to carry out a scoping review with the purpose of mapping the scientific evidence on the use of the neutropenic diet in neutropenic pediatric cancer patients.
Source of dataThe scoping review protocol was prepared in accordance with the PRISMA-ScR and the checklist before the literature search was performed. Articles on nutritional management in adults or on the treatment of other diseases, and articles that were not in Portuguese or English and published before the year 2000, were excluded. Data were extracted based on the Cochrane Consumer and Communication Review Group form.
Summary of the findingsThree hundred and forty scientific articles were identified, with the final sample of this review consisting of nine studies. Although the neutropenic diet has been part of the nutritional management of pediatric cancer patients for more than 20 years, there is still great variation in the criteria for indicating use and starting and discontinuing it, as well as in the nutritional composition of the diet. Furthermore, there is no consensus on the impact of using a neutropenic diet on different clinical and nutritional outcomes.
ConclusionIn the absence of guidelines that standardize the use of a neutropenic diet in pediatric patients with neutropenia, there are heterogeneous approaches reported in the literature, even within the same institution. The available literature presents an absence of evidence on the use, viability, and effectiveness of the neutropenic diet in oncological children with neutropenia. More studies are needed to identify the real impact of the neutropenic diet on clinical and nutritional outcomes.
Pediatric cancer accounts for 1 %–3 % of all malignant tumors that affect most populations. Therefore, it is considered a rare disease when compared to cancers that affect the adult population. However, it is one of the main causes of mortality in children and adolescents in developed countries and in Brazil.1,2
Childhood cancer has its own histopathological characteristics that impact disease progression and clinical behavior. Pediatric cancers, for the most part, present with short latency periods, but are more aggressive and progress rapidly. On the other hand, they respond better to antineoplastic treatments and are generally considered to have a good prognosis.1
The most frequent tumors in the pediatric population are leukemias (representing about 25 % to 35 % of cases), tumors that affect the central nervous system and lymphomas.1,2 The base of antineoplastic treatment is chemotherapy, radiotherapy, and surgery. With the most modern therapeutic modalities, treatment with hematopoietic stem cell transplantation, immunotherapy, monoclonal antibodies, and specific drugs against regulatory proteins of the abnormal cellular mechanism of the neoplasia is also possible.2
Progress in cancer treatment has been remarkable in recent decades, with cure rates reaching 80 %, as a result of scientific advances, the evolution of antineoplastic therapy, and the improvement in the quality of care support through multidisciplinary teams that provide assistance to patients. Despite the evolutions in antineoplastic treatments, the adverse effects arising from the therapies continue to have an important impact on the morbidity and mortality rates of children with cancer. Among the infectious complications resulting from antineoplastic treatments, febrile neutropenia (absolute neutrophil count < 1500 cells/mm³) caused by chemotherapy treatment is one of the most serious and with the highest potential for treatment-related mortality.3–7
Neutrophils constitute around 70 % of the white blood cells in the human body. Thus, when there is a drop in circulating levels, there is an increased risk of infections and sepsis.8 Since most infections present in neutropenic patients result from the intestinal microbiota itself, due to the presence of mucosal cell damage caused by certain chemotherapeutic agents, several nutritional strategies have been developed over the years. The most described is the neutropenic, which aims to reduce the introduction of microorganisms into the intestine from dietary restrictions mainly raw and/or uncooked foods.9 Despite being widely used in clinical contexts, there is no consensus in the available scientific literature on the use, viability, and impact on clinical outcomes of the neutropenic diet in oncological children with neutropenia. In this sense, the objective of this study was to gather scientific evidence about the use of the neutropenic diet in pediatric oncological patients with neutropenia to help with clinical and nutritional strategies, in addition to contributing to a better quality of life for these patients.
MethodsScoping reviews aim to map the literature on a given topic or area of study. The scoping review protocol was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extension for Scoping Reviews (PRISMA-ScR) and the checklist before the literature search and data extraction were performed. Five steps were followed: identification of the research question (“What scientific evidence was found regarding the use of the neutropenic diet in pediatric cancer patients with neutropenia?”); a search for relevant studies; selection of studies; extraction and grouping of data; and presentation of results.10,11
Search strategyThe PCC strategy (P: Population, C: Concept and C: Context) was adopted to elaborate the research question and search strategy.10 The searches were carried out independently by two reviewers (APCA and HGSA), in April and May 2022, and updated in February 2023, in the PubMed, LILACS, Google Scholar, and SciELO databases. Health Sciences Descriptors (DeCS) and Medical Subject Headings (MeSH) were consulted, with DeCS used to define keywords in Portuguese and MeSH to obtain their synonyms in English. The descriptors Child, Adolescent, Oncology, Neutropenic Patient, Neutropenic Diet, and Low Bacterial Diet and their corresponding keywords were used.
To maintain coherence in the search for articles and avoid possible biases, the descriptors and keywords were used separately and associated, respecting the specific characteristics of each of the selected databases. Searches in the databases were performed using keywords with the connector terms AND and OR. The searches were limited to the period from 2000 to 2023, due to the specificity of the theme, with a limited number of publications available. Manual searches were performed in the references of the included studies in order to locate relevant research.
