To translate, culturally adapt, and evaluate the psychometric properties of Adolescent Barriers Questionnaire for use in Brazilian adolescents with cancer aged 12 to 18 years, based on the original American Adolescent Barriers Questionnaire which was designed to measure the extent to which adolescents with cancer have concerns about reporting pain and using analgesics.
MethodsThe study analyzed the psychometric properties reliability (internal consistency and test-retest) and validity (known groups and convergent) of Adolescent Barriers Questionnaire in adolescents between 12 and 18 years of age with a diagnosis of cancer who were using or who had used analgesic medication (opioid or not) in a pediatric public health institution. It was estimated 64 adolescents as sample size and the research was conclude with 48.
ResultsResults of pre-test suggest good understanding (content validity index >0.9). The internal consistency value Cronbach's α was 88%. The convergent validity values ranged between -0.400 and -0.450. Analysis of known groups showed that the instrument discriminated groups of patients with solid vs. hematologic tumors. The intraclass correlation coefficient obtained after retest was 0.863.
ConclusionAfter the process of translations, validations and analysis of psychometric properties, the Brazilian Portuguese version of Adolescent Barriers Questionnaire could be considered culturally adapted, valid, and reliable for the Brazilian adolescent population with cancer aged 12 to 18 years and it can be useful in practical clinic, offering the health professionals the opportunity to understand which barriers the adolescent with cancer can encounter and offer, thus, all the support to overcome them.
Pediatric cancers are a set of diseases that have their own characteristics, representing 1–3% of all malignant tumors.1 In adolescents and young adults, cancer is a heterogeneous group of tumors. There were an estimated 1.2 million cancer cases and 400,000 cancer-related deaths among 15–39-year-olds in 2018.2
Despite advances in the cancer treatments, many patients progress to worse severity. Pain is the most common symptom experienced by children with cancer.3 Regardless of its etiology, it is an unpleasant experience that is part of the child and adolescent cancer context4 and warrants close attention from professionals, because its inadequate management can have a negative impact on patients and their family, including the patient's social relationships, family relationships, and quality of life.5 Adherence to medication is one of the many factors that impact clinical outcomes in the adequate management of cancer pain and should be considered a fundamental factor in the success of therapy and adequate pain control.6,7
To better understand the factors that affect treatment adherence in the oncological population, it is necessary to understand the barriers that prevent adherence, especially in adolescence. To identify the barriers that interfere with appropriate pain treatment, the use of specific assessment instruments is necessary and may have practical implications. Understanding the factors that influence the pain experienced by cancer patients is important to assist healthcare professionals in developing relevant intervention strategies to improve the outcomes of this therapy.8 However, it is noteworthy that the literature addressing the topic of opioid adherence in pediatric or pediatric oncology is still scarce.9 One tool, the Barriers Questionnaire II (BQII), is used to evaluate such barriers, but it was developed and validated for oncology patients aged over 18 years.10
Identifying the ideal assessment instrument for a given health outcome is not always easy, especially in Brazil, as most tools originate from other countries. The Adolescent Barriers Questionnaire (ABQ) was developed in the United States and initially validated in 2010. This questionnaire identifies the barriers that impact adolescents’ ability to cope and assesses their beliefs about pain and the use of analgesics to relief cancer pain.11
The objective of this study was to translate, culturally adapt, and evaluate the psychometric properties of ABQ for adolescents with cancer in Brazil.
MethodsThis was a cross-sectional, descriptive and methodological study (patient-reported outcome validation study), that followed to the methodology proposed by Beaton et al.12 Adolescents aged 12–18 years who were diagnosed with cancer and were using or had used medication to control pain (opioid, anti-inflammatory, with or without adjuvant medication) were included in the study. Those with cognitive impairments were not excluded.
This research was approved by the Research Ethics Committee of the Barretos Cancer Hospital under protocol 1503/2017. The author of the original instrument granted permission for the entire validation process in Brazil. The assent of the adolescents and the consent of their parents or guardians were obtained. Consent forms and questionnaires were administered in a private room.
