This study aimed to describe the effect of prophylactic phototherapy in the treatment of infants with Neonatal Hemolytic Disease.
MethodA retrospective cohort study was carried out with 199 RhD-positive infants, born to RhD-negative mothers, alloimmunized for RhD antigen, between January 2009 and December 2018.
ResultsThe incidence of exchange transfusions in the study population was 9.5%, with a mean maximum bilirubin value of 11.3 mg % (± 4.3mg %). Bilirubin's maximum peak was achieved with a mean of 119.2 life hours (± 70.6h).
ConclusionThe low incidence of exchange transfusion, the extended maximum bilirubin peak for later ages, and the low mean of the maximum bilirubin values may indicate a positive effect of prophylactic phototherapy in the treatment of this disease. Further studies must be carried out to confirm these findings.
Phototherapy is an essential therapy in treating unconjugated hyperbilirubinemia in infants, with irreversible photoconversion of bilirubin into water-soluble compounds whose excretion is independent of liver metabolization, allowing its elimination via the kidneys. There are two mechanisms for photoconversion of bilirubin: photooxidation, with a small contribution, and photoisomerization, which is the main mechanism to decrease serum bilirubin during phototherapy.1 Critical bilirubin levels are toxic in the basal ganglia and brainstem, causing bilirubin encephalopathy with short, medium, or long-term clinical repercussions.2-4 Phototherapy efficacy is related to the light wavelength employed, source intensity, total irradiance dose (duration of phototherapy and amount of exposed skin), and bilirubin level at the onset of phototherapy, without severe adverse effects.5-7 Currently, there are methods for measuring phototherapy irradiance, and it has been demonstrated that the amount of irradiation correlates positively with the production of bilirubin isomers, varying with the wavelengths provided by the different phototherapy devices.8,9
Temperature control, water loss through evaporation, and retinal damage are the main concerns. Current phototherapy uses light-emitting diode (LED) lamps and is less likely to increase body temperature than fluorescent lamps used previously.2,6,10 In 1994, the American Academy of Pediatrics (AAP) published hyperbilirubinemia management guidelines considering the evidence available to date.11 These guidelines were revised in 2004 by the American Academy of Pediatrics, including the management of hyperbilirubinemia in infants over 35 weeks of gestational age, and defining intensive phototherapy as an irradiance of 30µW/cm2/nm, measured at the center of the light incidence, where a minimum irradiance level of 8-10μW/2/nm is recommended.2 Curves with values indicating the limit between acceptable bilirubin levels and those indicating phototherapy or exchange transfusion (ET) were made available by lifetime and risk factors in each case.2 In 2016, Bhutani et al. published curves of indications for phototherapy and exchange transfusion for preterm infants with less than 35 weeks of gestational age.12
A systematic review by Cochrane (2012) on the use of prophylactic phototherapy in preterm infants concluded that implementing phototherapy soon after birth can prevent bilirubin from reaching the exchange transfusion level and reduce the risk of involving the central nervous system. However, the authors highlighted the negligible number of well-planned studies to assess the long-term effects of this intervention.13
Prophylactic phototherapy as a clinical practice was initiated at the Fernandes Figueira Institute (IFF) in 2006 in infants with perinatal hemolytic disease due to RhD. Professionals identified a declining need for ET with this measure. They hypothesized that phototherapy initiated soon after birth would act quickly to change the bilirubin to water-soluble compounds, reducing the rate of ascent to the maximum serum bilirubin peak, thus reducing the need for ET. Thus, this study aimed to describe the effect of this measure on the clinical development of infants with neonatal hemolytic disease by RhD, evaluating the incidence of ET while using this protocol.
Material and methodsStudy design and locationA retrospective prognostic study was carried out in RhD-positive infants, children of RhD-negative mothers, alloimmunized for the RhD antigen, born at the IFF from January 2009 to December 2018. The Institution's Ethics Committee approved the project under CAAE number 12708919.8.0000.5269. The study was conducted at the IFF. The informed consent was obtained from those responsible for the patients, and information was retrieved from the patients’ medical records. The use of information from the medical records of the children and their mothers was authorized when contact with the person responsible was unsuccessful after several attempts.
Patient selectionThe study inclusion criteria were RhD antigen alloimmunized mother and Rh-positive infant with positive direct Coombs test. Exclusion criteria were congenital malformation, inborn errors of metabolism, genetic syndromes, confirmed congenital infections, cases with an immediate indication for exchange transfusion at birth, infant with G6PD deficiency, and evidence of other antibodies in hemolysis except those of the Rh system. Additionally, pregnancies with reported abortions or stillbirth and births in another health unit were excluded.
