Through a literature review, make recommendations regarding immunizations in people living with Inborn Error of Metabolism (IEM) in Brazil, assess the possible impact on metabolic decompensations after immunization, and if this specific population may have an impaired immune response to vaccines.
Source of dataThe MeSH Terms vaccination OR vaccine OR immunization associated with the term inborn error of metabolism AND recommendation were used in combination with search databases. Only articles published after 1990, in the languages English, Spanish, French or Portuguese, human-related were included.
Synthesis of dataA total of 44 articles were included to make the following recommendations. Individuals with IEMs need to be up to date with their immunizations. Regarding which vaccines should be offered, children and adults should follow the routine immunization schedules locally available, including the COVID-19 vaccines. The only exception is the rotavirus vaccine for hereditary fructose intolerance. The benefit of immunization outweighs the very low risk of metabolic decompensation. Since not all patients will have an adequate immune response, measuring antibody conversion and titers is recommended
ConclusionsAll patients should receive age-appropriate immunizations in their respective schedules without delays. The only situation when vaccination may be contraindicated is with oral rotavirus vaccine in hereditary fructose intolerance. Monitoring the levels of antibodies should be done to detect any immune dysfunction or the necessity for boosters. A personalized immunization schedule is ideal for patients with IEMs. The reference organizations could improve their recommendations to address all IEMs, not only some of them.
The Inborn Errors of Metabolism (IEMs) are genetic disorders that can affect the intermediary metabolic pathway caused either by an enzymatic deficiency an abnormal transporter molecule or any other abnormal protein.1 Individually, each IEM is rare, but together they represent a considerable number of affected people.2
More than 1100 IEM disorders have been described and classified into 130 groups based on their biochemical alterations.3 A simplified classification divides them into three major groups, those involving: small molecules, complex molecules, and energy defects.4 The first group includes IEM where there is an accumulation of a normal or abnormal substrate due to a metabolic block. The second group includes disorders that affect the synthesis, process, and catabolism of complex molecules, such as glycogen, triglycerides, glycosaminoglycans, long-chain fatty acids, cholesterol, and oligosaccharides among others. The latter group is caused by a defect in the production and/or use of the energy necessary for the cells.2
IEM can manifest at any age, from the antenatal period until adulthood, and can affect any organ/tissue in the body.5 Since these disorders may have a wide range of manifestations, their diagnosis requires a high clinical suspicion and can be made using a range of diagnostic tools, from simpler laboratory tests to genetic sequencing. Some disorders can now be diagnosed in a pre-symptomatic phase through the development of newborn screening programs (NBS).5
In general, IEM cannot be cured, but different modalities of treatments are available, and others are under development. The available therapies include regulation of substrate ingestion through special diets, administration of co-factors, or enzyme replacement therapy.6 Other therapies are hematopoietic stem-cell transplantation and liver transplantation. The newest therapy under development is gene therapy to correct the IEM, where the gene to be corrected is delivered to the patient or corrected within the genome.5
With a piece of the intermediate metabolism missing, leading to various manifestations, the immune system can also be affected in individuals with IEM. Indeed, reports of immune abnormalities show decreased T and B cells, and decreased levels of immunoglobulin (Ig) G (IgG) and IgM, especially when in metabolic decompensation, i.e., in the state of metabolic acidosis.7 Another example is neutropenia with impairment of neutrophil adhesion, decreased neutrophil survival, and metabolic burst.8–10 Innate immune cells may also have dysfunction.11–13 With these affections in the immune system, IEM individuals can be more susceptible to infections. That is why this group of diseases needs to be carefully looked at, with respect to recommendations regarding priming and boosting immunizations.
In Brazil, the National Immunization Program (PNI – Programa Nacional de Imunizações) offers 48 different immunobiological, 20 of them available in the Basic Health Care Units to the general population. The remaining are accessible through the Reference Center for Special Immunobiologicals (CRIE), to provide care to individuals with special clinical conditions, such as patients with cancer, with chronic diseases that render them more susceptible to infections, patients who received transplants, individuals living with HIV, among others.14 IEM patients receive their routine immunizations in the Basic Health Care Unit and should be referred to CRIEs to receive additional vaccines according to their pathology. However, the CRIE's manual, the Brazilian Society for Immunization (SBIm), and the Brazilian Society of Pediatrics (SBP) only refer to storage diseases, not IEMs in general. For storage diseases the following vaccines are indicated as an addition to the age-appropriate schedule: influenza, hepatitis A and B, 23-valent pneumococcal, meningococcal C/ACWY, Hemophilus influenzae b.14,15 The primary objective of this study was to be able, through a literature review, to make recommendations regarding immunizations in people living with IEM in Brazil. Other objectives were to assess the possible impact on metabolic decompensations after immunization and if this specific population may have an impaired immune response to vaccines.
Collection of dataA literature review was conducted with the MeSH Terms vaccination OR vaccine OR immunization associated with the term inborn error of metabolism AND recommendation were used in combination with the databases, which included Medline/PubMed, Scientific Electronic Library Online (SciELO), and Science Direct. The articles were uploaded to the Mendeley Reference Manager.
Articles included needed to be published after 1990 and in English, Spanish, French or Portuguese. No exclusion was made by the type of publication. Articles not humans related were excluded, those published before 1990, and in another language apart from the four mentioned above.