The ABQ is a self-administered questionnaire assessing the presence of barriers to the use of medication for the relief of cancer-related pain in adolescents with cancer. It is composed of 45 items that assess 11 barriers to reporting pain and using analgesics: monitoring symptoms; being a good patient; fear of addiction; fear of drug tolerance; fatalism; unwanted side effects; unwanted parental reaction; treatment decisions; restriction of social activities; taking medication in public; and undesirable tests. Each barrier has three to six items and is scored on a 0-to-5 Likert scale, where 0 means “do not agree at all”, and 5 means “strongly agree”. The final score for each barrier is the mean of its items and the total score is the mean of all barriers. A higher score means more barriers. The American ABQ was shown to be reliable, with a Cronbach's α of 0.91, and valid with significant relationships between the barriers and coping.13
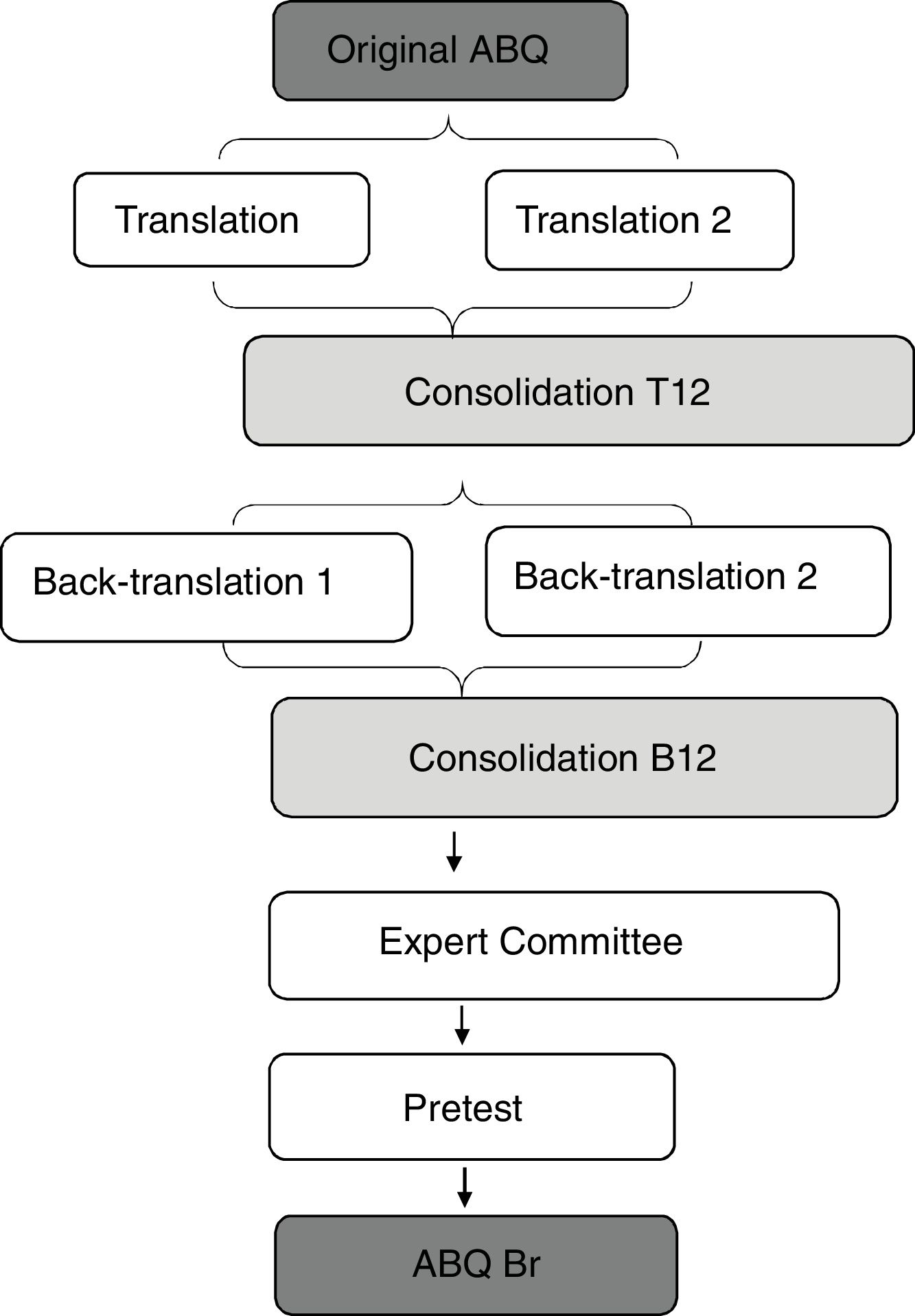
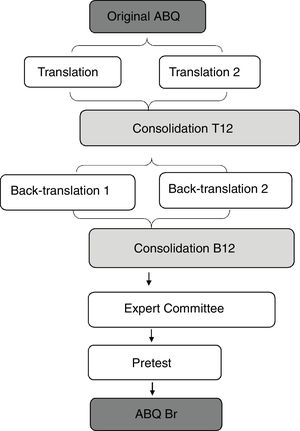
Translation and cultural adaptationThis process followed the methodology proposed by Beaton et al.12,14 (Fig. 1). The questionnaire was translated from English into Brazilian Portuguese by two independent translators, both native speakers of Brazilian Portuguese and fluent in English, producing T1 and T2 translation versions. After this process, these versions were consolidated into a single version, called T12, which was back-translated by two other translators who were native speakers of English and fluent in Brazilian Portuguese. The back-translated versions were named B1 and B2 and were later consolidated into a single version, B12. This version was sent to the author of the original instrument for evaluation and considerations. After this process, a Brazilian Portuguese version of the ABQ was created, called “pre-final”.
Expert committeeAn expert committee comprising five healthcare professionals with expertise in pediatric oncology and research analyzed the pre-final version regarding its semantic, conceptual, and cultural equivalence. The items of this pre-final version were scored from 1 to 4 according to the representativeness – i.e., the experts rated