Eligibility criteriaThe inclusion and exclusion criteria of the studies were defined before the beginning of the searches. Literature review articles or original articles, with a quantitative or qualitative approach, published in Portuguese and English, were included. Articles that discussed nutritional management in adults or the treatment of diseases other than cancer were excluded. Articles that were not in Portuguese or English and that were published before the year 2000 were also excluded.
The titles and abstracts of the articles found were organized in EndNote Web software and those that were duplicated were excluded. Two independent reviewers (APCA and HGSA) screened the articles by reading the titles and abstracts to identify those that were relevant. Disagreements to include or exclude publications were resolved by consensus with an additional reviewer (SFM).
Data extractionArticles selected based on the eligibility criteria were read in full by both reviewers in order to select the final review sample. Data from these studies were extracted based on the Cochrane Consumer and Communication Review Group form.12 The information of the articles on authorship, year of publication, origin, type of study, objective, characteristics and sample size, methods, main results, and observations were used and presented in a qualitative way. Such data were also descriptively analyzed by two researchers, who elaborated on the main themes to facilitate an overview of the entire literature.
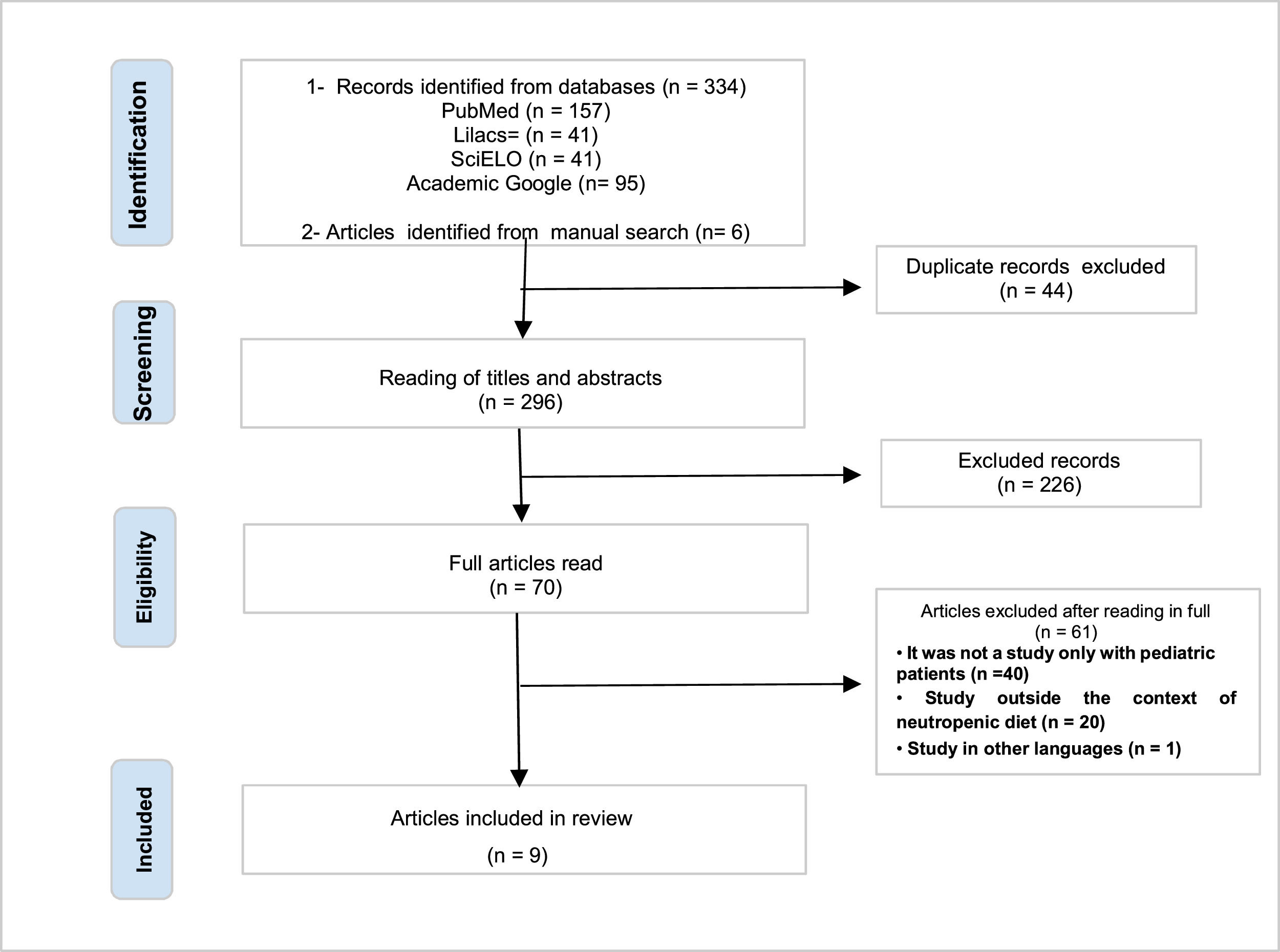
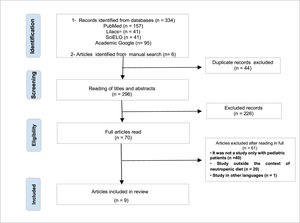
ResultsAfter searching the articles on the platforms and manually searching, 340 scientific studies were identified. Forty-four duplicate publications were excluded. Of the 296 remaining works, the titles and abstracts were read by the two reviewers, again independently, resulting in the exclusion of 226 articles that did not meet the inclusion criteria of this review. Next, the 70 articles were read in full, in order to identify those that responded to the objectives of the study. After full reading, 61 articles were excluded, as they did not address neutropenia in pediatric patients or did not present an oncological (hospitalized or in an outpatient chemotherapy setting) and nutritional context. The final review sample consisted of nine articles (Figure 1).