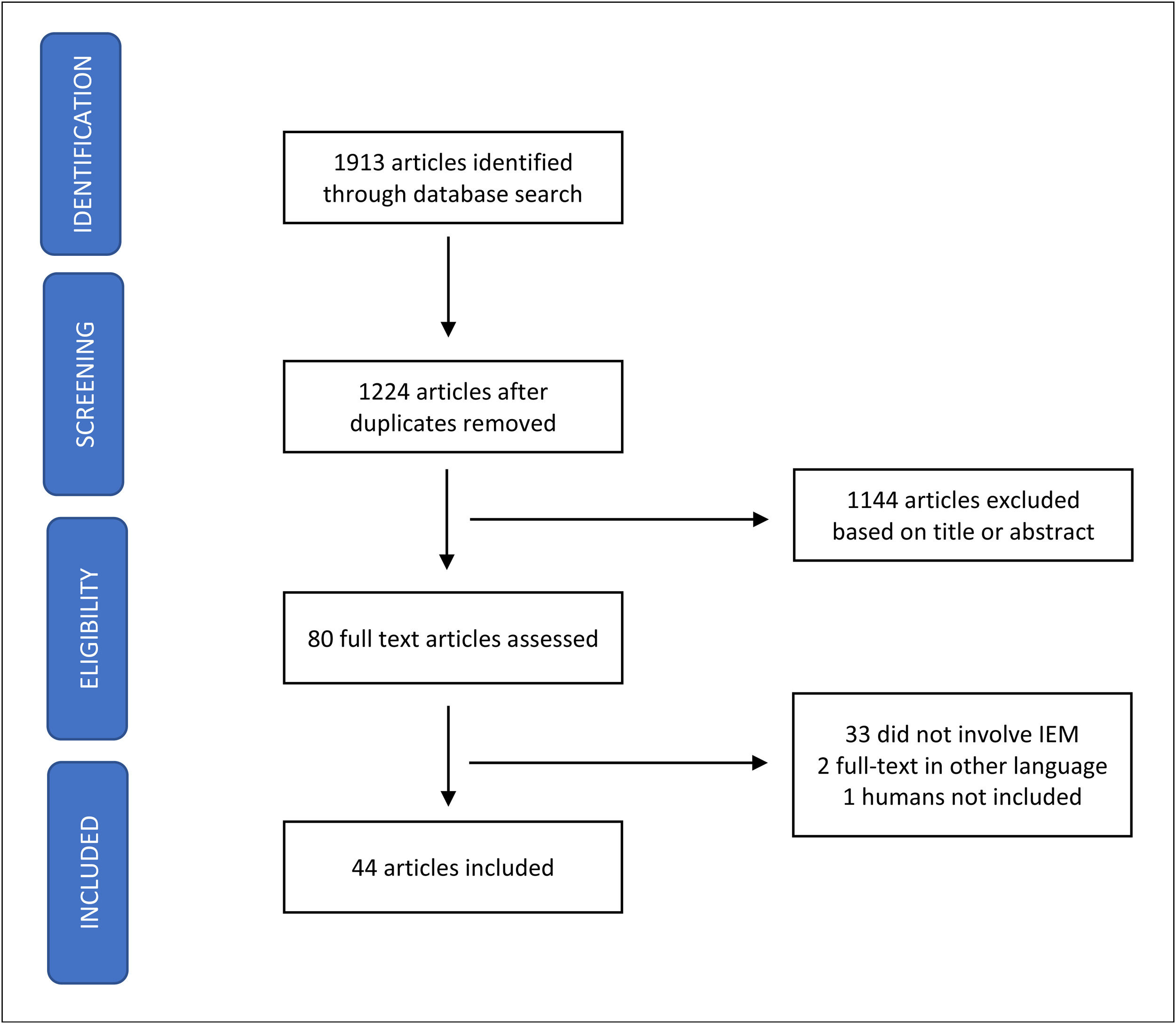
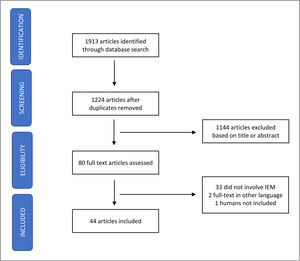
Synthesis of dataPubMed, SciELO, and ScienceDirect databases were searched, between April 6 and September 30th, 2022, for relevant publications using a combination of the search terms. A total of 1913 articles were initially identified and 1224 remained after the removal of duplicates. After analysis of the title and abstract, 1144 articles were excluded (discoursed about IEM but not vaccination; were related to inborn errors of immunity; or chronic diseases plus immunization), and the remaining 80 selected were full-text read. Thirty-three (33) papers were excluded because they did not approach IEM specifically, two were written in another language than English, Spanish, French and Portuguese and one did not involve humans. In the end, 44 articles were included in the analysis (Figure 1). Table 1 gathers the studies that were selected and are covered in the present paper. To facilitate their presentation, the data will be divided into the following topics.
