Among the mechanisms proposed for the development of bronchopulmonary dysplasia is the increase in the pulmonary inflammatory process and oxidative stress. Thus, the control of this process may result in improvements in bronchopulmonary dysplasia-related outcomes. This study aims to analyze the current scientific evidence regarding the use of budesonide, a potent anti-inflammatory drug, associated with a pulmonary surfactant to prevent bronchopulmonary dysplasia.
MethodsA systematic review of the literature was performed on the Embase and MEDLINE platforms, and studies that compared budesonide with pulmonary surfactant versus pulmonary surfactant for treating respiratory distress syndrome were included. The primary outcome was a reduction in bronchopulmonary dysplasia or death.
ResultsFour randomized clinical trials and two observational studies were included in this systematic review. Three of the randomized clinical trials found a reduction in bronchopulmonary dysplasia or death in the use of budesonide with the surfactant, all the other studies (1 clinical trial and 2 observational studies) found no statistical differences between the groups for the primary outcomes. The three main studies showed a reduction in the primary outcome; however, all studies showed great heterogeneity regarding the type of surfactant (poractant or beractant) and the method of administration.
ConclusionRobust clinical studies, in a heterogeneous population, using porcine surfactant associated with budesonide, with administration by a minimally invasive technique are necessary for there to be a recommendation based on scientific evidence for its widespread use.
Bronchopulmonary dysplasia (BPD) is one of the main chronic diseases of newborns born prematurely and, despite the development of medicine since its initial description more than 50 years ago, little has changed regarding the incidence of this disease.1–3 The increasing survival of extremely premature newborns with lung development in the transition from the canalicular to saccular phase, leading to disruption of alveolarization and vascular dysmorphism, may explain these findings.1–3 This pathophysiological process of chronic lung disease in the newborn differs from that described by Northway et al.,4 in which large areas of cell death and fibrosis were observed, resulting from intense pulmonary aggression caused by mechanical ventilation.2–4
A mechanism proposed for developing the “new” BPD is the increase in the systemic inflammatory process and oxidative stress secondary to chorioamnionitis, placental insufficiency, neonatal sepsis, volutrauma associated with invasive mechanical ventilation, and hyperoxia in the postnatal period, decreasing the release of growth factors with the cessation of lung development.1,2 Thus, interventions capable of controlling the systemic and pulmonary inflammatory process would lead to better pulmonary development in the postnatal period, with consequent improvement in BPD.3
Among the medications related to the control of the inflammatory process, corticosteroids stand out because they have a potent anti-inflammatory power. Systemic administration of dexamethasone to preterm newborns was associated with a lower incidence of BDP, however, it was also associated with worsening neurodevelopment.5,6 Thus, its use is warranted only for those newborns at high risk for developing BDP.5,6 Moreover, the administration of inhaled corticosteroids before 14 days of life was associated with higher mortality in this population, and, therefore, its use is not recommended.3,5,6
Nevertheless, studies with animals have shown a lower pulmonary inflammatory response and a lower rate of BPD or death when budesonide was associated with pulmonary surfactant in the treatment of Neonatal Respiratory Distress Syndrome (NRDS), with no effect on the neurodevelopment of those subjects.7,8
This study aims to analyze the current evidence regarding the use of budesonide associated with pulmonary surfactant for treating neonatal respiratory distress syndrome of the newborn and its prevention of BPD or death.
MethodsIn the present study, the authors performed a systematic review of the literature on the databases Embase® and MEDLINE (PubMed®), according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) recommendation.9 The following search strategy was employed: ("surfactant" OR "poractant alfa" OR "curosurf" OR "survanta") AND "budesonide" AND ("respiratory distress syndrome" OR "bronchopulmonary dysplasia" OR "hyaline membrane disease"). Only articles written in English or Portuguese were selected for a complete reading. Articles published from conception until May 15, 2022, were included for review.
The inclusion criteria for the articles aimed to answer the following PI(E)CO question:
- (1)
Population: Humans; Preterm newborns younger than 32 weeks of gestational age or very low birth weight (VLBW - less than 1500 g).
