to review the evolution of acute respiratory distress syndrome (ARDS) definitions and present the current definition for the syndrome.
Data sourcea literature review and selection of the most relevant articles on ARDS definitions was performed using the MEDLINE®/PubMed® Resource Guide database (last ten years), in addition to including the most important articles (classic articles) that described the disease evolution.
Data synthesisthe review included the following subjects: introduction; importance of definition; description of the first diagnostic criterion and subsequently used definitions, such as acute lung injury score; definition by the American-European Consensus Conference, and its limitations; description of the definition by Delphi, and its problems; accuracy of the aforementioned definitions; description of most recent definition (the Berlin definition), and its limitations; and practical importance of the new definition.
ConclusionsARDS is a serious disease that remains an ongoing diagnostic and therapeutic challenge. The evolution of definitions used to describe the disease shows that studies are needed to validate the current definition, especially in pediatrics, where the data are very scarce.
revisar a evolução das definições de síndrome do desconforto respiratório agudo e apresentar a proposta atual para a mesma.
Fontes dos dadosrevisão bibliográfica e seleção de publicações mais relevantes sobre as definições de síndrome do desconforto respiratório agudo, utilizando a base de dados MEDLINE®/PubMed® Resources Guide (últimos dez anos), além da inclusão dos artigos mais importantes (artigos clássicos) que descrevem a evolução da doença.
Síntese dos dadosrevisão incluiu os seguintes tópicos: introdução; importância da definição; descrição do primeiro critério diagnóstico e das definições utilizadas subsequentemente, como o escore de lesão pulmonar aguda, definição da Conferência de Consenso Americana- Europeia e suas limitações, descrição da definição de Delphi e seus problemas; acurácia das definições citadas e descrição da definição mais recente (Definição de Berlim) e suas limitações; e importância prática da nova definição.
Conclusõesa síndrome do desconforto respiratório agudo é uma doença grave, que consiste em um contínuo desafio diagnóstico e terapêutico. A evolução das definições utilizadas para descrever a doença evidencia que estudos são necessários para validar a definição atual, principalmente em pediatria, onde os dados são muito escassos.
Acute respiratory distress syndrome (ARDS) is clinically a diagnostic and therapeutic challenge, especially for pediatric intensivists, as there are few studies performed in children and there are reasons to believe the disease is different in adults and children. It is known that ARDS in response to a viral infection is much more common in children than in adults,1 and, histopathologically, there are three distinct patterns of lung injury: bronchiolitis, acute interstitial pneumonia, and classic diffuse alveolar damage,2 which may have different clinical outcomes. However, the diagnostic criteria established in consensuses that addressed the definitions of ARDS in adults have been used in pediatrics.
Initially described as “acute respiratory disorder in adults”,3 the disease subsequently became known as ARDS because it affected adults and children alike.4 It is noteworthy that the first publication described 12 patients, one of whom was 11 years old.
It is still controversial whether the data obtained from studies in adults can be fully used in studies performed in children. Certainly, the transfer of knowledge depends on each patient, etiology of pulmonary disease, presence of comorbidities, and age and weight of patients.5
ARDS is a form of acute respiratory failure that may be caused by different pulmonary and extrapulmonary conditions. Classically, there is the involvement of gas exchange units due to an inflammatory process, with the development of non-cardiogenic pulmonary edema. Consequently, patients have varying degrees of hypoxemia refractory to oxygen administration.
This review presents the evolution of definitions of ARDS and their future implications for clinical and experimental research.
Importance of a precise definition of ARDSA definition is a “clear determination of the limits of anything, especially a disease process”.6 In general, definitions circumscribe a condition, providing limits between what is and what is not its essential nature.7 It so happens that sometimes diseases, and especially syndromes, have imprecise limits, making the diagnosis difficult, as occurs with ARDS.
Considering that, despite recent advances in the monitoring and treatment of critically-ill patients with ARDS, mortality remains high for the syndrome,5 especially in more severe cases, it is important to continually improve diagnostic criteria in order to reach a definition that has greater applicability in the clinical setting. Additionally, the review of the disease definition is important for research, for clinicians in daily practice, and for administrators.