an item as 1 if they considered that the item was not correctly translated or adapted; as 2, if the item or statement needed considerable changes; as 3, if the item needed minor changes; or as 4, if the item was believed to be properly translated and adapted, and, thus, ready for inclusion in the final version of the questionnaire). The more items scored 3 or 4, the higher the content validity index, and values >0.8 are considered acceptable.15
PretestThe version was applied to a pretest sample, which consisted of a small group that met the profile of people to whom the questionnaire was intended: adolescents with cancer who used oral pain medication. At this stage, the goal was to assess whether the adolescents understood the ABQ items and response scales. They were asked about their understanding of the questionnaire, whether they comprehended the meaning of the words and what they understood about the statements. Thus, if an item was unclear, they could make suggestions to improve its understanding.
Assessment of psychometric propertiesIn the second stage, two different instruments were used. Correlating validated instruments with an instrument with intended validate is part or process of assessment of psychometric property measure. The adolescents of this stage were different from those of the first group. In that phase, besides ABQ, they needed to complete the following questionnaires according to their age: Pediatric Quality of Life Inventory™ (PedsQL 4.0; 8–12 years),14 PedsQL 4.0 (13–18 years),16 Pediatric Pain Questionnaire (PPQ) – PedsQL 3.0 (8–12 years),17 and PPQ–PedsQL 3.0 (13–18 years).17
The PedsQL 4.0 is a generic questionnaire composed of parallel forms, divided by age group. It evaluates the perception of health-related quality of life, including psychosocial aspects, school life, and feelings. It uses a five-level Likert scale (0–4), where 0 is assigned to “never” and 4 is assigned to “almost always.”16
The PPQ–PedsQL 3.0 evaluates the intensity and location of pain. It contains an open question and a graphic representation indicating the intensity of pain at the time of the interview.17
The psychometric properties evaluated were reliability and validity.