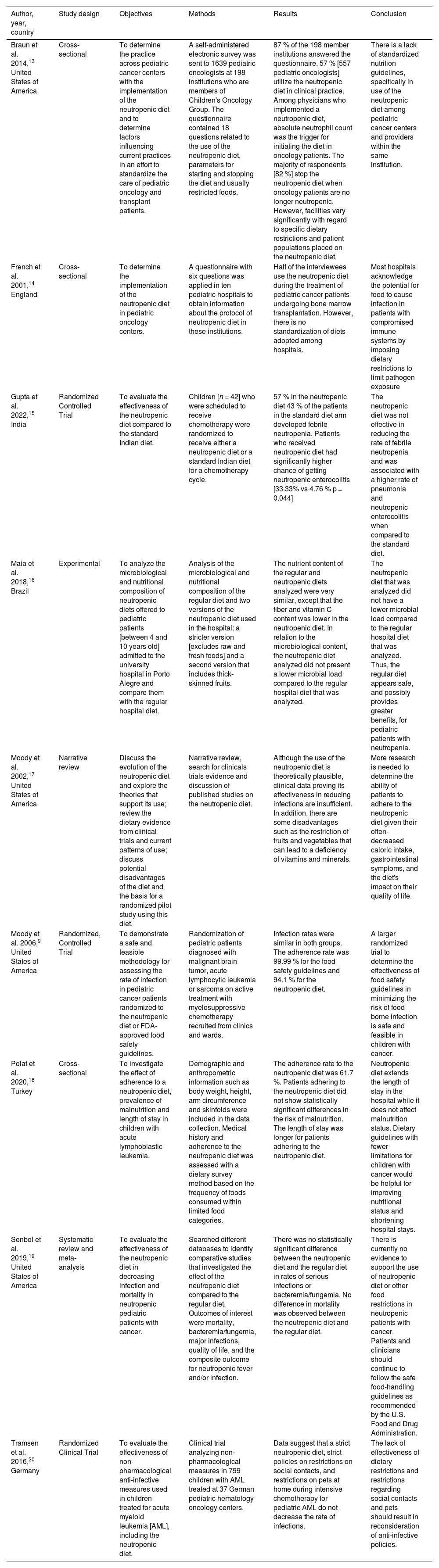
The main characteristics of the studies,13–20 such as year of publication, location, objectives, methods, and results are described in Table 1.
Main characteristics of the studies included in the scoping review, 2023.
| Author, year, country | Study design | Objectives | Methods | Results | Conclusion |
|---|---|---|---|---|---|
| Braun et al. 2014,13 United States of America | Cross-sectional | To determine the practice across pediatric cancer centers with the implementation of the neutropenic diet and to determine factors influencing current practices in an effort to standardize the care of pediatric oncology and transplant patients. | A self-administered electronic survey was sent to 1639 pediatric oncologists at 198 institutions who are members of Children's Oncology Group. The questionnaire contained 18 questions related to the use of the neutropenic diet, parameters for starting and stopping the diet and usually restricted foods. | 87 % of the 198 member institutions answered the questionnaire. 57 % [557 pediatric oncologists] utilize the neutropenic diet in clinical practice. Among physicians who implemented a neutropenic diet, absolute neutrophil count was the trigger for initiating the diet in oncology patients. The majority of respondents [82 %] stop the neutropenic diet when oncology patients are no longer neutropenic. However, facilities vary significantly with regard to specific dietary restrictions and patient populations placed on the neutropenic diet. | There is a lack of standardized nutrition guidelines, specifically in use of the neutropenic diet among pediatric cancer centers and providers within the same institution. |
| French et al. 2001,14 England | Cross-sectional | To determine the implementation of the neutropenic diet in pediatric oncology centers. | A questionnaire with six questions was applied in ten pediatric hospitals to obtain information about the protocol of neutropenic diet in these institutions. | Half of the interviewees use the neutropenic diet during the treatment of pediatric cancer patients undergoing bone marrow transplantation. However, there is no standardization of diets adopted among hospitals. | Most hospitals acknowledge the potential for food to cause infection in patients with compromised immune systems by imposing dietary restrictions to limit pathogen exposure |
| Gupta et al. 2022,15 India | Randomized Controlled Trial | To evaluate the effectiveness of the neutropenic diet compared to the standard Indian diet. | Children [n = 42] who were scheduled to receive chemotherapy were randomized to receive either a neutropenic diet or a standard Indian diet for a chemotherapy cycle. | 57 % in the neutropenic diet 43 % of the patients in the standard diet arm developed febrile neutropenia. Patients who received neutropenic diet had significantly higher chance of getting neutropenic enterocolitis [33.33% vs 4.76 % p = 0.044] | The neutropenic diet was not effective in reducing the rate of febrile neutropenia and was associated with a higher rate of pneumonia and neutropenic enterocolitis when compared to the standard diet. |