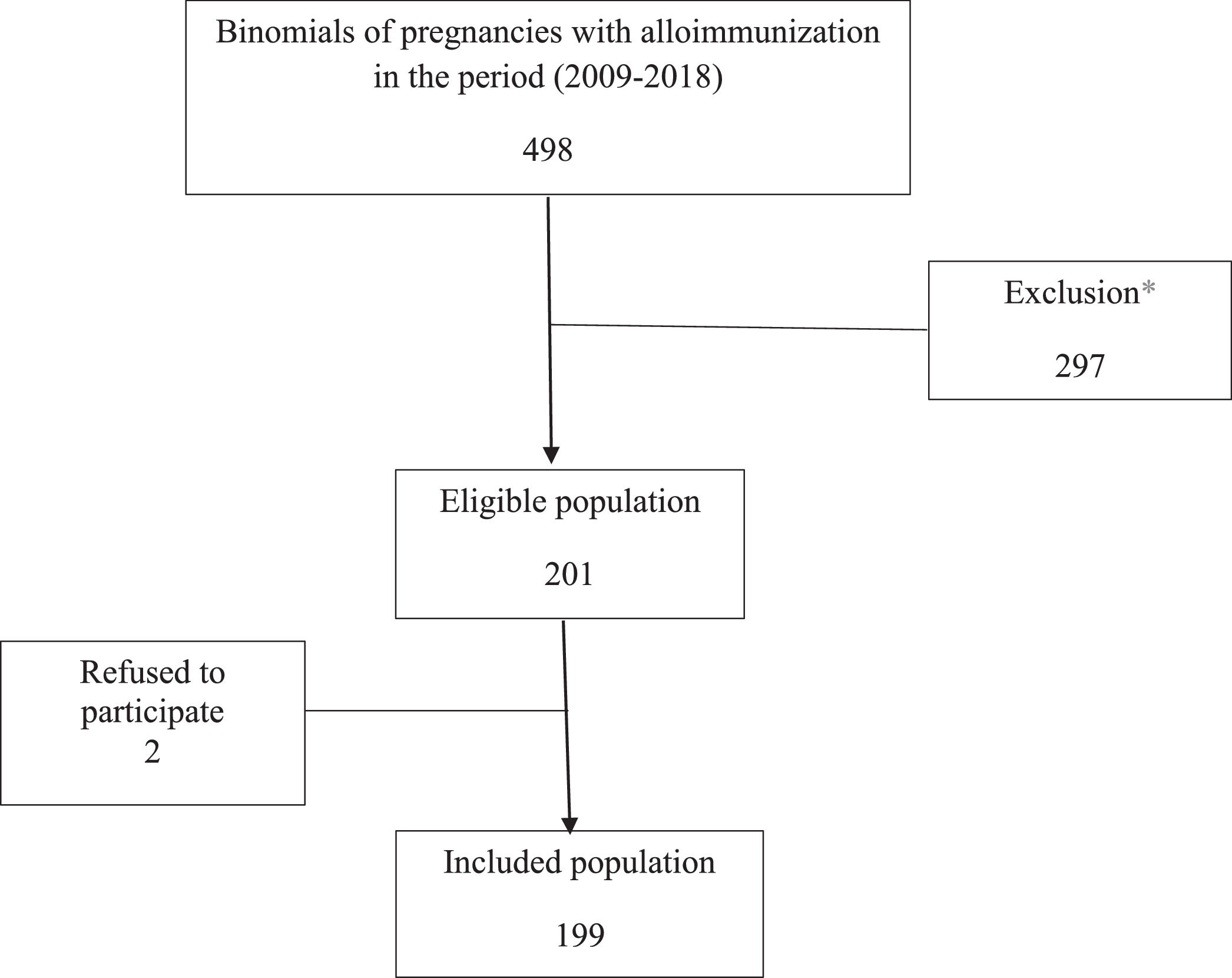
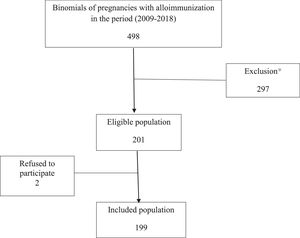
The database of alloimmunized pregnant women from the Hemotherapy sector of the IFF was used to search for patients. Patients treated at the Institute between January 2009 and December 2018 were listed, thus identifying infants’ medical records. Infants were analyzed separately in multiple pregnancy cases. Therefore, each research record is a mother-infant binomial. A total of 498 binomials were listed, of which 297 were excluded, leaving 201 eligible. Two participant refusals were recorded, which resulted in a final sample of 199 binomials (Figure 1).