- (2)
Intervention/Exposure: Use of pulmonary surfactant associated with budesonide for treating NRDS.
- (3)
Control: Use of pulmonary surfactant for treating NRDS.
- (4)
Outcomes: Main: Bronchopulmonary dysplasia or death. Secondary: Length of mechanical ventilation, neurodevelopmental delay, neonatal sepsis.
Only original articles (randomized clinical studies, cohort studies, case-control studies) were included. The following types of studies were excluded from this systematic review: Systematic review, narrative review, letters to editors, comments, preprints, abstracts presented at congresses, and case reports.
The exclusion criteria were articles written in languages other than Portuguese and English in which the authors could not be able to extract data from the abstract.
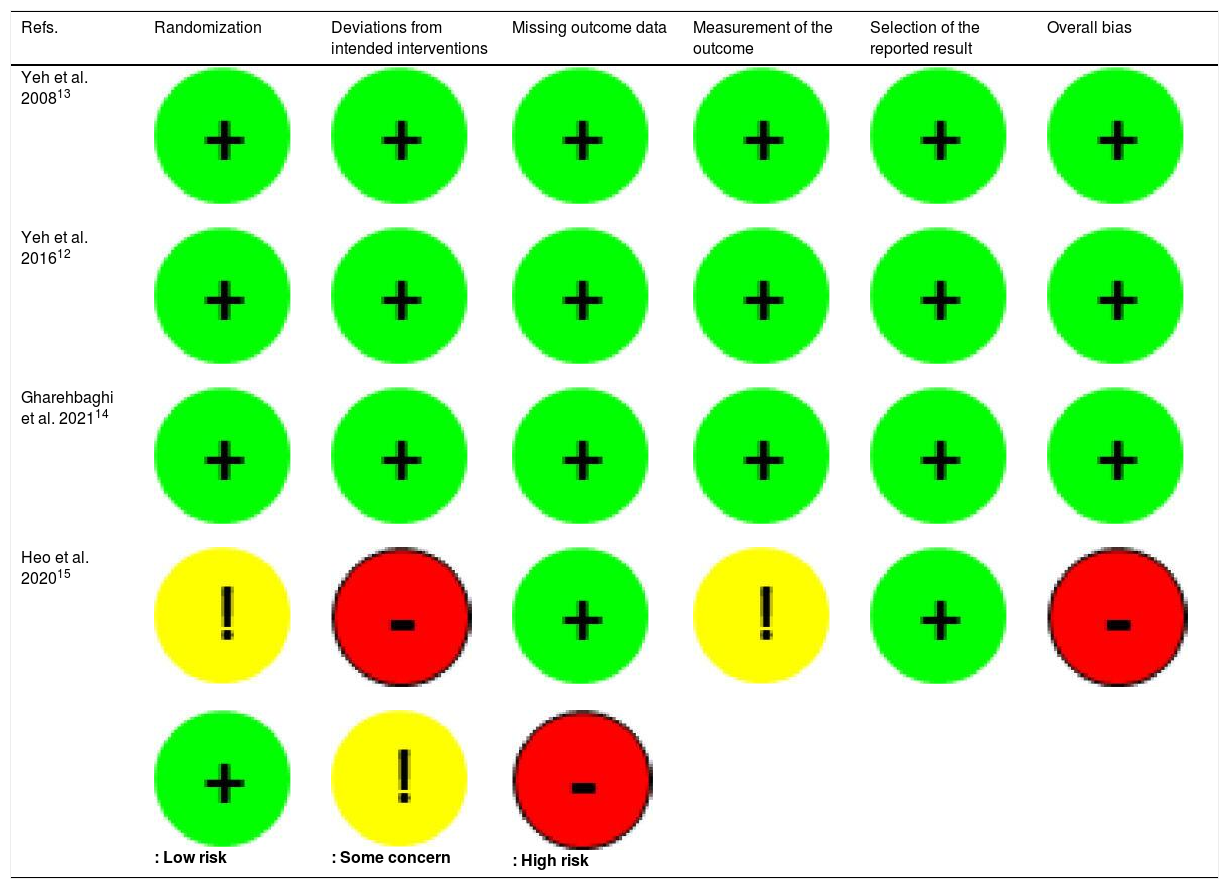
Two different investigators screened all the studies for the risk of bias. The quality of randomized clinical trials was assessed using the instrument RoB 2 (Risk of Bias Tool version 2.0)10 from the Cochrane collaboration® by L.H.A.M and R.M.D.C. The analysis of observational studies was performed using the Newcastle-Ottawa Quality Assessment Scale11 by L.H.A.M and G.P.N.S.B.