As for research, it is useful to obtain new information about the pathogenesis of ARDS, seeking connection between basic science and the clinical setting, as therapeutic modalities can be constantly tested in the basic research environment and sometimes transported into daily practice. Moreover, a more precise definition would allow the comparison of findings from several clinical studies with higher degree of certainty.8
Regarding the actual clinical practice, a precise definition would allow for earlier institution of established and tested therapeutic methods, such as the use of lung-protective ventilation with limited tidal volume and pressure plateau.9,10 Additionally, the assessment of individual prognosis would be improved, facilitating the relationship with patients’ relatives and enhancing information given to them.
In the field of hospital administration, it is understood that epidemiological studies on ARDS are crucial to provide data on its incidence and frequency, which are useful elements for administrators to allocate the limited resources of the health system for the treatment of these patients. Obviously, these studies are based on a specific definition of the disease,11 and many researchers have raised the possibility that the reported differences regarding the mortality rate of ARDS are primarily due to the variability in its definition.12
The definitionsFrom Ashbaugh to Murray Score (Acute Lung Injury)Ever since the description of the disease was published by Ashbaugh et al.3 in 1967, several non-standard definitions were used in clinical studies. In 1976, Bone et al.13, when describing the association between DIC and ARDS, used as diagnostic criteria of the syndrome the presence of arterial partial pressure of oxygen (PaO2) ≤ 70mmHg with a fraction of inspired oxygen (FiO2) ≥ 0.5 and of positive-end expiratory pressure (PEEP), whose value was not specified.
In 1982, Pepe et al.,14 when studying clinical predictors of the syndrome in 136 adult patients, defined the disease when PaO2<75mmHg with FiO2 ≥ 0.5, in the presence of diffuse bilateral infiltrates on the chest X-ray, with the involvement of all lung fields, pulmonary capillary pressure (PCP) < 18mmHg not due to heart failure, pleural effusion, or bacterial pneumonia. In the following year, Fowler et al.15, in a study with several centers investigating 68 patients with ARDS, used the following as diagnostic criteria: acute onset of bilateral pulmonary infiltrates; PCP ≤ 12mmHg; lung compliance ≤ 50mL/cmH2O; and PaO2 and alveolar oxygen pressure ratio (PaO2/PAO2) ≤ 0.2.
Given the diversity of criteria used for the diagnosis of ARDS and seeking to expand the definition of the syndrome, in 1988 Murray et al.16 incorporated risk factors to the definition, as well as the relative brevity of the disease process and severity measures. Regarding risk factors, the authors pointed to the need for identifying whether the syndrome was caused by aspiration pneumonia, medications, or inhalation of toxic gases, or if it was associated with systemic events such as sepsis, multiple trauma, or acute pancreatitis.
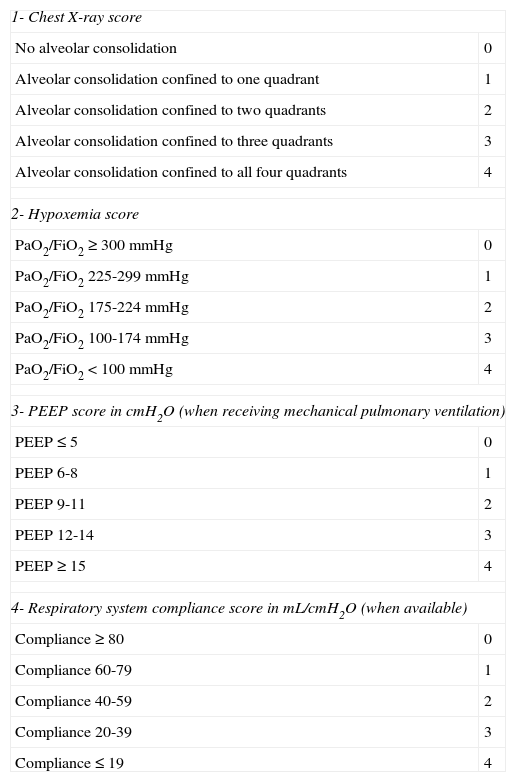
Severity was assessed using the lung injury score (LIS), incorporating physiological data indicators of oxygenation, PEEP values, and compliance and distribution of radiological lesions (Table 1). The score included the evaluation of four criteria: 1) chest X-ray, 2) hypoxemia score (PaO2/FiO2); 3) PEEP level; and 4) respiratory system compliance score (Crs) (when available).