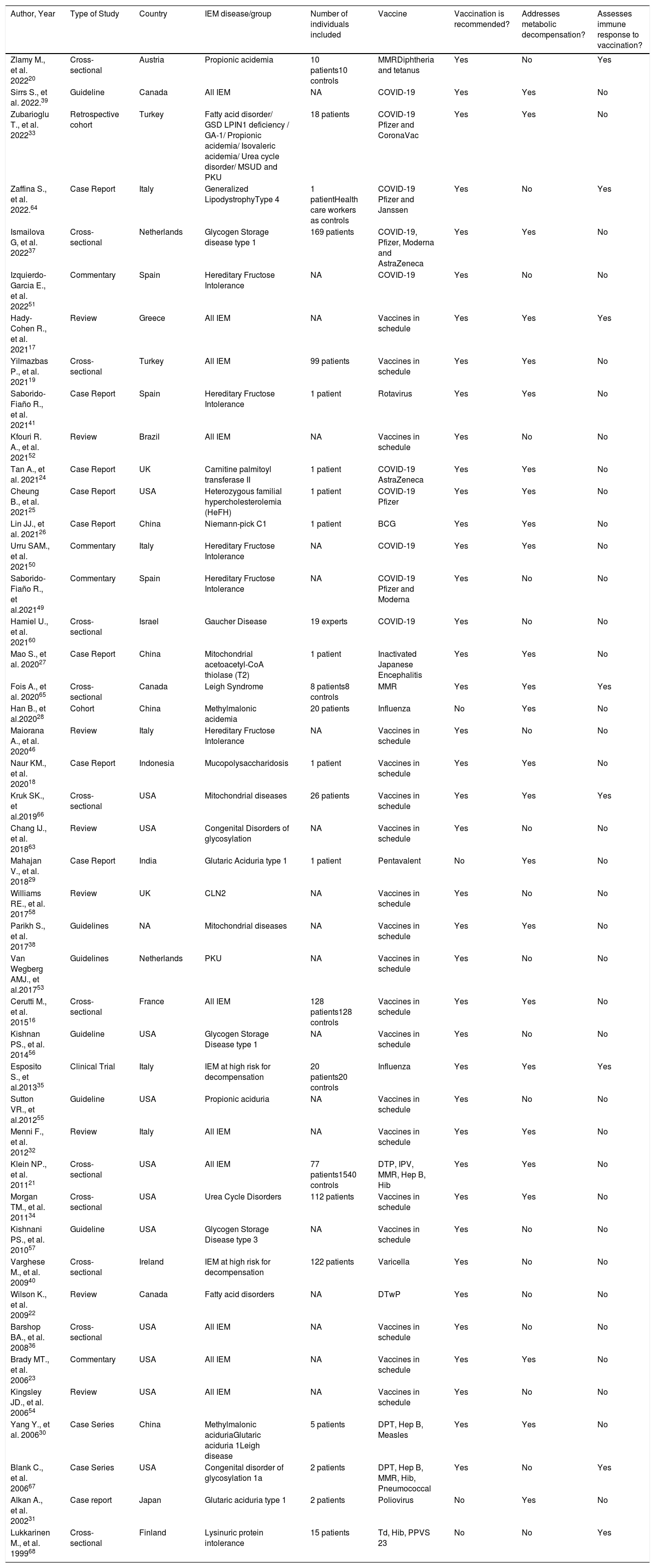
Articles included in the analysis and their characteristics.
| Author, Year | Type of Study | Country | IEM disease/group | Number of individuals included | Vaccine | Vaccination is recommended? | Addresses metabolic decompensation? | Assesses immune response to vaccination? |
|---|---|---|---|---|---|---|---|---|
| Zlamy M., et al. 202220 | Cross-sectional | Austria | Propionic acidemia | 10 patients10 controls | MMRDiphtheria and tetanus | Yes | No | Yes |
| Sirrs S., et al. 2022.39 | Guideline | Canada | All IEM | NA | COVID-19 | Yes | Yes | No |
| Zubarioglu T., et al. 202233 | Retrospective cohort | Turkey | Fatty acid disorder/ GSD LPIN1 deficiency / GA-1/ Propionic acidemia/ Isovaleric acidemia/ Urea cycle disorder/ MSUD and PKU | 18 patients | COVID-19 Pfizer and CoronaVac | Yes | Yes | No |
| Zaffina S., et al. 2022.64 | Case Report | Italy | Generalized LipodystrophyType 4 | 1 patientHealth care workers as controls | COVID-19 Pfizer and Janssen | Yes | No | Yes |
| Ismailova G, et al. 202237 | Cross-sectional | Netherlands | Glycogen Storage disease type 1 | 169 patients | COVID-19, Pfizer, Moderna and AstraZeneca | Yes | Yes | No |
| Izquierdo-Garcia E., et al. 202251 | Commentary | Spain | Hereditary Fructose Intolerance | NA | COVID-19 | Yes | No | No |
| Hady-Cohen R., et al. 202117 | Review | Greece | All IEM | NA | Vaccines in schedule | Yes | Yes | Yes |
| Yilmazbas P., et al. 202119 | Cross-sectional | Turkey | All IEM | 99 patients | Vaccines in schedule | Yes | Yes | No |
| Saborido-Fiaño R., et al. 202141 | Case Report | Spain | Hereditary Fructose Intolerance | 1 patient | Rotavirus | Yes | Yes | No |
| Kfouri R. A., et al. 202152 | Review | Brazil | All IEM | NA | Vaccines in schedule | Yes | No | No |
| Tan A., et al. 202124 | Case Report | UK | Carnitine palmitoyl transferase II | 1 patient | COVID-19 AstraZeneca | Yes | Yes | No |
| Cheung B., et al. 202125 | Case Report | USA | Heterozygous familial hypercholesterolemia (HeFH) | 1 patient | COVID-19 Pfizer | Yes | Yes | No |
| Lin JJ., et al. 202126 | Case Report | China | Niemann-pick C1 | 1 patient | BCG | Yes | Yes | No |
| Urru SAM., et al. 202150 | Commentary | Italy | Hereditary Fructose Intolerance | NA | COVID-19 | Yes | Yes | No |
| Saborido-Fiaño R., et al.202149 | Commentary | Spain | Hereditary Fructose Intolerance | NA | COVID-19 Pfizer and Moderna | Yes | No | No |
| Hamiel U., et al. 202160 | Cross-sectional | Israel | Gaucher Disease | 19 experts | COVID-19 | Yes | No | No |
| Mao S., et al. 202027 | Case Report | China | Mitochondrial acetoacetyl-CoA thiolase (T2) | 1 patient | Inactivated Japanese Encephalitis | Yes | Yes | No |
| Fois A., et al. 202065 | Cross-sectional | Canada | Leigh Syndrome | 8 patients8 controls | MMR | Yes | Yes | Yes |
| Han B., et al.202028 | Cohort | China | Methylmalonic acidemia | 20 patients | Influenza | No | Yes | No |
| Maiorana A., et al. 202046 | Review | Italy | Hereditary Fructose Intolerance | NA | Vaccines in schedule | Yes | No | No |
| Naur KM., et al. 202018 | Case Report | Indonesia | Mucopolysaccharidosis | 1 patient | Vaccines in schedule | Yes | Yes | No |
| Kruk SK., et al.201966 | Cross-sectional | USA | Mitochondrial diseases | 26 patients | Vaccines in schedule | Yes | Yes | Yes |
| Chang IJ., et al. 201863 | Review | USA | Congenital Disorders of glycosylation | NA | Vaccines in schedule | Yes | No | No |
| Mahajan V., et al. 201829 | Case Report | India | Glutaric Aciduria type 1 | 1 patient | Pentavalent | No | Yes | No |
| Williams RE., et al. 201758 | Review | UK | CLN2 | NA | Vaccines in schedule | Yes | No | No |
| Parikh S., et al. 201738 | Guidelines | NA | Mitochondrial diseases | NA | Vaccines in schedule | Yes | Yes | No |
| Van Wegberg AMJ., et al.201753 | Guidelines | Netherlands | PKU | NA | Vaccines in schedule | Yes | No | No |
| Cerutti M., et al. 201516 | Cross-sectional | France | All IEM | 128 patients128 controls | Vaccines in schedule | Yes | Yes | No |
| Kishnan PS., et al. 201456 | Guideline | USA | Glycogen Storage Disease type 1 | NA | Vaccines in schedule | Yes | No | No |
| Esposito S., et al.201335 | Clinical Trial | Italy | IEM at high risk for decompensation | 20 patients20 controls | Influenza | Yes | Yes | Yes |
| Sutton VR., et al.201255 | Guideline | USA | Propionic aciduria | NA | Vaccines in schedule | Yes | No | No |
| Menni F., et al. 201232 | Review | Italy | All IEM | NA | Vaccines in schedule | Yes | Yes | No |