The articles that underwent full reading had their results summarized to answer the PI(E)CO question. All three main investigators (L.H.A.M, R.M.D.C, and G.P.N.S.B.) performed the data extraction independently and, in cases of disagreement, a consensus was reached by altogether.
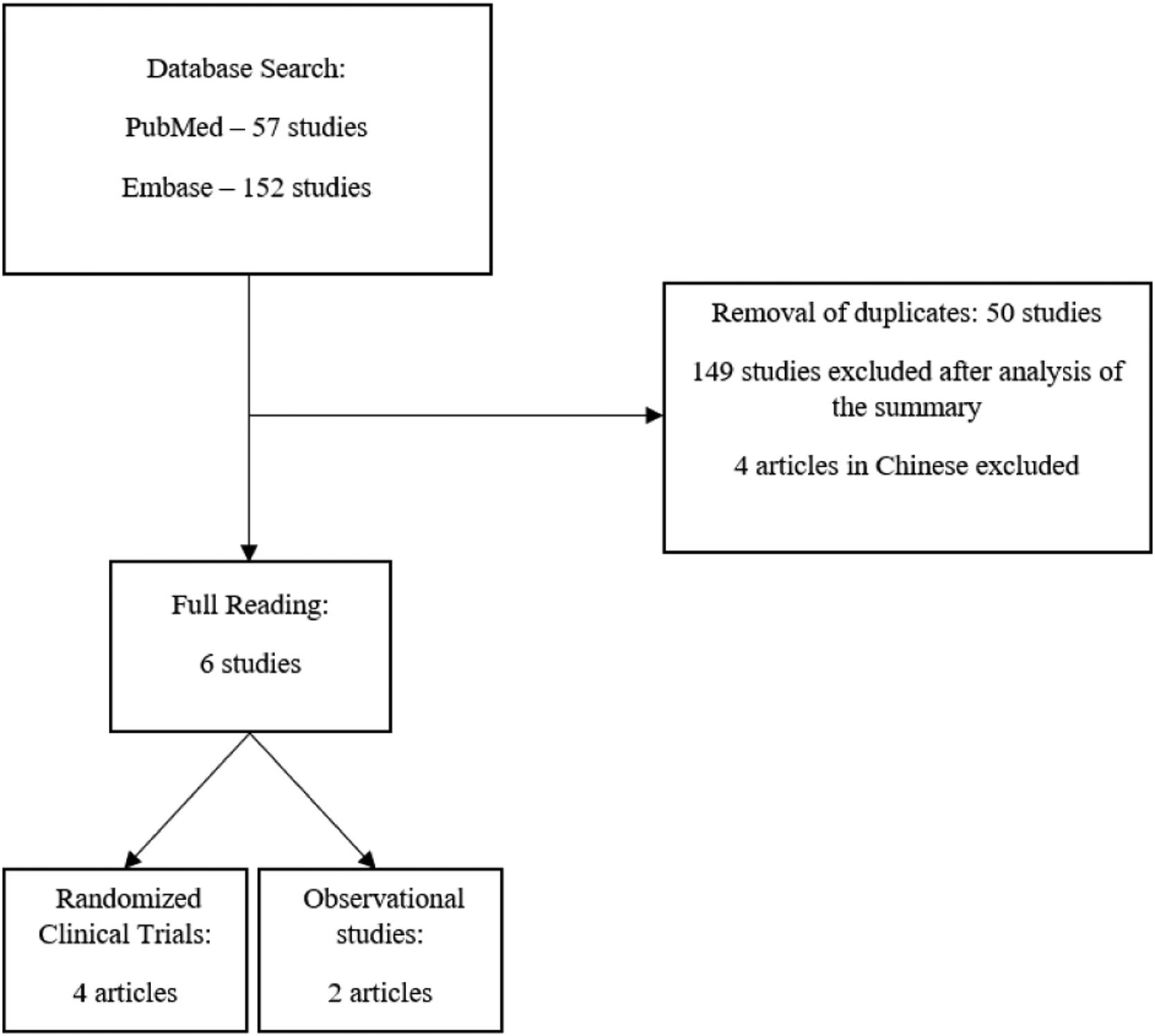
ResultsAfter the initial search, 57 articles were found on the PubMed® platform and 152 articles on the Embase platform. After the removal of duplicates, the abstracts of the 159 remaining articles were read. Of these, 6 articles were selected for full reading, 4 of which were randomized clinical trials and 2 were retrospective observational studies (Figure 1). The main results of the included studies are summarized in Table 1. The risks of bias are summarized in Table 2 (Randomized Clinical Trials) and Table 3 (Observational Studies).