Information retrieved from the medical recordsMother and newborn's blood typing, maternal serology in the studied pregnancy, identification of maternal anti-erythrocyte antibodies and their titration, number of previous pregnancies and whether immunoglobulin was used, intrauterine transfusion (IUT) in the studied pregnancy, infant's gestational age (GA) (in complete weeks), infant's gender, delivery type, birth weight (in grams), APGAR at birth, perinatal asphyxia, diagnosis of hydrops at birth, total serum bilirubin (TSB – mg/dL) and hematocrit (HT) at birth, number and indication criteria for ET, blood transfusions during hospitalization and follow-up, maximum TSB value (mg/dL) and hours of life at this time, minimum HT, phototherapy duration (days), number of devices used and complications, discharge HT and TSB, length of hospital stay (days), serology (Cytomegalovirus, human immunodeficiency virus and Hepatitis B and C) with one year of life in the case of blood transfusion.
Protocol for using phototherapy in the serviceThe phototherapy equipment are frequently calibrated and regularly checked with a radiometer (Drager®) by the clinical engineering team, setting up the irradiance meters supplied or recommended by the manufacturer. The lamps are replaced if the irradiance falls below the recommended range.
Phototherapy is started in the first four hours of life after confirming that the infant has a positive Rh factor with positive direct Coombs. Two to three high-intensity devices combined are used.
The devices used at the institution during the study period were initially conventional phototherapy, using fluorescent lights (Octofoto® 006-OFL and Biliberço® 006-FB, FANEM® Inc. Brasil) or halogen light spots (Bilispot® 006 – BP, FANEM® Inc. Brasil). They were replaced by devices using a high radiance “Light Emitting Diode” light type, which is more effective, over the study years. In 2011, the use of Bilitron (Bilitron® 3006, FANEM® Inc. Brasil) replaced Octofoto® and Bilispot®. In 2014, the conventional repalBiliberço® was replaced by the Biliberço with LED light (Bilitron® Bed 4006, FANEM® Inc. Brasil). In 2017, the use of Bilisky (Bilitron® Sky 5006, FANEM® Inc. Brasil) was started as an alternative to simple bilitron.
Indications for ETThe indications for ET used at the service are: i – TSB level ≥ 4.5 mg/dL or hemoglobin level ≤ 30% at birth (immediate indication for ET); ii – hemolysis rate: increase in TSB value ≥ 0.5 mg/dL/h in the first 36 hours of life despite intensive phototherapy; iii – TSB at the ET level according to the American Association of Pediatrics curves for GA, risk and lifetime.1 The following indications are used for the transfusion of packed red blood cells: i – HT between 25 and 30% with signs of significant hemodynamic repercussions; ii – in all infants with HT ≤ 25%.
Statistical analysisThe Epi Info™ 7.2.3.1 software was used for data recording and the IBM® SPSS® version 19 software for statistical analyses. Descriptive analyses of the population were performed, and the frequencies or means of the variables of interest to the study were calculated. Pearson or Mann-Whitney tests were used to compare the proportions, means, or medians of the variables over the years of the study or between groups. A p-value of 0.05 was considered for statistical significance.
ResultsThe final sample consisted of 199 binomials (Figure 1). The mothers’ median age was 31 years (IQR: 28-36), and most (51.3%) completed high school. Most pregnant women were alloimmunized only for the RhD antigen (87.9%), and the number of previous pregnancies with alloimmunization ranged from 0 to 1. Antibody titers were equal to or greater than 16, with a median of 32 (IQR:16-128) in 89.3% of pregnant women.
In the sample, 8% of pregnancies required IUT, which was very frequent among pregnancies with mothers immunized by anti-D and anti-C together (16.7% versus 6.9% in pregnancies with immunization only by RhD). However, there was no statistical significance (p-value = 0.07). The median IUTs performed per pregnancy was 3.5 (IQR: 2.0-5.5).
The mean birth weight was 2,930.79 g (± 558.4 g), with a mean GA of 36.6 weeks (± 2 weeks) and male predominance (56.8%) (Table 1). In this sample, two infants had perinatal asphyxia, and three had severe hydrops with cavity effusions requiring drainage after birth.