| Klein NP., et al. 201121 | Cross-sectional | USA | All IEM | 77 patients1540 controls | DTP, IPV, MMR, Hep B, Hib | Yes | Yes | No |
| Morgan TM., et al. 201134 | Cross-sectional | USA | Urea Cycle Disorders | 112 patients | Vaccines in schedule | Yes | Yes | No |
| Kishnani PS., et al. 201057 | Guideline | USA | Glycogen Storage Disease type 3 | NA | Vaccines in schedule | Yes | No | No |
| Varghese M., et al. 200940 | Cross-sectional | Ireland | IEM at high risk for decompensation | 122 patients | Varicella | Yes | No | No |
| Wilson K., et al. 200922 | Review | Canada | Fatty acid disorders | NA | DTwP | Yes | No | No |
| Barshop BA., et al. 200836 | Cross-sectional | USA | All IEM | NA | Vaccines in schedule | Yes | No | No |
| Brady MT., et al. 200623 | Commentary | USA | All IEM | NA | Vaccines in schedule | Yes | Yes | No |
| Kingsley JD., et al. 200654 | Review | USA | All IEM | NA | Vaccines in schedule | Yes | No | No |
| Yang Y., et al. 200630 | Case Series | China | Methylmalonic aciduriaGlutaric aciduria 1Leigh disease | 5 patients | DPT, Hep B, Measles | Yes | Yes | No |
| Blank C., et al. 200667 | Case Series | USA | Congenital disorder of glycosylation 1a | 2 patients | DPT, Hep B, MMR, Hib, Pneumococcal | Yes | No | Yes |
| Alkan A., et al. 200231 | Case report | Japan | Glutaric aciduria type 1 | 2 patients | Poliovirus | No | Yes | No |
| Lukkarinen M., et al. 199968 | Cross-sectional | Finland | Lysinuric protein intolerance | 15 patients | Td, Hib, PPVS 23 | No | No | Yes |
BCG – Bacillus Calmette-Guerin; CLN2 – Neuronal ceroid lipofuscinosis 2; COVID-19 – Coronavirus Disease 2019; DTP – Diphtheria, tetanus and pertussis; DTwP – diphtheria, tetanus and whole pertussis; GSD – Glycogen Storage Disease; Hep B – Hepatitis B; Hib – Hemophilus influenzae b; IEM – Inborn Error of Metabolism; IPV – Inactivated poliovirus; MMR – Measles, Mumps and Rubella; MSUD – Maple Syrup Urine Disease; PKU – Phenylketonuria; PPSV23 – Pneumococcal polysaccharide vaccine 23; Td – tetanus and diphtheria; UK – United Kingdom; USA – United States of America; NA – Not applicable.
In the past there was a lack of confidence in the use of vaccines in patients with IEM, mainly because of single case reports of adverse events and lack of a systematic review. If the possibility of having an AE with a healthy child already makes parents hesitant to vaccinate their children, the hesitancy is higher in parents with special children. Indeed, a study showed that one of the main reasons given by parents with children with IEMs to not vaccinate or to delay vaccination was the possibility of metabolic deterioration and the occurrence of other AEs.16 A review from 202117 also stated that parents have major concerns about their child having a metabolic crisis after immunization.
Not only do parents demonstrate their fear, but healthcare workers also may have questions and doubts regarding the safety of vaccines. A two-month-old boy with an IEM had his routine vaccination schedule delayed because the primary health staff was afraid of a possible AE from the vaccine. The child was then referred to a specialized center to be authorized to receive vaccines.18 These issues usually delay vaccination in IEM patients, making them more susceptible to infectious diseases.17,19,20 Only one study showed that patients with IEMs received vaccines on the same immunization schedule as their healthy controls, without delays.21
Some IEMs have the tendency to decompensate more easily than others, as well as the small molecule group and the energy defect group. The metabolic crisis can occur with any intercurrent event, such as fasting, acute infections, and even the following vaccination, and may be severe enough to have the patient admitted to the hospital.2,4
It is unusual to see descriptions of patients suffering metabolic decompensation after receiving a vaccine. The rationale for a metabolic decompensation after immunization is that the vaccine would trigger a catabolic state in the organism which breaks the subtle balance that these patients live with.22,23
The authors found eight different publications reporting this situation, in 15 patients, most of them from China.24–31 Yet, the authors must keep in mind that, apart from the two events related to a COVID-19 vaccine,24,25 many of the individuals had not been previously diagnosed as having an IEM, and therefore were not in their best metabolic homeostasis, increasing the risk of suffering decompensation.
