averaged 25.3 days (range: 9–28 days).
Feeding toleranceFormula intake at maximal enteral feeding (128.4±6.7 vs. 112.3±7.2mL/kg/day, p<0.05) was higher in the S. boulardii than in the control group, and the time needed to reach full enteral feeding (0.4±0.1 vs. 1.7±0.5 day, p<0.05) was shorter in the intervention than in the control group (Table 2).
Comparison of weight gain, growth (mean+1 SD), feeding tolerance, adverse events (sepsis, gastro-intestinal symptoms), and duration of hospitalization between the S. boulardii and control group.
| S. boulardii | Control | p | |
|---|---|---|---|
| Weight gain (g/day) | 16.14±1.96 | 10.73±1.77g | 0.02 |
| Max enteral feeding (mL/kg/day) | 128.44±6.67 | 112.29±7.24 | 0.03 |
| From start of feeding, time needed until full enteral feeding (days) | 0.37±0.13 | 1.70±0.45 | <0.01 |
| HC increase (cm/week) | 0.74±0.03 | 0.72±0.04 | 0.67 |
| Linear growth (cm/week) | 0.89±0.04 | 0.87±0.04 | 0.17 |
| Incidence of sepsis (n [%]) | 4 (7.8%) | 6 (12.2%) | 0.06 |
| Incidence GI symptoms (n [%]) | 7/51 (13.7%) | 10/49 (20.4%) | 0.05 |
| Hospital stay (days) | 23.3±1.6 | 28.0±1.8 | 0.035 |
HC, head circumference; GI, gastrointestinal symptoms: regurgitation, vomiting, gastric residuals; S, Saccharomyces.
The weight gain in the S. boulardii group was 16.14±1.96g/kg/day versus 10.73±1.77g/kg/day (p<0.05) in the control group. There was no significant difference in linear growth, head circumference growth, incidence of abdominal distension, and incidence of sepsis (Table 2). Hospital stay in the S. boulardii group was shorter (p=0.035) (Table 2). No infants developed NEC.
Adverse effectsNo preterms developed fungemia, and no adverse reactions to S. boulardii were reported.
DiscussionThis study demonstrated that S. boulardii can safely be administered to preterm infants, and that it improves oral feeding tolerance and weight gain. In term infants, formula supplemented with Lactobacillus (L.) rhamnosus GG was shown to increase weight gain, but formulas supplemented with Bifidobacterium (B.) longum, B. animalis subsp. lactis, and L. reuteri did not.11–13 In preterm infants, administration of B. breve also improved weight gain.14 The mechanisms by which weight gain is affected are not yet clear.
S. boulardii is effective in the treatment of a number of GI disorders related to the presence of bacterial and viral pathogens.15 It competes with pathogens for binding sites and produces a wide range of antimicrobial substances.16S. boulardii has the ability to produce polyamines, which are substances essential for cell growth and differentiation and enhance intestinal maturation, what is reflected in increased levels of enzyme expression.17S. boulardii is a yeast that significantly increases the activity of metabolic enzymes in the intestinal mucosa, stimulates the secretion of disaccharide enzymes, participates in the metabolism and absorption of carbohydrates, and stimulates secretory IgA production as the result of a trophic effect on intestinal mucosa.18 In addition, S. boulardii promotes the stability of the intestinal microbiome and reduces the possibility of malabsorption caused by GI disorders.19 Translocation of S. boulardii has not been reported; on the contrary, S. boulardii was reported to reduce bacterial translocation.20 Based on these properties, it was hypothesized that S. boulardii could improve growth and clinical outcomes in preterm or low birth weight infants.