| Maia et al. 2018,16 Brazil | Experimental | To analyze the microbiological and nutritional composition of neutropenic diets offered to pediatric patients [between 4 and 10 years old] admitted to the university hospital in Porto Alegre and compare them with the regular hospital diet. | Analysis of the microbiological and nutritional composition of the regular diet and two versions of the neutropenic diet used in the hospital: a stricter version [excludes raw and fresh foods] and a second version that includes thick-skinned fruits. | The nutrient content of the regular and neutropenic diets analyzed were very similar, except that the fiber and vitamin C content was lower in the neutropenic diet. In relation to the microbiological content, the neutropenic diet analyzed did not present a lower microbial load compared to the regular hospital diet that was analyzed. | The neutropenic diet that was analyzed did not have a lower microbial load compared to the regular hospital diet that was analyzed. Thus, the regular diet appears safe, and possibly provides greater benefits, for pediatric patients with neutropenia. |
| Moody et al. 2002,17 United States of America | Narrative review | Discuss the evolution of the neutropenic diet and explore the theories that support its use; review the dietary evidence from clinical trials and current patterns of use; discuss potential disadvantages of the diet and the basis for a randomized pilot study using this diet. | Narrative review, search for clinicals trials evidence and discussion of published studies on the neutropenic diet. | Although the use of the neutropenic diet is theoretically plausible, clinical data proving its effectiveness in reducing infections are insufficient. In addition, there are some disadvantages such as the restriction of fruits and vegetables that can lead to a deficiency of vitamins and minerals. | More research is needed to determine the ability of patients to adhere to the neutropenic diet given their often-decreased caloric intake, gastrointestinal symptoms, and the diet's impact on their quality of life. |
| Moody et al. 2006,9 United States of America | Randomized, Controlled Trial | To demonstrate a safe and feasible methodology for assessing the rate of infection in pediatric cancer patients randomized to the neutropenic diet or FDA-approved food safety guidelines. | Randomization of pediatric patients diagnosed with malignant brain tumor, acute lymphocytic leukemia or sarcoma on active treatment with myelosuppressive chemotherapy recruited from clinics and wards. | Infection rates were similar in both groups. The adherence rate was 99.99 % for the food safety guidelines and 94.1 % for the neutropenic diet. | A larger randomized trial to determine the effectiveness of food safety guidelines in minimizing the risk of food borne infection is safe and feasible in children with cancer. |