Namely, an AE following immunization (AEFI), was reported in India after a child presented with fever and seizures post-immunization with the pentavalent vaccine (Hib, hepatitis B, tetanus, diphtheria, and pertussis vaccine). Subsequently to the child's discharge, she had a cardiac arrest. Images from the brain were highly suggestive of glutaric aciduria type 1, previously undiagnosed.29 A similar case happened in Turkey, where two patients were diagnosed with glutaric aciduria succeeding an acute encephalopathy after they were immunized with the poliovirus vaccine.31
In China, a child received the Bacillus Calmette Guerin (BCG)vaccine as recommended. At 6 months she was diagnosed by a polymerase chain reaction confirmed BCGitis, leading to an investigation for underlying disease, culminating with the diagnosis of Niemann-Pick C1. The authors postulated that the Niemann-pick cells are a favorable environment for the survival and viability of the mycobacteria.26 Another case from China presented with unconsciousness, convulsion, and severe metabolic acidosis one day after receiving the second dose of the inactivated Japanese encephalitis vaccine. During the child's admission, a beta-ketothiolase deficiency (T2) was diagnosed. Of note, this subject also presented with symptoms of an upper respiratory tract infection, not detected during immunization.27
Authors from China reported a metabolic crisis in a child after immunization with the influenza vaccine. Before this episode, no IEM had been suspected. He was investigated and diagnosed with methylmalonic academia28. A distinct Chinese group outlined a series of subjects that presented with metabolic decompensation three to twelve hours after receiving a vaccine. All but one of them had not been previously diagnosed as having an IEM.30
Despite the apparent safety of the COVID-19 vaccine, there were two IEM cases reporting decompensation. One patient with familial hypertriglyceridemia showed a marked increase in triglycerides the day after the second dose of the mRNA Pfizer vaccine. Because of this elevation, he was not able to complete his routine LDL-apheresis, which was successfully completed a week later.25 The second case happened following the receipt of the replication-deficient adenoviral vector vaccine (AstraZeneca), where a subject with carnitine palmitoyl transferase II deficiency suffered from rhabdomyolysis.24
However, many other studies have already stated that these patients have a low risk of decompensation after immunization,16,21,27,32–34 including one that was followed up three months after the influenza vaccine, with no metabolic crisis reported35. The authors can then say that the more stable the condition, the safer the immunization.19 To guarantee stability, the first step is to perform the diagnosis and then initiate treatment. The advance in newborn screening made it possible to detect some of the IEMs in a pre-symptomatic phase, assuring a better prognosis, because of the rapid institution of specific management of the disease.5
A cross-sectional study from Turkey19 evaluated 99 patients. They found no reports of AEs, emergency department visits, hospitalization, or metabolic decompensation after immunization. In another study,16 this time from France, 128 patients were assessed for serious adverse events (SAE) after vaccination. The safety profile was similar between patients and no SAE was observed. A study,21 analyzed the AEs of 271 vaccinated IEM subjects. Receiving a vaccine did not increase the risk of AEs during the 30 days following immunization, although children between one and four years, with the group for higher risk of metabolic decompensation, may have had increased frequencies of hospitalization during the first two weeks after vaccination.
In the United States, 112 patients with diagnosed UCD showed no evidence of hyperammonemia episodes following routine vaccinations.34 When practitioners were asked if they had experienced a decompensation due to immunization, three-quarters answered that they had never seen a case.36
Regarding COVID-19 vaccines, two studies analyzed the possible risk of metabolic crisis after vaccination. The first one37 approached patients diagnosed with Pompe disease. Fifty patients were immunized for COVID-19, and about 60% had mild AEs, like pain in the injection site, myalgia, headache, fever, and fatigue. One patient developed perimyocarditis two weeks after the second dose of the Moderna mRNA COVID-19 vaccine. The other study33 examined 18 adolescents with different types of IEMs. All adolescents received the Pfizer mRNA COVID-19 vaccine, but one, received CoronaVac because of an existing long-QT syndrome. Two patients with phenylketonuria showed an elevated level of phenylalanine 24 h after the second dose. No clinical or other laboratory alterations occurred in these. Both groups conclude that the COVID-19 vaccine is recommended for IEM patients, but it is wise to monitor them closely for signs of decompensation or any other AE.33,37
As with any other person, patients with IEM are very susceptible to infectious diseases, and vaccination reduces the morbidity and mortality caused by vaccine-preventable diseases in patients with IEM, since they may develop a metabolic crisis requiring hospitalization more frequently. Thus, the benefit of immunization outweighs the very low risk of metabolic decompensation, not to mention that children with IEMs are not at increased risk of having AEs from the vaccine as compared to the general population.18,19,21,23,30,32,34,38 Two researchers recommend always considering assessing whether the individual has not a concomitant infection at the time of immunization, since the infection may cause a decompensation, which could be potentiated by the vaccine.27,39 Furthermore, a close clinical and/or laboratory follow-up can be done next to vaccination, particularly in subjects with a more unstable condition, to ensure no signs or symptoms of decompensation occur.16,17,22,24,27,33,40
The special case of hereditary fructose intoleranceThe literature review found single case report of a 2-month old child41 that presented with a metabolic decompensation two hours after receiving Rotarix vaccine. Later on he was diagnosed with HFI. Therefore, the benefit and risk needs to be weight against each Other, and if vaccination is done, extra cautious and observation is need. Yet this observation is not to all vaccines
Patients diagnosed with HFI need to have a fructose-free diet to avoid metabolic decompensation. Moreover, sucrose and sorbitol must be avoided as well. This fructose intake restriction also applies to drugs and vaccines. According to the Instituto Superiore di Sanità of Italy, an amount up to 2.4 mg/kg at each dose of medication or vaccine is safe and does not cause a metabolic crisis.42 Unfortunately, many vaccines have in their formulation some form of sugar, which most often is sucrose. Therefore, any vaccine dose administered within this 2.4 threshold is recommended for those patients.