Although several clinical trials strongly suggest a place for S. boulardii in the prevention and treatment of several GI diseases in adults and children, data in preterm infants are limited.18S. boulardii supplemented formula was shown to be well tolerated by preterm infants and to have beneficial effects on the GI microbiome, bringing it closer to that of breastfed babies.11 Clinical trials in preterm infants also suggested that S. boulardii improved feeding tolerance and reduced the risk of sepsis.21,22 In order to achieve optimum growth for a preterm infant, the goal is to mimic intrauterine growth while obtaining a functional outcome comparable to term infants.23 A gain in weight of 15–20g/kg/day, in length of 0.7–1.0cm/week, and in head circumference of 0.7cm/week is recommended.24,25 In the S. boulardii group, the average weight gain was 16.14g/kg/day, linear growth was 0.9cm/week, and head circumference increase was 0.7cm/week. Weight gain in the control group was 10.73g/kg/day, which is below the recommendation. The number of days to reach full enteral nutrition was shorter in the S. boulardii than in the control group. The better weight gain is likely to be related to the improvement of feeding tolerance. It was observed that the incidence of vomiting, gastric residual volume, and abdominal distension (“GI symptoms”, Table 2) were decreased in the intervention group in comparison to the control group, although there was no statistical significant difference. The total hospital stay in the S. boulardii group was shorter than that in the control group.
No significant difference was observed in linear growth and head circumference evolution, which could be related to the relative short intervention period of 1 month. Other limitations of this study are the lack of information on postnatal clinical characteristics of the neonates that could have been factors influencing the outcome, such as the ratio of patent ductus arteriosus, intraventricular hemorrhage, and others. Information on the number of infants with predisposing factors for NEC, sepsis, or other problems such as pre-eclampsia, antenatal steroid use, premature rupture of membranes, and caesarian birth are missing. The absence of breastfeeding is another weakness of the study.
A recent Cochrane review reported on 24 trials on probiotics in preterm infants and concluded that the trials were highly variable with regard to enrollment criteria (birth weight, gestational age), baseline risk of NEC, timing, dose, formulation of the probiotics, and feeding regimens.8 Enteral supplementation with probiotics significantly reduced the incidence of severe NEC (stage II or more) (typical relative risk [RR] 0.43, 95% confidence interval [CI] 0.33–0.56; 20 studies, 5529 infants) and mortality (typical RR 0.65, 95% CI 0.52–0.81; 17 studies, 5112 infants).8 According to this meta-analysis, there was no evidence for a significant reduction of nosocomial sepsis (typical RR 0.91, 95% CI 0.80–1.03; 19 studies, 5338 infants).8 In the present trial, there were no preterms who developed NEC; this is likely to be related to the fact that gestational age for inclusion was 30–37 weeks and that NEC occurs more frequently in infants born with a lesser gestational age. Previous clinical trials showed that S. boulardii supplementation did not reduce the incidence of death or NEC in very low birth weight infants, but improved feeding tolerance and reduced the risk of clinical sepsis, while adverse effects related to the intake of S. boulardii were not observed.21,22
S. boulardii has a protective effect against various enteric pathogens by two main mechanisms: production of factors that neutralize bacterial toxins and modulation of the host cell signaling pathway implicated in proinflammatory response during bacterial infection.18,19 In addition, S. boulardii can increase the activity of regulatory T cells and secretion of IgA of intestinal epithelial and crypt cells, improving intestinal protection through immune regulation.18 In this study, there was no statistically significant difference in the incidence of sepsis between the two groups (4/51 vs. 6/49). This finding is in agreement with the Cochrane analysis, showing that the included trials reported no systemic infection with the supplemental probiotics organism.8S. boulardii fungemia has been reported in patients with deep central venous access.18 In this clinical trial, there were no cases of fungemia, and no side effects occurred. The authors of the recent Cochrane review concluded that the updated review of available evidence strongly supports a change in practice, meaning that probiotics should be given to preterm infants to decrease the risk for NEC and mortality.8
In conclusion, the results of the present study show that prophylactic use of S. boulardii in preterm infants accelerates weight gain and improves feeding tolerance. These data confirm a recent retrospective analysis concluding that probiotics improve feeding tolerance, leading to better overall growth in preterm infants.26 It is the first time that better weight gain of preterm infants provided with S. boulardii has been demonstrated. Future double-blinded placebo-controlled trials are needed to confirm these data.
Conflicts of interestY. Vandenplas is a consultant for United Pharmaceuticals and Biocodex. The others authors declare no conflicts of interest.
Please cite this article as: Xu L, Wang Y, Wang Y, Fu J, Sun M, Mao Z, et al. A double-blinded randomized trial on growth and feeding tolerance with Saccharomyces boulardii CNCM I-745 in formula-fed preterm infants. J Pediatr (Rio J). 2016;92:296–301.