Summary of the studies (PICO question).
| Refs. | Country | Type of Study | Population Inclusion/Exclusion criteria | Method of administration | Intervention/Exposure | Control | Outcomes |
|---|---|---|---|---|---|---|---|
| Yeh et al. 200813 | Taiwan/USA | Randomized clinical trial, double-blinded, controlled (pilot study) | Weight <1500g+ severe NRDS+ Invasive Mechanical Ventilation+ FiO2 ≥ 0.6+ absence ofsevere congenital anomalies or lethalcardiopulmonary disorder | After intubation | n = 60Budesonide 0.25 mg/kg + Survanta 100 mg/kg | n = 56Beractant 100 mg/kg | Intervention:Fewer patients receiving mechanical ventilation at 1st and 2nd weeks in the intervention group (p = 0.001 and p = 0.015, respectively).Reduction in death and BPD (combined) (p = 0.003).Similar adverse effects in both groups |
| Yeh et al. 201612 | Taiwan/USA | Randomized clinical trial, double-blinded, controlled | Weight <1500g+ severe NRDS (radiographic evidence)+ Invasive Mechanical Ventilation+ FiO2 ≥ 0.5+ absence ofsevere congenital anomalies or lethalcardiopulmonary disorder | After intubation | n = 131Budesonide 0.25 mg/kg + Beractant 100 mg/kg | n = 134Beractant 100 mg/kg | Intervention:Reduction in the incidence of death or BPD (RR 0.58; CI 95% 0.44 - 0.77; p < 0.001). NNT 4.1 (CI 95% 2.8 – 7.8)Reduction in BPD (RR 0.70; CI 95% 0.56 – 0.86; p < 0.001), NNT 4.7Lower levels of IL – 1, IL – 6 and IL – 8 (median) in the tracheal aspirate.Similar adverse effects in both groups |
| Gharehbaghi et al. 202114 | Iran | Randomized clinical trial, double-blinded, controlled | Preterm newborns < 30 wk and weight <1500g+ NRDS with indication of surfactant replacement(CPAP 5 FiO2 > 30% or indication for intubation) | INSURE | n = 64Poractant alfa 200 mg/kg + budesonide 0.25 mg/kg | n = 64Poractant alfa 200 mg/kg | Intervention:Reduction in BPD (p = 0.04), fewer administration of second dose of surfactant (p = 0.01), shorter duration of invasive mechanical ventilation (p = 0.006).Similar adverse effects in both groups |
| Heo M, Jeon GW. 202015 | South Korea | Randomized clinical trial | Weight <1500g+ severe NRDS (radiographic evidence)+ Invasive Mechanical Ventilation+ FiO2 > 0.5+ absence ofSevere or lethal congenital anomalies | Endotracheal administration with orogastric tube | n = 16Calfactant 105 mg/kg + budesonide 0.25 mg/kg | n = 18Calfactant105 mg/kg | No statistically significant differences between the control and intervention groups.Similar adverse effects in both groups |
| Moschino et al. 202117 | Italy | Retrospective cohort | Preterm newborns < 28 wk and weight <1500g+ FIO2 > 30%+ exclusion of preterm newborns < 23 weeks and < 400g | - | n = 18Curosurf 200 mg/kg + budesonida 0.25 mg/kg | n = 18Curosurf 200 mg/kg | Lower rate of hypotension and less hospital stay in the intervention group.No statistical differences in BPD or mortality between groups. |
| Kothe et al. 202016 | USA | Retrospective cohort | Weight ≤ 1250g+ CPAP 5 FiO2 21%orintubation in delivery room+ exclusion of preterm newborns < 23 weeks and < 500g | - | n = 173Budesonide 0.25 mg/kg + Beractant 100 mg/kg | n = 294Beractant 100 mg/kg | Fewer patients in the intervention group on invasive mechanical ventilation on days 3, 7 and 28.Lower BPD severity in the intervention group.Lower rate of PCA with repercussions in the budesonide group.No statistical differences in BPD or mortality between groups. |
For all the studies, the main objective was to analyze the effect of endotracheal administration of budesonide and the incidence of BDP or death.