Many pediatric vaccines respect this limit, except for oral rotavirus suspensions Rotarix® (oral suspension has 1073 mg of sucrose per dose)43 and Rotateq® (contains 1080 mg of sucrose per dose),44 both with a total of sucrose above the safety limit. Rotarix® white powder and solvent have 9 mg of sucrose and 13.5 mg of sorbitol at each dose,45 so it may be administered if the child weighs more than 9.3 kg, to avoid adverse events.46 One needs to bear in mind that when the rotavirus vaccine is administered according to the schedule, children do not have this weight and probably do not even have a diagnosis of HFI.41
For measles, mumps and rubella (MMR) vaccines, typically there are no issues, as at time of vaccination, a child weights more than 6 kg. The M-M-RVAXPRO® contains 14.4 mg of sorbitol per dose47 and Proquad® has in its composition 16 mg of sorbitol per dose.48 Other vaccines given in childhood are safe for children with HFI.46
Caution needs to be taken also with COVID-19, which is now being approved for the pediatric age group – they should also respect the limit of 2.4 mg/kg/dose of sucrose. The Pfizer mRNA COVID-19 vaccine contains 6 mg/dose of sucrose and thus can be given to children with this condition, unlike the Moderna mRNA COVID-19 vaccine, which may only be given to children with a weight above 18.2 kg due to the amount of sucrose, which is of 43.5 mg/dose.49 However, both vaccines and the viral-vector vaccines (AstraZeneca and Janssen), can be used in adolescents and adults with HFI,50 without restrictions. The inactivated COVID-19 CoronaVac, approved for children in some countries, was not mentioned in any of the correspondences, probably because this publication come from Europe, where the vaccine is not approved.
Interestingly, one paper commented that parental drugs or vaccines with sucrose in their formulation can be administered to patients with HFI because sucrose is not metabolized in the bloodstream and is excreted unchanged in the urine. The contraindication would arise only with the parental administration of drugs/vaccines that contains fructose or sorbitol, as well as oral sucrose if the amount exceeds the threshold for fructose intake.51 More studies are needed to help understand the safe amount of sucrose in parental drugs to be used in HFI individuals.
Recommendations of immunizationOf the publications analyzed, almost all stated that the routine vaccination schedule should be carried out in patients with IEM. Despite this recommendation, many authors suggest measures to prevent a possible IEM decompensation.
Vaccines that are not included in the routine immunization schedule should be considered in some patients with IEM. Considerations for the underlying pathology of the IEM and its predisposition to specific infections can be useful to decide which vaccines may provide an additional benefit.52 For example, it was demonstrated that children with IEM had a higher rate of hospitalization for varicella infection when compared to healthy children. Therefore, vaccinating IEM patients for varicella is beneficial in reducing the burden of hospitalization.40 Furthermore, in a survey done with practitioners, 75% responded that they recommend the routine schedule associated with the annual influenza vaccine.36
Individuals with aminoacidopathy, like those with phenylketonuria and tyrosinemia type 1, should follow the routine childhood vaccination schedule, but markers of disease should be monitored, according to one paper,17 since it has been previously reported an increased level of phenylalanine, although not symptomatic and transient. The European guideline for phenylketonuria only says that these patients should receive routine immunizations, without mentioning monitoring by laboratory markers.53 Receiving therapy with 2-nitro-4-trifluoromethylbenzoyl]−1,3-cyclohexanedione (NTBC) is not a contraindication to vaccination, hence tyrosinemia patients should receive all vaccines.54 People with organic acidemias should also receive all age-appropriate vaccines available, including the annual influenza vaccine.20,54,55
Children with galactosemia, a disorder in carbohydrate metabolism, after diagnosis and treatment initiation, should follow the routine schedule for childhood vaccines.17,54 The same recommendation is made for those with glycogen storage diseases.54,56,57 The pneumococcal and influenza vaccines should be considered in the latter patients because of the risk of having hypoglycemia caused by the infections.56,57
These last two vaccines should also be considered in patients with mitochondrial diseases with associated pulmonary conditions. If a pulmonary condition is not present, they should follow the routine vaccination schedule.38
Patients that have urea cycle disorders are recommended to receive all age-appropriate vaccines. Two studies17,54 suggest monitoring ammonia levels after immunization, but in another study, there was no increased risk for episodes of hyperammonemia.34
For lysosomal storage disorders, the recommendation is to vaccinate according to the immunization schedule and add the pneumococcal and meningococcal vaccines.17 A paper for the management of neuronal ceroid lipofuscinosis type 2 (CLN2) was the only one to also recommend vaccination against influenza and pneumococcal infection to family members.58
Since the beginning of the Sars-CoV-2 pandemic in December 2019, there have been 634 million reported cases and 6.6 million deaths worldwide.59 It is still debated whether having an IEM poses an increased risk for severe COVID-19. Data assessing this issue is still limited, but even if the IEM does not provoke a more severe course of infection, the infection itself may cause a metabolic crisis leading to higher morbidity.39,54 Two studies, one with patients with Pompe disease37 and the other with patients with Gaucher Disease60 showed that most of them that acquired COVID-19 had an asymptomatic or mild disease. However, because of possible decompensation due to the Sars-CoV-2 infection, receiving a COVID-19 vaccine is of extreme importance to these patients33,37 and the benefit outweighs the risk of vaccination. In general mRNA vaccine will be preferred over other licensed COVID-19 vaccines, but other label warnings need to be considered, like myocarditis with the mRNA vaccines61 and the thrombosis with thrombocytopenia syndrome with viral vectors vaccines.62
Besides recommending and ensuring that patients with IEM are vaccinated, it is important that they receive the vaccines at the proper time when they are offered, since a delay may put these patients at risk of acquiring an infection. One study16 demonstrated that for seven different vaccines, IEM patients had lower coverage for five (pneumococcal conjugate, meningococcal conjugated, MMR, influenza, and diphtheria-tetanus-pertussis booster vaccines) and that there also was a delay in receiving four vaccines (pneumococcal conjugate, meningococcal conjugated, second dose of MMR and BCG vaccines).