Demographic and clinical characteristics of infants (n = 199).
* Asphyxia is defined as the presence of three of the following five elements: (I) arterial pH less than 7.1 in the first hour of life; (II) APGAR score ≤ 5 in the 10th minute; (III) failure to breathe spontaneously within 10 minutes; (IV) evidence of multiple organ failure.
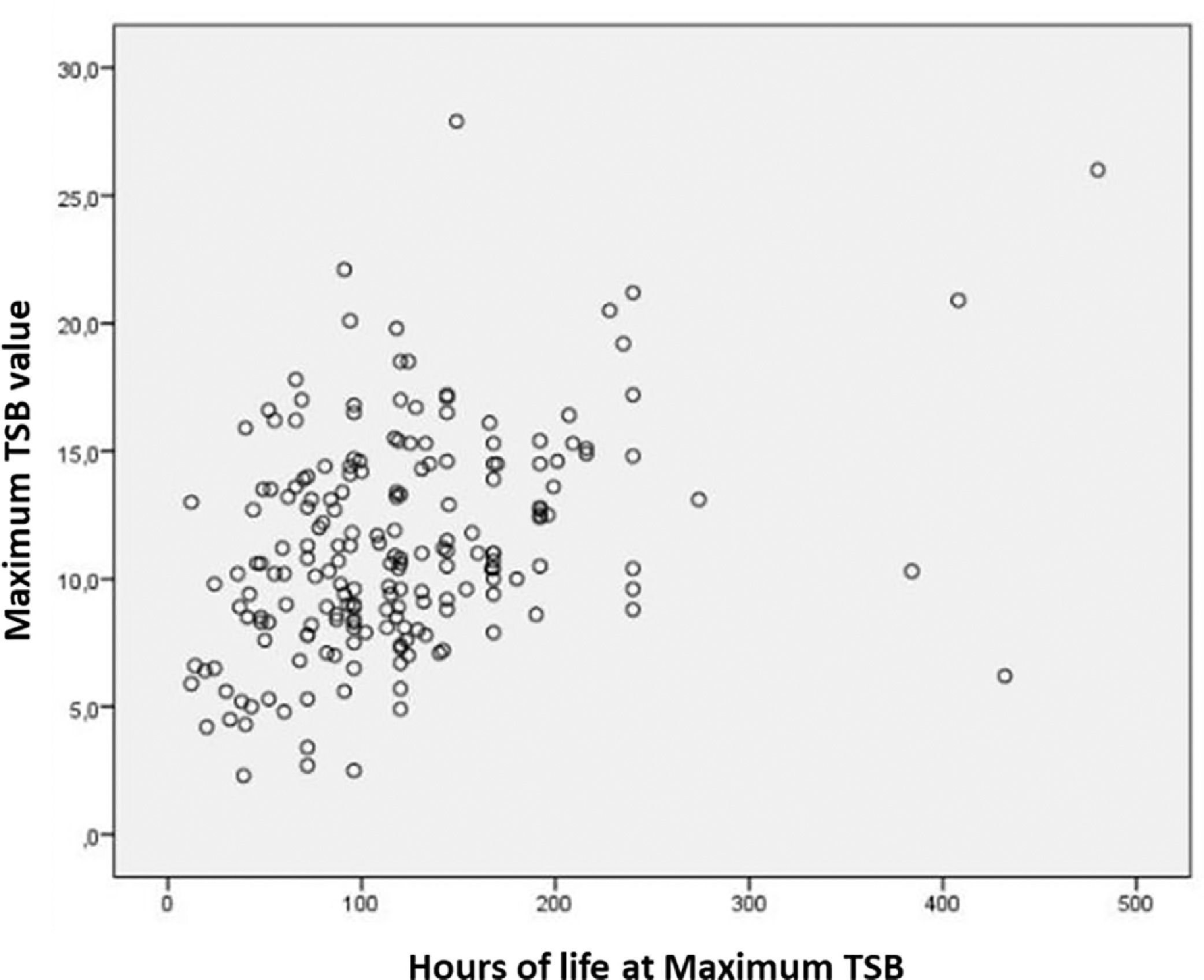
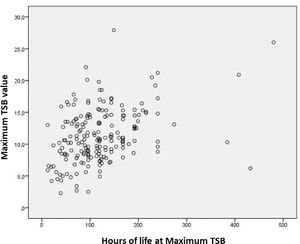
The median maximum TSB value in the study population was 10.7mg (IQR = 8.4-14.2mg), and the maximum TSB value found was 27.9 mg/dL. The maximum TSB peak was reached with a median of 113.5h of life (IQR = 42-144h).