Randomized clinical trialsIn all randomized clinical trials, the population included was VLBW and premature newborns. In both studies conducted by Yeh et al.12,13 the mean gestational age was less than 28 weeks, and the mean birth weight was less than 1000 g. In the studies conducted by Gharehbaghi et al.14 and by Heo et al.15 the population had a mean gestational age greater than 28 weeks and a mean birth weight greater than 1000 g.
Yeh et al.12,13 found a decrease in BPD or death in both studies, but an isolated reduction in BPD was observed only in the study performed in 2016 (RR 0.7 CI95% 0.58 – 0.86, p < 0.001).12 Gharehbaghi et al.14 found a lower rate of BPD (58.9% vs 37.5%, p = 0.004) and a shorter duration of invasive mechanical ventilation in the intervention group (2.8±0.6 vs. 0.8±0.1 days, p = 0.006).14 In the study by Heo et al.,15 there were no statistically significant differences between the control group and the intervention group.
There was no statistically significant difference between the secondary outcomes in the aforementioned studies.
Observational studiesThe results of the observational studies are also summarized in Table 1.
In the study by Kothe et al.16 there was no statistical difference in the primary outcome of BPD or death. (Odds ratio (OR) 0.94; CI 95%, 0.62–1.42; p = 0.75). Regarding the secondary outcomes, Kothe et al. observed that fewer patients remained on invasive mechanical ventilation on the third day of life (DOL) (43% vs. 58%; OR 0.55; 0.37–0.81; p < 0.01), on the DOL 7 (RC 0.56; 0.38–0.82; p < 0.01) and by 28 DOL (3% vs. 10% OR 0.52; 0.31–0.86; p = 0.015).16 There were no statistically significant differences in the remaining secondary outcomes.
In the study by Moschino et al.17 no differences were found for the primary and secondary outcomes.
DiscussionThe use of corticosteroids in the postnatal period continues to be of great interest to researchers due to their potential effect on decreasing BPD rates and improving the quality of life of newborns.5,6
Regarding the PI(E)CO question, all the studies analyzed included preterm and VLBW newborns and, in all of them, there was a comparison between the use of budesonide with surfactant versus surfactant alone. Those studies aimed to identify the potential anti-inflammatory effect of budesonide rather than an effect on gas exchange, as both groups (control and intervention) received surfactant therapy.
After full reading and analysis, the authors found a decrease in the rate of BPD or death in only 3 of the 6 studies.12–14 However, these studies were clinically randomized and controlled trials, with a low risk of bias, which gives them greater clinical and statistical power.