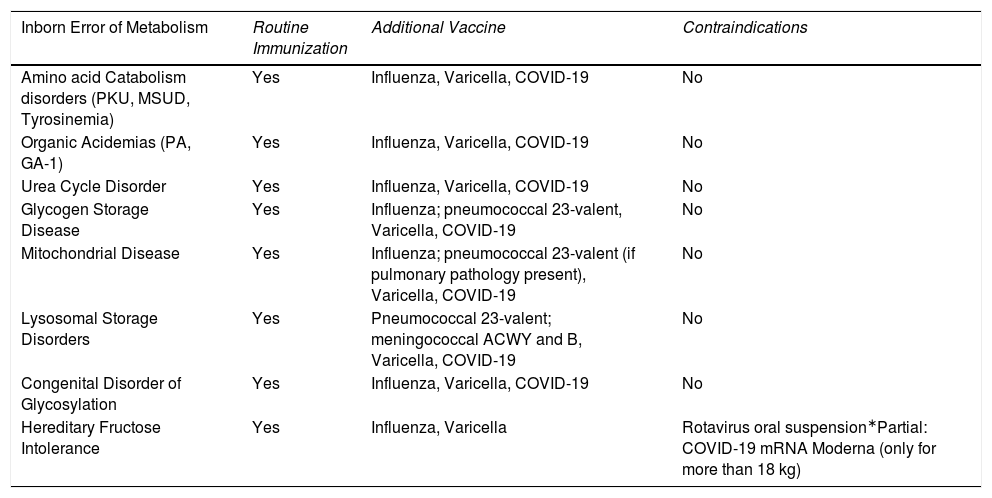
To summarize, all individuals with IEMs need to be up to date with their immunizations. Regarding which vaccines should be offered, most of the guidelines and publications17,22,32,35,38,53.55–58,63 concerning different metabolic disorders recommend that children and adults should follow the routine immunization schedules locally available. This includes the COVID-19 vaccines.33,37,39Table 2 summarizes the recommendation for immunization and contraindications for the main groups of IEM.
Recommendations for immunization in patients with IEM (main groups).
| Inborn Error of Metabolism | Routine Immunization | Additional Vaccine | Contraindications |
|---|---|---|---|
| Amino acid Catabolism disorders (PKU, MSUD, Tyrosinemia) | Yes | Influenza, Varicella, COVID-19 | No |
| Organic Acidemias (PA, GA-1) | Yes | Influenza, Varicella, COVID-19 | No |
| Urea Cycle Disorder | Yes | Influenza, Varicella, COVID-19 | No |
| Glycogen Storage Disease | Yes | Influenza; pneumococcal 23-valent, Varicella, COVID-19 | No |
| Mitochondrial Disease | Yes | Influenza; pneumococcal 23-valent (if pulmonary pathology present), Varicella, COVID-19 | No |
| Lysosomal Storage Disorders | Yes | Pneumococcal 23-valent; meningococcal ACWY and B, Varicella, COVID-19 | No |
| Congenital Disorder of Glycosylation | Yes | Influenza, Varicella, COVID-19 | No |
| Hereditary Fructose Intolerance | Yes | Influenza, Varicella | Rotavirus oral suspension∗Partial: COVID-19 mRNA Moderna (only for more than 18 kg) |
COVID-19 – Coronavirus Disease 2019; GA-1 – Glutaric Aciduria type 1; mRNA – messenger RNA; MSUD – Maple Syrup Urine Disease; PA – Propionic Aciduria; PKU – Phenylketonuria.
A point to consider when patients with IEM are vaccinated is that not all may mount an adequate immune response to the antigen. The production of antibodies and memory cells may be impaired because the accumulation of a substrate or deficiency of an enzyme can also affect immune cells. Not many studies have been conducted on the immunogenicity of vaccines in IEM disorders and even fewer on immune function. In general, the papers that assessed this information show that some disorders may indeed be associated with immune dysfunction or a poorer antibody response.
In Propionic Acidemia (PA) there is an impairment in the maturation and proliferation of the immune cells because of the accumulation of propionic acid.20 This impairment is more pronounced during a catabolic crisis. Patients with mitochondrial disorders (MDs) have a range of abnormalities in the immune system, such as low immunoglobulin G (IgG) titers, poor response to vaccine antigens, and low memory B-cells.17,38 In the same way that MDs may show unusual B-cell numbers, hyperlysinuric protein intolerance can also present with B and T-cells abnormalities.54 In addition, immunological disarrangements have been reported in congenital disorders of glycosylation and lysosomal storage disorders.17
The most recent study addressing immunological response in IEMs was done in PA patients.20 It included 10 patients and their matched healthy controls to evaluate IgG titers and avidity to five vaccine antigens (measles, mumps, rubella, diphtheria, and tetanus). Patients had lower antibody concentrations for all five antigens when compared to controls, but this was not statistically significant. No correlations for age, sex and time since vaccination could be done due to the small sample size. One important conclusion for this paper is that all patients showed immune protection for at least one of the antigens tested. So, evaluation of the immune system is critical to detect a non-respondent and also indicate a booster.