ReliabilityReliability was verified through internal consistency by calculating Cronbach's α. Values between 0.7 and 0.95 were considered to be acceptable.18 The ABQ was reapplied to the same sample seven to 14 days after the first application to measure test–retest reproducibility by calculating intraclass correlation coefficient (ICC). This value checks for big differences between answers in the interval. ICC values >0.7 were considered to be acceptable.18
ValidityValidity was assessed through construct validity (convergent validity) through correlations between ABQ, PPQ 3.0, and PedsQL 4.0 were hypothesized a priori by researcher's judgment based on clinical routine and literature. Then, it was not necessary to correlate all the items. (Spearman and Pearson correlation was certificated a priori about normality test and, the correlations were made between questionnaire and the instruments cited above. Coefficients ≥0.4 were considered acceptable.19 The content validity through value obtained by committee of experts or judges.
The statistical analysis of data was subjected to descriptive inferential analysis within SPSS v.20 software. The level of significance was set at 5%.
ResultsTranslation, cultural adaptation, and expert committeeThe process of translation and cultural adaptation is summarized in the flowchart of Fig. 1.
The content validity index values were >0.9. The pretest was performed on 16 adolescents, with a mean age of 15 years. A slight predominance of males was observed (n=9; 56.3%). There were representatives from four of the five regions of the country (North, South, Southeast, Midwest, and Northeast), with a predominance of the Northern region (n=9; 56.3%). It is important to include adolescents from different regions to ensure that there are no different regional interpretations that could interfere with the understanding of the questionnaire. Regarding education level, the same proportion of patients attended both Elementary School and High School (n=5; 31.3% for each group). In the Brazilian education system, Elementary School comprises children aged between 6 and 15, while High School typically is attended by students aged between 15 and 18. The most common bracket of monthly family income was zero (no income) to two Brazilian wages (n=11; 68.5%). All adolescents had solid tumors, and osteosarcoma had the highest incidence (n=9; 56.3%). Half the participants had metastases at diagnosis (n=8; 50%).
Of the 45 items included in the ABQ, the adolescents made suggestions for 21 (46%) items. In each in those 21 items, one to ten participants requested changes. Nine items were altered; the other items for which the adolescents suggested changes were not modified because grammatical errors or a change in meaning would have been introduced.
Assessment of psychometric propertiesA total of 56 adolescents were recruited, of whom eight refused to participate for various reasons, including “they were tired”, “they were not up for it”, “research takes too long”, or “laziness”. Six adolescents were not contacted because their guardians/parents did not allow them to participate in the study. Thus, the psychometric properties were assessed in 48 participants. In both samples (pretest e assessment of psychometric properties), all of adolescents had cancer and used oral pain medication.
The clinical and sociodemographic characteristics indicated there was a slight predominance of males (n=26; 54.2%). The mean (min–max) age was 15.7 years (12.6–18.9). There were representatives from all five regions of the country, with a predominance of the southeastern region (n=16; 33.33%). The majority had not completed High School (not completed Elementary School: n=23; 47.9%; completed Elementary but not High School: n=21; 43.8%), and the monthly family income was 1 to 2 minimum wages (n=19; 39.6). Regarding the clinical characteristics of patients, most patients had solid tumors (n=31; 77%), and 27 (56.3%) patients had no metastasis at diagnosis.