Nevertheless, several factors can impact the external validation in both studies conducted by Yeh et al.12,13 First, the pulmonary surfactant used was the Survanta® (beractant) instead of poractant-alfa. A systematic review with meta-analysis of different types of animal-derived surfactants found that a bovine-derived surfactant may be associated with worse outcomes (BPD or death and length of mechanical ventilation) compared to the porcine-derived surfactant (poractant alfa – Curosurf®).18 Nonetheless, the authors concluded that the effect could not be fully attributed to the type of surfactant, since the studies with poractant alfa had higher doses of surfactant (200 mg/kg) compared with those with beractant (100 mg/kg).18
Still, regarding the dose of surfactant, a recent retrospective study conducted in Italy19 showed that a higher initial dose of surfactant (200 mg/kg) had an advantage over the lower dose (100 mg/kg), with a lower incidence of BPD or duration of mechanical ventilation.19
Moreover, the European Consensus Guidelines on the Management of Respiratory Distress Syndrome (2019), recommends the use of poractant alfa at an initial dose of 200 mg/kg for the treatment of NRDS (level A1).20 The Canadian Pediatric Society follows a very similar recommendation.21
Additionally, the surfactant administration in both studies by Yeh et al.12,13 was through orotracheal intubation followed by mechanical ventilation, without specifying the time of extubation. There is currently robust scientific evidence to recommend the use of the INSURE method (intubation, surfactant administration, extubating within 1 h) or the administration of surfactant by thin catheter (LISA, MIST) as the preferred methods for administering surfactant.20,21 However, these data do not nullify the findings of these studies, they only make it difficult to extrapolate the data to the population outside the clinical research.
Thus, the method of administration (INSURE) and the type of surfactant (poractant alfa) used by Gharehbaghi et al.14 give a better possibility of external validation compared to the studies conducted by Yeh et al.12,13
However, the homogeneous population included in the study by Gharehbaghi et al.,14 in which all the newborns were Iranian, also hampers its external validation.
The study by Heo et al.15 has a high risk of bias, mainly due to the nonblinding and nonrandomization of the process. Furthermore, it does not present a sample size calculation or post-HOC analysis of the data, thus undermining the power of the study.
In a recent systematic review with meta-analysis, conducted by Yi et al.,22 a pooled reduction of 50% in the incidence of BPD was found (OR = 0.52, CI 95%: 0.39–0.68, p < 0.00001) when the budesonide was administered in association with pulmonary surfactant. However, this meta-analysis included 10 studies, 9 of which were performed in China, which makes it difficult to extrapolate data outside this population. Another relevant fact is the heterogeneity regarding the dose of surfactant used in the included studies.
Regarding observational studies, despite having lower statistical and scientific power when compared to randomized clinical studies, both were carried out with diverse populations, which could bring strength in the sense of external validation of the results.
However, the study by Moschino et al.17 has a major limitation regarding the number of patients included. The pairing process between the control and intervention groups to reduce potential confounders drastically reduced the number of patients included, reducing the statistical power of the study.
In contrast, the study by Kothe et al.,16 which included many newborns in the United States, whose population is heterogeneous, has greater power. Despite that, they did not discuss the method of surfactant administration. Additionally, the surfactant used was the beractant, at a dose of 100 mg/kg.16
Among the limitations of the present study, the authors can mention the exclusion of articles in Chinese. Those articles underwent the reading of their abstracts; however, the authors could not extract relevant data from those readings. Another limitation was the choice to search in only two electronic databases (PubMed® and EMBASE®). Nonetheless, those are the biggest health databases, and the authors probably haven´t missed any relevant articles. Moreover, the choice to not perform a meta-analysis can also be considered a limitation of this study. However, there is in the current literature a recent meta-analysis,22 and, therefore, performing a new meta-analysis would not add relevant information to the scientific community.
ConclusionThe administration of budesonide associated with surfactant for treating NRDS appears to be associated with a lower rate of BDP incidence, lower duration of mechanical ventilation, and mortality. However, these studies have limitations that prevent the widespread use of this combination in clinical practice. Among them, the authors can cite the predominantly Asian population, only a few studies using the most recommended surfactant in the literature, the Poractant-alfa (Curosurf®), the non-standardization of the dose of the surfactant (100 mg/kg versus 200 mg/kg) and heterogeneous surfactant administration method between the main studies found. A prospective, randomized, double-blind, and controlled study in a heterogeneous population, using surfactant of porcine origin (poractant-alfa) combined with budesonide, at a dose of 200 mg/kg, through administration by INSURE or minimally invasive (LISA, MIST), is urgently necessary for its recommendation based on scientific evidence.