| Polat et al. 2020,18 Turkey | Cross-sectional | To investigate the effect of adherence to a neutropenic diet, prevalence of malnutrition and length of stay in children with acute lymphoblastic leukemia. | Demographic and anthropometric information such as body weight, height, arm circumference and skinfolds were included in the data collection. Medical history and adherence to the neutropenic diet was assessed with a dietary survey method based on the frequency of foods consumed within limited food categories. | The adherence rate to the neutropenic diet was 61.7 %. Patients adhering to the neutropenic diet did not show statistically significant differences in the risk of malnutrition. The length of stay was longer for patients adhering to the neutropenic diet. | Neutropenic diet extends the length of stay in the hospital while it does not affect malnutrition status. Dietary guidelines with fewer limitations for children with cancer would be helpful for improving nutritional status and shortening hospital stays. |
| Sonbol et al. 2019,19 United States of America | Systematic review and meta-analysis | To evaluate the effectiveness of the neutropenic diet in decreasing infection and mortality in neutropenic pediatric patients with cancer. | Searched different databases to identify comparative studies that investigated the effect of the neutropenic diet compared to the regular diet. Outcomes of interest were mortality, bacteremia/fungemia, major infections, quality of life, and the composite outcome for neutropenic fever and/or infection. | There was no statistically significant difference between the neutropenic diet and the regular diet in rates of serious infections or bacteremia/fungemia. No difference in mortality was observed between the neutropenic diet and the regular diet. | There is currently no evidence to support the use of neutropenic diet or other food restrictions in neutropenic patients with cancer. Patients and clinicians should continue to follow the safe food-handling guidelines as recommended by the U.S. Food and Drug Administration. |
| Tramsen et al. 2016,20 Germany | Randomized Clinical Trial | To evaluate the effectiveness of non-pharmacological anti-infective measures used in children treated for acute myeloid leukemia [AML], including the neutropenic diet. | Clinical trial analyzing non-pharmacological measures in 799 children with AML treated at 37 German pediatric hematology oncology centers. | Data suggest that a strict neutropenic diet, strict policies on restrictions on social contacts, and restrictions on pets at home during intensive chemotherapy for pediatric AML do not decrease the rate of infections. | The lack of effectiveness of dietary restrictions and restrictions regarding social contacts and pets should result in reconsideration of anti-infective policies. |
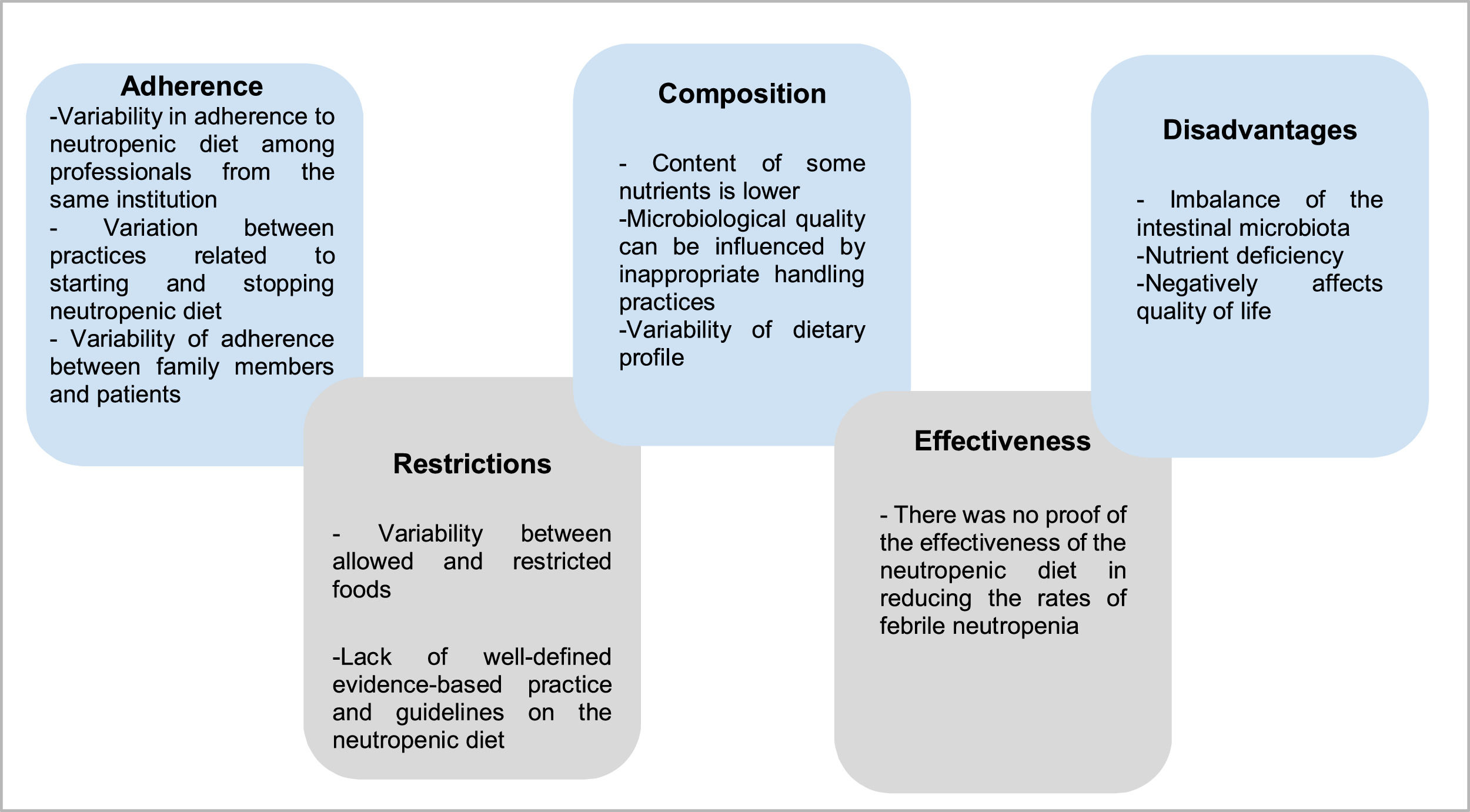
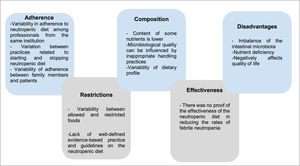
Figure 2 presents the five themes constructed based on qualitative analysis of the results of the included studies.