Nineteen infants (9.5%) required ET. The main indication was a hemolysis rate greater than 0.5 mg/h (78.9%), followed by TSB at the ET level according to specific curves for a lifetime, risk, and GA (15.8%) and drop in HT above 10 points (5.3%). Only one case required two ETs (Table 2). A weak correlation was obtained between the variables (Spearman's test: 0.348) when the maximum TSB values were correlated with the lifetime (in hours) in which it occurred, but with relevant statistical significance (p-value = 0.0001) (Figure 2).
Clinical course and therapeutic intervention in infants with Perinatal Hemolytic Disease during the study period.
| n (%) | Median (IQR) | |
|---|---|---|
| Hydrops at birth | 3 (1.5) | |
| Duration of phototherapy (days) | 6 (5-7) | |
| Duration of hospitalization (days) | 8 (6-7) | |
| Maximum TSB value (mg/dL) | 10.7 (8.4-14.2) | |
| Age at maximum TSB (hours) | 113.5 (42-144) | |
| Infants requiring blood transfusiona | 86 (41.7) | |
| During hospitalization | 33 (16.6) | |
| At follow-up | 62 (31.2) | |
| Infants requiring ET | 19 (9.5) | |
| Infants with TSB > 20 | 8 (4) | |
| Phototherapy complication | 75 (37.7) | |
| Skin burns | 1 (0.5) | |
| Hypothermia | 64 (32.2) | |
| Hyperthermia | 20 (10.1) |
TSB, total serum bilirubin; ET, exchange transfusion.
The total phototherapy duration ranged from 1 to 22 days with a median of 6 days (IQR = 5-7). Approximately 37.7% of the cases evidenced phototherapy complications, and hypothermia was the most common (32.2%), followed by hyperthermia (10.1%). A single case reported skin burns.
The median length of stay was eight days (IQR: 6-11). Considering the entire period observed, 41.7% of children received at least one red blood cell transfusion: 16.6% of the cases during hospitalization and 31.2% during follow-up after hospital discharge. The median was one transfusion (IQR = 1-1). All blood-transfused patients had negative serology for HIV, VDRL, and hepatitis B and C at one year of life.
Three deaths in preterm infants born by emergency deliveries due to acute fetal distress initiated during the IUT procedure were recorded. One infant was born hydropic at 28 weeks GA, underwent two ETs, and died at 22 days of life. Another was born at 32 weeks GA, with asphyxia, and died at 21 days of life. The third was born at 31 weeks GA, with hydrops and asphyxia, and evolved to death at 11 days of life. The last two were not submitted to ET. Six infants developed neonatal sepsis, but only one had been submitted to ET. Concerning the 11 cases of cholestasis, only three underwent ET.
Considering separately the results of each research year and comparing them with each other, no significant difference was observed in the annual variation in the number of ETs performed (p-value = 0.82), the maximum TSB value (p-value = 0.33), phototherapy duration (p-value = 0.87), hospitalization (p-value = 0.35) and the number of blood transfusions, either during hospitalization or during follow-up (p-value = 0.25). Moreover, no significant difference in these same variables was observed when cases were gathered in groups by year of birth following the time intervals in which devices were changed (2009 to 2010, 2011 to 2013, 2014 to 2016, and 2017 to 2018).