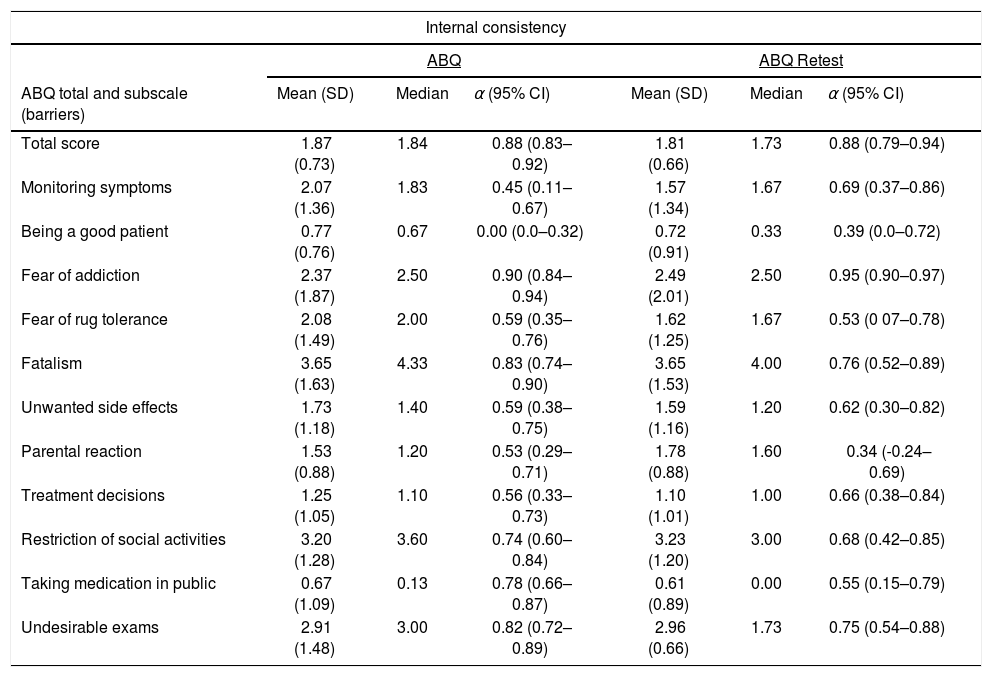
The internal consistency reliability was adequate, with Cronbach's α of 0.88 at baseline and 0.88 at retest. The Cronbach's α coefficients were calculated for each barrier in the questionnaire in both applications. These values and their 95% confidence intervals are shown in Table 1.
Analysis of reliability using Cronbach's α at two time-points of questionnaire application (n=48).
| Internal consistency | ||||||
|---|---|---|---|---|---|---|
| ABQ | ABQ Retest | |||||
| ABQ total and subscale (barriers) | Mean (SD) | Median | α (95% CI) | Mean (SD) | Median | α (95% CI) |
| Total score | 1.87 (0.73) | 1.84 | 0.88 (0.83–0.92) | 1.81 (0.66) | 1.73 | 0.88 (0.79–0.94) |
| Monitoring symptoms | 2.07 (1.36) | 1.83 | 0.45 (0.11–0.67) | 1.57 (1.34) | 1.67 | 0.69 (0.37–0.86) |
| Being a good patient | 0.77 (0.76) | 0.67 | 0.00 (0.0–0.32) | 0.72 (0.91) | 0.33 | 0.39 (0.0–0.72) |
| Fear of addiction | 2.37 (1.87) | 2.50 | 0.90 (0.84–0.94) | 2.49 (2.01) | 2.50 | 0.95 (0.90–0.97) |
| Fear of rug tolerance | 2.08 (1.49) | 2.00 | 0.59 (0.35–0.76) | 1.62 (1.25) | 1.67 | 0.53 (0 07–0.78) |
| Fatalism | 3.65 (1.63) | 4.33 | 0.83 (0.74–0.90) | 3.65 (1.53) | 4.00 | 0.76 (0.52–0.89) |
| Unwanted side effects | 1.73 (1.18) | 1.40 | 0.59 (0.38–0.75) | 1.59 (1.16) | 1.20 | 0.62 (0.30–0.82) |
| Parental reaction | 1.53 (0.88) | 1.20 | 0.53 (0.29–0.71) | 1.78 (0.88) | 1.60 | 0.34 (-0.24–0.69) |
| Treatment decisions | 1.25 (1.05) | 1.10 | 0.56 (0.33–0.73) | 1.10 (1.01) | 1.00 | 0.66 (0.38–0.84) |
| Restriction of social activities | 3.20 (1.28) | 3.60 | 0.74 (0.60–0.84) | 3.23 (1.20) | 3.00 | 0.68 (0.42–0.85) |
| Taking medication in public | 0.67 (1.09) | 0.13 | 0.78 (0.66–0.87) | 0.61 (0.89) | 0.00 | 0.55 (0.15–0.79) |
| Undesirable exams | 2.91 (1.48) | 3.00 | 0.82 (0.72–0.89) | 2.96 (0.66) | 1.73 | 0.75 (0.54–0.88) |
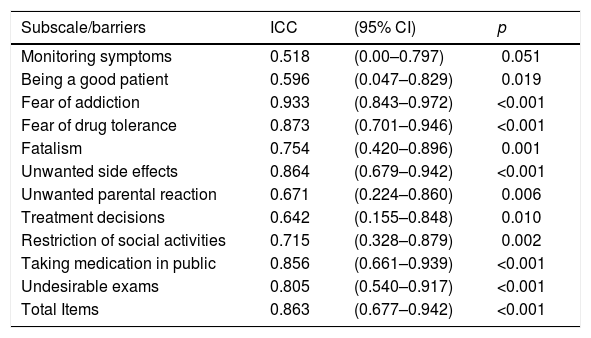
The test–retest reliability was evaluated in 23 patients seven to 14 days after first evaluation (Table 2). Although some barriers presented ICC values <0.7 (monitoring symptoms, being a good patient, unwanted parental reaction, treatment decisions), the overall instrument presented an ICC >0.8.