Definition, implementation and adherence to the neutropenic dietThe foundation of the neutropenic diet is to limit the introduction of potentially harmful pathogens into the gastrointestinal tract by restricting foods that may harbor these organisms.20 However, the lack of evidence and guidelines regarding the neutropenic diet results in a great variability of patterns that are described in the literature. Dietary restrictions are highly variable. According to the analyzed articles, the most commonly restricted foods include raw vegetables, fresh and dried fruits, raw meat, chicken, eggs, seafood, and condiments.21 It has also been noted that some hospitals recommend restricting herbs and sprouts, aged cheeses, sushi, fast food, and raw honey. In addition, it is noteworthy that restrictions vary not only between different hospitals but also within the same institution.13
Considering the articles included in this review, some institutions, in the absence of well-defined evidence-based practice and guidelines on the neutropenic diet, use the guidelines published by the Food and Drug Administration (FDA) or the Centers for Disease Control and Prevention (CDC) regarding food safety practices.17 For immunocompromised patients, these organizations advise the consumption of only pasteurized juice, milk or cheese and hard-boiled eggs, poultry, meat and cooked fish.17
In two studies included in this review, the timing of the introduction of the neutropenic diet was defined by pediatric oncologists. There was variation in relation to the beginning of the use of the neutropenic diet. According to the studies, most respondents reported starting the diet based on the absolute neutrophil count, usually when the rate is less than 500 cells/mm³.13,14
A cross-sectional study carried out in the United States with 557 pediatric oncologists identified, through the application of a questionnaire, that 57 % of respondents use the neutropenic diet in clinical practice. However, there is great variation regarding specific dietary restrictions, the characteristics of the patients for whom it is indicated, and parameters for starting and stopping the diet. The results of this research indicate that there is variability in the use of the neutropenic diet among physicians at the same institution.13
This finding corroborates the study by French et al., where 5 of the 10 pediatric hospitals evaluated indicate the use of a neutropenic diet for bone marrow transplant patients, but without consensus among professionals. Only three institutions report following the local guidelines on low-bacteria diets. In addition, hospitals use different criteria for when to start or discontinue the diet, with no standardization.14
Few studies have investigated adherence to the neutropenic diet from the point of view of patients and their guardians. Moody et al. observed that patient adherence varies according to the types of restrictions: the greater the restriction, the lower the adherence. The authors also inquired about the continuation of the diet during treatment. All evaluated patients reported that they were able to maintain the diet and most stated that they could follow it during all chemotherapy cycles. Among the cited reasons that would lead to the inability to continue the diet for more than one cycle, the following were included: difficulties in following many restrictions and the various steps that the family must follow to guarantee the production of meals consistent with the neutropenic diet. In this sense, families who reported having received a list of guidelines on what to do and not to do on the neutropenic diet had less difficulty in following it for a longer period of time.9
In 2020, Polat and collaborators investigated the effect of adherence to the neutropenic diet on malnutrition and length of hospital stay in children with acute lymphoblastic leukemia. The authors observed that the rate of adherence to the neutropenic diet was 61.7 %. In addition, patients adhering to the neutropenic diet did not show statistically significant differences in relation to the risk of malnutrition, and the length of hospital stay was longer for patients who adhered to the neutropenic diet.18
Nutritional and microbiological composition of the neutropenic dietThe nutritional and microbiological composition of neutropenic diets varies greatly according to the studies and guidelines analyzed. In Brazil, the National Cancer Institute (INCA) addresses the recommendations for nutritional management in neutropenia through a summary table. The INCA recommendations are: do not use probiotics, clean all fruits and vegetables with sanitizers, use potable, boiled, or mineral water in non-reusable packaging, eat only cooked condiments and grains, sterilized or pasteurized milk and pasteurized derivatives and well-cooked meat and eggs, do not consume oilseeds (chestnuts, almonds, walnuts), do not consume teas in sachets or dried leaves (or boiled), use preparations produced by establishments that take all the necessary care for food safety, prefer industrialized foods in packages for individual consumption immediately, avoid industrialized meat and prefer processed foods in individual packages.1
On the other hand, dietary guidelines from the FDA, CDC,22 and National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Prevention and Treatment of Cancer-Related Infections23 do not recommend the neutropenic diet due to the scarcity of evidence proving its independent effect on the rate of opportunistic infection in patients.9,24
The American Cancer Society also does not make specific recommendations regarding the use of the neutropenic diet.25 Recommendations focus on food-handling tips (wash hands with warm, soapy water for 20 s before and after preparing food and before eating), avoiding cross-contamination (use a clean knife to cut different foods), cooking foods well [put a meat thermometer into the middle of the thickest part of the food to test for doneness], grocery shopping tips (do not eat foods that are bought from self-serve or bulk containers) and dining out tips (ask for pasteurized fruit juices; avoid “fresh-squeezed” juices in restaurants). They also emphasize that raw vegetables fruits and fresh herbs are safe to eat if washed under running water and lightly scrubbed with a vegetable brush.25
The United States Department of Agriculture (USDA) recommendations for food safety for people with cancer include the following: (a) consumption only of pasteurized milk and dairy products; (b) meat, poultry and seafood cooked to a safe internal temperature; (c) cooked eggs with a firm yolk; (d) washed fresh or cooked produce. Notably, there is no recommendation for the restriction of fresh fruits and vegetables.26 Thus, there is no reference regarding the nutritional and microbiological composition of the neutropenic diet defined by these institutions. Instead, they recommend standard safe food-handling methods that actually allow washing of fresh fruits and vegetables.22,23,25,26