A patient with generalized lipodystrophy type 4 received two doses of mRNA Pfizer vaccine, but when immune response was assessed, he had a minimal response when compared to healthcare workers. However, he had a satisfactory response after a single dose of the Jansen viral-vector vaccine.64
A study from Canada analyzed the antibody response to MMR in patients diagnosed with Leigh Syndrome.65 The seroconversion rate for measles in the eight patients analyzed was very similar to the matched controls. However, the overall response rate to MMR and to the mumps and rubella-specific antigens was lower. Taking only the seropositive patients and comparing them with controls, the antibody titers were the same. Authors encourage an additional dose of MMR in those patients that are considered non-responders.65
Twenty-six patients with MD had their serum antibodies evaluated for a variety of vaccine-preventable diseases.66 It is important to note that the vast majority received a complete vaccination schedule. Overall, more than half did not have antibodies to two or more antigens. More than 60% of the patients were seronegative for varicella, followed by 55% negative for hepatitis B, 33% for Hemophilus influenzae b, 20% for measles, and less than 15% were negative for mumps, rubella, poliomyelitis, diphtheria, and tetanus. A tendency to have lower IgG values was observed in the patients that had three or more negative antigens. A guideline on the management of MD endorses the need to assess the immune system function in these patients, especially in the ones that have recurrent and/or severe infections.38
Lower antibody titers are recognized as one of the alterations that CDG patients can have. Two siblings presenting with recurrent infections had their antibody titers evaluated for several vaccine-preventable antigens (diphtheria, tetanus, pertussis, measles, mumps, rubella, hepatitis B, Hemophilus influenzae b, and pneumococcal) after receiving the complete vaccination schedule. At first analysis “sibling 1″ did not have protective antibody titers for diphtheria, pertussis, and seven pneumococcal serotypes. He was revaccinated and successfully seroconverted for all previously negative antigens, excluding pertussis. Albeit a few years later, he lost protection to hepatitis B and pneumococcal antigens. His-younger sibling received immunization according to schedule but had achieved protective titers to hepatitis B, varicella, and seven pneumococcal serotypes. Both brothers had non-specific low IgG levels and high numbers of B-cells.67 Two reviews express that serological levels of antibodies should be monitored in these patients and, if needed, a booster should be given.17,63
Hyperlysinuric protein intolerance can present with abnormalities in the B and T-cell compartments, inflicting a poorer humoral response to antigens. A Finnish study with 15 patients demonstrated that a good response was present to the Hemophilus influenzae vaccine. A somewhat good response to the tetanus and diphtheria vaccine was seen, which improved after a booster dose was given. However, the response to polysaccharide antigens, in the 23-valent pneumococcal vaccine, was poor.68 An assessment of their immune response would be a strategy to recommend booster doses, as a review pointed out.54
The last study determined the response rate against the influenza trivalent inactivated vaccine. It included 20 patients with IEM alongside 20 healthy controls. The immune response was similar in both groups, with patients maintaining the protective titers at the last point assessed (three months post-vaccination) just as the controls. It is worth noting that the included patients had to be metabolically stable for at least three months before receiving the vaccine, so this stability may have improved their immune system function.35
A review and position paper recommends finding the best time for vaccination, in order that the best immune response can be achieved, especially in IEM patients that may already have an impairment in the immune system.52
By these scarce studies the authors can assume that not all patients will have an adequate immune response, so measuring antibody conversion is a good strategy, since it not only can detect susceptible individuals but also help understand better the functionality of their immune cells. These types of laboratory assays are not widely available in Brazil but are found in almost all the specialized centers where the patient is referred to after diagnosis, an example of multidisciplinary patient care, involving the primary system where the vaccine is given and the specialized center.
ConclusionIndividually rare, but jointly numerous, the IEMs are increasingly being studied all around the world, and new ways of diagnosing and treating them emerge every year, with the objective of reducing morbidity and mortality and the burden of the disease. One way to achieve this is to provide the earliest diagnosis possible. This can be achieved by implementing NBS programs and educating healthcare staff in considering these disorders as a differential diagnosis. Another way of improving the health of those with IEMs is by preventing intercurrent events, which can disproportionately increase mortality. One of the most important events is infectious diseases. Against some of these, we have one of the most efficient ways of prevention: vaccination.
All patients should receive age-appropriate immunizations in their respective schedules, without delays. It is important to ensure, prior to immunization, that the patient has his/her baseline condition stabilized and that no acute illness is present. This is to ensure minimal chances of AEs and the best possible response to the vaccine. A follow-up with the primary or specialized physician may be necessary to guarantee that any signs of metabolic decompensation can be caught early. The only situation when vaccination may be contraindicated is that of the oral rotavirus vaccine in hereditary fructose intolerance patients with less than 9 kg due to the amount of sucrose in the formulation. This should be an alert to be written in the Ministry of Health manual and SBIm and SBP recommendations in Brazil.
Additional vaccines than those already in the basic immunization calendar, like influenza, 23-valent pneumococcal, meningococcal B, as well as additional booster doses, should be considered based on the underline pathology, history of infections and impairment of the immune system. A personalized immunization schedule is ideal for patients with IEMs.
The Brazilian immunization program (PNI) has one of the most complete schedules of vaccines in the world. It is accessible and offers a high variety of vaccines. Individuals with special conditions/diseases, or who had any AE following immunization, can have access to biologicals in the CRIEs. Nonetheless, only IEMs classified as storage disorders are mentioned in its manual, as well as in the immunization calendar from the SBIm and SBP. However, as discussed here, other IEMs disorders may also benefit from additional vaccines or additional boosters. Our reference organizations could improve their recommendations to address all IEMs, not only a portion of them.