ABQ test–retest reproducibility in the seven-to-14-day interval (n=48).
| Subscale/barriers | ICC | (95% CI) | p |
|---|---|---|---|
| Monitoring symptoms | 0.518 | (0.00–0.797) | 0.051 |
| Being a good patient | 0.596 | (0.047–0.829) | 0.019 |
| Fear of addiction | 0.933 | (0.843–0.972) | <0.001 |
| Fear of drug tolerance | 0.873 | (0.701–0.946) | <0.001 |
| Fatalism | 0.754 | (0.420–0.896) | 0.001 |
| Unwanted side effects | 0.864 | (0.679–0.942) | <0.001 |
| Unwanted parental reaction | 0.671 | (0.224–0.860) | 0.006 |
| Treatment decisions | 0.642 | (0.155–0.848) | 0.010 |
| Restriction of social activities | 0.715 | (0.328–0.879) | 0.002 |
| Taking medication in public | 0.856 | (0.661–0.939) | <0.001 |
| Undesirable exams | 0.805 | (0.540–0.917) | <0.001 |
| Total Items | 0.863 | (0.677–0.942) | <0.001 |
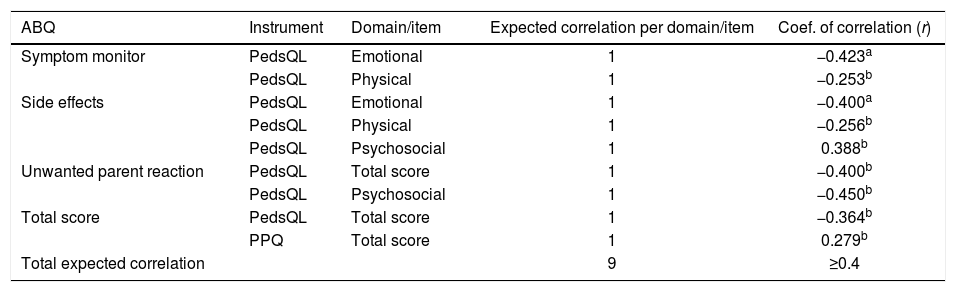
Of the nine correlations hypothesized in the convergent validity (construct validity), four were confirmed, and two presented values very close to the expected values. The hypothesized and confirmed correlations are listed in Table 3.
Expected correlations between the ABQ domains and the domains of the other instruments used in the study (PedsQL and PPQ; n=48).
| ABQ | Instrument | Domain/item | Expected correlation per domain/item | Coef. of correlation (r) |
|---|---|---|---|---|
| Symptom monitor | PedsQL | Emotional | 1 | −0.423a |
| PedsQL | Physical | 1 | −0.253b | |
| Side effects | PedsQL | Emotional | 1 | −0.400a |
| PedsQL | Physical | 1 | −0.256b | |
| PedsQL | Psychosocial | 1 | 0.388b | |
| Unwanted parent reaction | PedsQL | Total score | 1 | −0.400b |
| PedsQL | Psychosocial | 1 | −0.450b | |
| Total score | PedsQL | Total score | 1 | −0.364b |
| PPQ | Total score | 1 | 0.279b | |
| Total expected correlation | 9 | ≥0.4 |
As for the known groups validity, researchers expected that the instrument could discriminate patients by tumor type (solid or hematological), metastasis (presence or absence), and age group (12–14 years or >15 years) based on hypothesis researchers made a priory. The instrument was able to discriminate patients with solid vs. hematological tumors in the treatment decisions domain (p=0.02) of ABQ. The other hypothesized groups did not present significant discrimination values.