Maia et al. analyzed the microbiological and nutritional composition of the neutropenic diet and regular diet used in an oncology hospital. The regular diet and two versions of the neutropenic diet were evaluated: a stricter version, in which raw and fresh foods were excluded, and another version, in which thick-skinned fruits were included. The study showed that the nutrient content of the regular and neutropenic diets analyzed was very similar, except that the fiber and vitamin C content was lower in the strict neutropenic diet. As for the microbiological content, the analyzed neutropenic diets did not present a lower microbial load compared to the regular hospital diet. The authors assume that all contamination found after food is prepared is due to food handling and inadequate temperature control.16
Efficacy of the neutropenic dietAlthough the use of the neutropenic diet is a common practice, there is not enough clinical and scientific data available to prove its effectiveness in reducing infections in neutropenic patients. It is important to highlight that the use of a neutropenic diet has important disadvantages in the context of pediatric cancer patients with neutropenia. Restriction of fruits and vegetables can disrupt the balance of the gut microbiota and increase the risk of bacterial overgrowth and translocation. In addition, limited food choices can compromise the general nutritional status of these patients and contribute to increased mortality.17
The studies included in this review did not prove the effectiveness of the neutropenic diet. A clinical trial carried out with 799 children with acute myeloid leukemia evaluated the effectiveness of non-pharmacological anti-infective measures. The results of this study suggest that a strict neutropenic diet strict policies on restrictions on social contacts and restrictions on pets at home during intensive chemotherapy for pediatric acute myeloid leukemia do not decrease infection rates.20
The findings by Gupta et al. are similar. The authors performed a randomized clinical trial with 42 children who were scheduled to receive chemotherapy. The neutropenic diet was not effective in reducing the rate of febrile neutropenia and was associated with a higher rate of pneumonia and neutropenic enterocolitis when compared with the standard diet.15
Moody et al. conducted a multicenter, prospective, randomized, controlled study in the United States, with the aim of evaluating the infection rate in pediatric cancer patients. Patients were randomized to receive either the neutropenic diet or the FDA-approved food safety guidelines. Results showed that infection rates were similar in both groups. In addition, the adherence rate was 99.99 % for food safety guidelines and 94.1 % for the neutropenic diet.9
Sonbol et al. evaluated the effectiveness of the neutropenic diet in decreasing infection and mortality in neutropenic children. The study showed that there was no statistically significant difference in the rates of serious infections between the neutropenic diet and the regular diet. In patients who underwent hematopoietic cell transplantation, the neutropenic diet was associated with a slightly increased risk of infections. No difference in mortality was observed between the neutropenic diet and the regular diet.19
Dietary restrictions & potential disadvantages of using the neutropenic dietRestriction of fruits and vegetables can alter the balance of the gut microbiota and increase the risk of overgrowth of harmful bacteria. Furthermore, the neutropenic diet, which limits food choices, may compromise the overall nutritional status of cancer patients and lead to increased morbidity. Specifically, children with cancer have been shown to have significantly lower levels of some micronutrients and antioxidants.17 Corroborating this, the restrictive nature of the neutropenic diet can put patients at risk of having multiple nutritional micro-deficiencies.15
The limitation of food intake in neutropenic oncological children may also contribute to the increased need for nutritional support. This situation can lead to an increased risk of developing infections and hospital costs, in addition to a reduction in quality of life.18 In a study included in this scoping review, Polat et al. also found that the hospital stay was significantly longer in patients adhering to the neutropenic diet compared to non-adherent patients.18
Furthermore, the potential nutritional drawbacks of the neutropenic diet and the practical recommendations may affect patients’ adherence to this diet.9 Dietary restrictions may not be well tolerated and may negatively affect patients’ quality of life, in addition to requiring more effort.21 It is worth mentioning that dietary guidelines from the FDA, CDC,22 and National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Prevention and Treatment of Cancer-Related Infections23 reinforce the lack of evidence for the use of the neutropenic diet. In addition, these institutions claim that this strategy can contribute to an increase in malnutrition since dietary restrictions may not be well tolerated.9,24
DiscussionThe main objective of this scoping review was to gather scientific evidence on the use of the neutropenic diet in pediatric patients with neutropenia with regard, above all, to restrictions, adherence, composition, efficacy, and disadvantages.
Although the role of nutrition is widely recognized as an essential component in the management of pediatric cancer patients, there is a lack of standardized nutritional guidelines, specifically in the management of patients with neutropenia. The neutropenic diet, despite being part of the nutritional practices of pediatric cancer patients for more than 20 years, does not have a standardized protocol. There is still variation in the criteria for indication, implementation, characteristics and impact on clinical outcomes related to the use of this diet.27 This variation was observed even within the same institution. A study published in the United Kingdom demonstrated that this variation in the recommendation of restrictions and prescriptions also occurs among nutritionists, among whom specialists in oncology and hematology are significantly more likely to prescribe a neutropenic diet than those who work in non-specialized posts.28 Therefore, although most of the professionals indicated in the studies adhere to the neutropenic diet, these data highlight that the differences in practice can be attributed to the lack of high-quality robust evidence.