DiscussionThe incidence of ET found in infants undergoing prophylactic phototherapy after birth was 9.5%. The study by Sá et al. (2009) at the same institution found 45% of ET between 2002 and 2007.14 However, that study included all infants undergoing ET at any given lifetime. The same parameters for indication of ET were used in both periods (2002-2007 and 2009-2018), and the only difference was the introduction of phototherapy prophylactic use after birth. A possible explanation for the lower incidence of ET would be the adoption of prophylactic phototherapy. However, the two studies are not comparable since, in the current study, the authors assessed the incidence of ET only in infants undergoing prophylactic phototherapy and not in the total population of infants with hemolytic disease of newborns (HDN). The comparison with results from other centers is hampered, due to different evaluation periods, populations, phototherapy devices, and protocols and guidelines, as described by Ree et al. in the 20-year experience (2000-2020) of ET in infants with gestational age equal to or greater than 35 weeks with HDN by Rh at the Leiden University Medical Center.15
The highest values of maximum TB peak were reached later with prophylactic phototherapy. The mean number of hours of life at the maximum peak was close to the fifth day of life. In hemolytic disease, due to Rh incompatibility, as hemolysis is intense, the values usually rise much faster and peak early, commonly within the first 48 hours of life. The bilirubin increase rate is reportedly an essential factor in the development of bilirubin encephalopathy.16 The neurotoxic effect of bilirubin is due to the amount of free bilirubin in serum, crossing the blood-brain barrier. The newborn infant's ability to cope with the excessive bilirubin load produced by hemolysis is determinant to decrease the risk of brain injury, and the bilirubin binding capacity is related to the gestational age.4
A systematic review by Cochrane (2012) on the efficacy and safety of prophylactic phototherapy for low birth weight preterm infants concluded that this therapy keeps a lower serum bilirubin concentration and may have an effect on reducing the need for ET and the risk of neurodevelopmental impairment.13 While the population of the studies included in the metanalysis differed significantly from the present sample, limiting comparisons, the results were similar to those reported in the Cochrane review.
In this study, the authors found a median of eight days (IQR = 6-11) for the length of stay and six days (IQR = 5-7) for the phototherapy duration. Sá et al. reported a mean of 10.4 ± 11.3 hospitalization days and 6.02 ±2.84 phototherapy days in the group born between 2000 and 2004.13 Ree et al. reported a median of 5 days (IQR = 4–6) of phototherapy in the group born between 2015 to 2020 included in their study.15 The values found in the current study are similar, although the difficulties of comparing with other studies are again highlighted.
An important finding in this sample was that most pregnant women were in their first pregnancy with proven alloimmunization, and antibody titers were already critical for developing fetal anemia (≥ 16) in 89.3% of them. A retrospective study evaluating pregnancies complicated by Rh alloimmunization between January 2000 and May 2013 reported more frequent anti-D antibodies (21.7%). In the rest of the population, the most common association was between anti-D and anti-C, with 50% of pregnant women in this group developing severe HDN, with an OR of 3.65 (95% CI, 1.84–7.33) compared to patients with just anti-D.17 In the present sample, most pregnant women (87.9%) were alloimmunized only for the RhD antigen; then, the authors found anti-D in combination with anti-C (12.1%). The frequency of IUT was higher in pregnancies with the anti-D and anti-C (16.7%) combination; it was 6.9% in those with anti-D only. However, this difference was not significant (p-value = 0.071), so it cannot be said that the combination of antibodies was associated with the need for IUT in this sample.
This study highlighted the high incidence of hypothermia in treated patients (32.2%). This incidence could be explained by the frequent use of Biliberço, which does not prevent the infants from losing heat, as they need to wear only a diaper to keep the largest area of the skin exposed. While it is a somewhat expected complication, hypothermia has a detrimental effect on infants. The use of air heating units (model Bair Hugger™775, 3M™ Company, USA), with the output end of the heated air duct positioned at one end of the Biliberço equipment, was initiated in the service in the last two years of this research. These devices keep circulating heated air in them. The authors could not measure the impact of this measure on the population due to the short period of use of these units in the institution.
During the study, the less effective equipment was replaced by devices using high-radiance LED light. Contrary to expectations, there was no significant difference in the number of ETs performed, maximum TSB value, phototherapy duration, length of stay, and the number of blood transfusions during hospitalization or follow-up over the years and between the temporal groups. Phototherapy effectiveness knowingly depends, among others, on the baby's body's surface area exposed to it.4 Thus, the authors can speculate that the combined use of devices throughout the entire period, mostly with simultaneous exposure of the ventral and dorsal regions, enhances the exposed body surface.
One of the limitations of this study is its retrospective nature and dependence on information from medical records, with possible information bias. As a result, the lack of some information in the analysis and the small sample size limited the study's power. However, the final sample was homogeneous concerning the intervention studied, which was already included in the service's routine work during the study period, allowing for a consistent assessment of this practice. Moreover, the same researcher retrieved data from the medical records, which standardizes the collection method.
The authors conclude by saying that the low incidence of ET may indicate a positive effect of prophylactic phototherapy. The authors observed an extended maximum bilirubin peak for later moments and a low mean of maximum TSB values, with no impact on the total duration of phototherapy and hospitalization at the institution. Although the results point to a potential benefit of this therapy, further studies are required to obtain consistent information on the impact of prophylactic phototherapy on infants in the short and medium-term. Prospective and multicenter studies would be critical to better assess the impacts of this measure.