DiscussionIn both studies (original questionnaire from United States and the Brazilian version), the two highest means were from the same barriers, restriction of social activities and undesirable tests; the barrier with lowest mean was also the same: taking medication in public. However, the internal consistence of two barriers in this study, being a good patient and taking medication in public, showed lower values; this can be justified by the fact that patients did not recognize that as a barrier or did not understand what that barrier meant. Considering that pain is a very common symptom, observed in more than 70% of cancer patients both in the pediatric and adult populations, it is important to note that there are still barriers that prevent patients from correctly using pain management mediations.3,4 The identification of these barriers may allow professionals of the multidisciplinary care team to apply personalized planning, ensuring success in drug adherence and reducing pain, bearing in mind that medicines are not the only resource to relieve pain.20
By using an internationally well-established and widely-used methodology for the process of translation and cultural adaptation of instruments12 and evaluation of psychometric properties, this study provided evidence of the validity of ABQ in its version for the Brazilian Portuguese language. This is believed to be the first study of cultural adaptation and evaluation of psychometric properties of an instrument for assessing barriers to the use of analgesic and opioid medications by adolescents with cancer in Brazil.
Evaluation instruments are useful in clinical practice and can be complementary measures or even contribute to the outcome of the care plan used by professionals.21 Thus, using the appropriate methodology for the validation process is of fundamental importance.13 The ABQ validation process for the Brazilian Portuguese language followed the methodology proposed to ensure adequate interpretation by adolescents and, consequently, identification of barriers.
Regarding the psychometric properties of ABQ, the present results indicated that it is a reliable and valid questionnaire for the evaluation of barriers to the use of analgesic and opioid medications by Brazilian adolescents in cancer treatment. ABQ reliability was assessed using the Cronbach's α test, considering values >0.7 acceptable. In the present study, α was satisfactory at 0.88, which corroborated the results of the original study (α=0.91).
The retest was performed seven to 14 days after the test, as recommended in the literature, although it is possible to make adjustments to this period, depending on the instrument adopted.19 The ICC values of this study were also similar to those of the original questionnaire: ABQ (USA) ICC=0.82 and ABQ (Brazil) ICC=0.86. Although some ICC values from individual barriers were lower than the general ICC, the validation process considers the general value.
In general, and especially in Brazil, there is a shortage of instruments that can be used in clinical practice involving the adolescent population with cancer, especially instruments that assess barriers to the use of analgesic and opioid medications. Most instruments are foreign and designed for patients older than 18 years. ABQ is a valid and reliable instrument for identifying these barriers.13 Once validated for use in Brazil, the authors believe that the ABQ will help professionals perform interventions according to the barrier identified, as non-adherence to drug protocols may have clinical consequences on the treatment and quality of life of adolescents.21
Although all steps necessary for validation of the Brazilian Portuguese version have been done, this study has some limitations. The first is the number of participants. Adolescents may not always want to take part in research. Another limitation is that the study was conducted in only one cancer center in Brazil, although this institution treats adolescents from all regions of the country. Nonetheless, it can be considered the first step to identify adolescent's barriers and join efforts to minimize cancer pain.
The Brazilian Portuguese version of ABQ can be considered culturally adapted for adolescents with cancer aged 12–18 years, and its psychometric properties are considered valid and reliable. Future studies are needed to evaluate ABQ in a larger sample in a multicenter setting.
FundingSão Paulo Research Foundation (FAPESP) for financial support (project no. 2018/09977-0).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the São Paulo Research Foundation (FAPESP) for its financial support (project no. 2018/09977-0); to the Researcher Support and Incentive Program of the Barretos Cancer Hospital; and to the Epidemiology and Biostatistics Center of the Barretos Cancer Hospital.