The same has been observed in the nutritional care of adult cancer patients. Wolfe et al. observed in randomized trials that restrictive diets are not superior in preventing infections than more liberalized diets. They also emphasized that adherence to the Safe Food-Handling guidelines issued by the FDA, a mandate for all hospital kitchens, provides adequate protection against food-borne infection, precluding the need for a neutropenic diet.27 Thus, it is observed in the literature that conduct is heterogeneous and consequently can significantly impact the clinical and nutritional outcomes of these patients.27
The primary reason for proposing the use of a neutropenic diet is the likely impact on reducing the risk of infections. However, the studies included in this review showed no impact or benefit of the neutropenic diet on clinical outcomes. This low impact could be associated with poor adherence to the neutropenic diet in the pediatric age group. However, Polat et al. demonstrated that most of the analyzed patients adhered to the neutropenic diet, especially preschool children. This can be attributed to the fact that at school age (7–12 years old), the act of eating becomes much more complex, involving physiological, psychological, social, and cultural factors, as food preferences change as a result of experiences and learning.28,29 Furthermore, a meta-analysis of randomized, controlled clinical trials with 388 adult and child patients diagnosed with acute myelogenous leukemia, acute lymphoblastic leukemia, and sarcoma, did not observe a statistically significant difference in the rate of infection between the neutropenic versus unrestricted diet groups. Thus, the use of the neutropenic diet was not associated with a decreased risk of infection in pediatric and adult neutropenic oncology patients, and the continued use of restrictive neutropenic diets should be carefully reviewed.30
Another relevant point is the impact of using specific diets on food consumption and the nutritional risk of children with cancer. Food is an important factor not only in the clinical outcomes of the disease but also in the quality of life.9 In the study carried out by Moody et al., it was observed that the reduction of dietary restrictions can result in improvements in caloric intake and in the quality of life of neutropenic patients. The denial of foods desired by patients using a neutropenic diet, such as fast food and fresh fruit, is frustrating for children, in addition to being difficult to implement dynamically, since there is a need to prepare all meals at home. The reduction in food consumption increases the risk of malnutrition, which consequently increases the chances of more intense side effects and interruption of clinical treatment.9 Pedretti et al.,31 also described that the absence of a systematic approach and recommendations standards for nutritional care in the pediatric population with cancer put them at nutritional risk, compromising the clinical outcome and quality of life. Considering this gap in clinical and scientific practice Fabozzi et al.,32 developed a framework for the assessment and optimal care of nutritional care in low-middle-income countries, with practical recommendations for caring for the nutritional status of pediatric cancer patients, given that it is an independent prognostic factor. This practical guide has the potential to guide research and clinical practice.
None of the studies analyzed demonstrated the effectiveness of the neutropenic diet. Most published studies on the subject reported limitations or weak evidence, concluding that neutropenic diets had little or no effect on infection rates in patients with neutropenia. Corroborating the findings of Moody et al., which were exposed in this review, new studies by the same author showed that this diet did not present a difference in the prevention of infection in pediatric and adult cancer patients compared with those who adopted FDA-approved food safety guideline recommendations (33% vs. 35 %, p = 0.78).21
Universally, in the scientific literature, there are questions and suggestions that additional research on the subject is necessary to prove the effectiveness of the diet.33,34 In addition to being exposed to the lack of proof of efficacy and the lower microbiological load of the neutropenic diet, the disadvantages of this conduct are also mentioned. Among them is the fact that restrictive diets interfere with the patient's food choices and may result in inadequate intake of nutrients during chemotherapy.35 It should be noted that the oncological disease itself and its treatments cause changes, mainly in nutrition, appetite, and eating habits.36 In addition, patients commonly have insufficient nutrient intake due to anorexia, vomiting, nausea, and changes in smell and taste.37 Therefore, the neutropenic diet can further aggravate this situation, which can be a possible obstacle to maintaining a good nutritional status during cancer treatment and this can lead to a lower tolerance of a pediatric oncology patient to chemotherapy, decreasing their quality of life and increasing the risk of infection. Given the above, the scientific literature discusses the possibility of adopting a standardized approach focused on the safe handling of foods capable of allowing a less restrictive diet in the context of immunosuppression and neutropenia. In addition to contributing to meeting caloric needs, these guidelines on safe food handling are easier for patients to adhere to and for health professionals to reproduce since the different socioeconomic, racial, and cultural realities of patients must be considered.21,34 The limitations of this scoping review must be considered. The theme is still incipient in the literature, as only nine articles were found in the four databases consulted. Of these few studies, it was observed that most of them have limitations such as (a) small sample size; (b) lack of measurement of other variables that determine the incidence of neutropenic infection; (c) low methodological quality. In addition, the present search was limited to publications in Portuguese and English, which excluded some studies and may have biased the findings. Another limitation is the lack of differentiation between pediatric cancer treatment and of patients post hematopoietic cell transplantation, in addition to the inclusion of different scenarios (hospital and outpatients’ scenario). However, despite these limitations, this review has the potential to encourage and support new investigations, as it demonstrates the need for research more well-delineated, with the aim of generating consistent scientific evidence, substantiating clinical practice and contributing to a better quality of life for these patients. In addition, encourages the implementation of better hygienic control at home, hospital, and institutional settings for neutropenic patients in accordance with the recommendations of local health authorities.
ConclusionAlthough part of the nutritional practices of pediatric cancer patients, the use of the neutropenic diet varies greatly among health professionals, resulting in heterogeneous practices. The available literature presents an absence of evidence on the use, viability, and effectiveness of the neutropenic diet in oncological children with neutropenia. More studies are needed to identify the real impact of the neutropenic diet on clinical and nutritional outcomes.
Authors’ contributionsAll the authors participated in the conception and design of the study, acquisition of data, analysis and interpretation of data and drafting of the article. All the authors approved the final version submitted.