Acute lung injury score - Murray score.
| 1- Chest X-ray score | |
| No alveolar consolidation | 0 |
| Alveolar consolidation confined to one quadrant | 1 |
| Alveolar consolidation confined to two quadrants | 2 |
| Alveolar consolidation confined to three quadrants | 3 |
| Alveolar consolidation confined to all four quadrants | 4 |
| 2- Hypoxemia score | |
| PaO2/FiO2 ≥ 300mmHg | 0 |
| PaO2/FiO2 225-299mmHg | 1 |
| PaO2/FiO2 175-224mmHg | 2 |
| PaO2/FiO2 100-174mmHg | 3 |
| PaO2/FiO2 < 100mmHg | 4 |
| 3- PEEP score in cmH2O (when receiving mechanical pulmonary ventilation) | |
| PEEP ≤ 5 | 0 |
| PEEP 6-8 | 1 |
| PEEP 9-11 | 2 |
| PEEP 12-14 | 3 |
| PEEP ≥ 15 | 4 |
| 4- Respiratory system compliance score in mL/cmH2O (when available) | |
| Compliance ≥ 80 | 0 |
| Compliance 60-79 | 1 |
| Compliance 40-59 | 2 |
| Compliance 20-39 | 3 |
| Compliance ≤ 19 | 4 |
The final score is attained by dividing the values obtained from the initial analysis by the number of elements used for the analysis. When the score value is zero, there is no lung injury; from 1 to 2.5, lung injury is considered to be mild to moderate; and when greater than 2.5, the diagnosis of ARDS is established.
Although the Murray score is still used, it has not been validated, that is, it has not been established whether patients with scores of the same value correspond to similar levels of lung injury, and thus, have the same prognosis. Additionally, the LIS has several problems, such as: it does not consider the effect of time on injury severity (acute or chronic event); the score is not specific for ARDS, patients with cardiogenic pulmonary edema are identified as having ARDS; and patients with mild volume overload, for any reason, can be diagnosed as having ARDS, as it is not mandatory to obtain the PCP levels to rule out cardiogenic edema.8,17,18
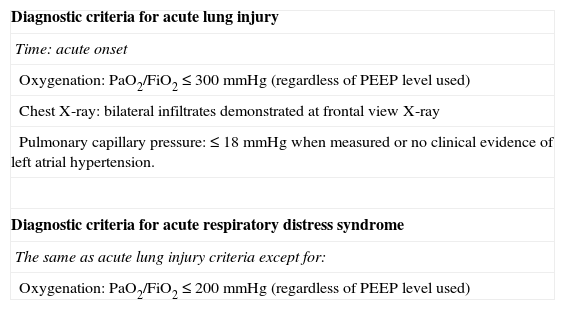
The American-European Consensus Conference (AECC) DefinitionIn 1994, experts from the United States and Europe met to improve the definition of ARDS, aiming to improve the standardization of research, the criteria for more accurately determining the severity, and disease prognosis.19 Formally, two different conditions were defined, acute lung injury (ALI) and ARDS itself (Table 2), as diseases in which there is a sudden and acute onset of respiratory distress, bilateral infiltrates on the chest X-ray in the frontal view, absence of left atrial hypertension (PCP ≤ 18mmHg when measured or no clinical evidence of left ventricular failure), and severe hypoxemia, evaluated by PaO2/FiO2 ratio. More specifically, ALI is diagnosed when PaO2/FiO2 ratio is less than or equal to 300, while ARDS is diagnosed when PaO2/FiO2 it is less than or equal to 200, regardless of the level of PEEP and FiO2 used.
AECC definition of acute lung injury and acute respiratory distress syndrome.
| Diagnostic criteria for acute lung injury |
| Time: acute onset |
| Oxygenation: PaO2/FiO2 ≤ 300mmHg (regardless of PEEP level used) |
| Chest X-ray: bilateral infiltrates demonstrated at frontal view X-ray |
| Pulmonary capillary pressure: ≤ 18mmHg when measured or no clinical evidence of left atrial hypertension. |
| Diagnostic criteria for acute respiratory distress syndrome |
| The same as acute lung injury criteria except for: |
| Oxygenation: PaO2/FiO2 ≤ 200mmHg (regardless of PEEP level used) |
AECC, American-European Consensus Conference; PEEP, ositive end-expiratory pressure; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen.
Although the AECC definition has had the merit of formalizing the diagnostic criteria for ARDS and is simple to use in daily practice, it has been questioned over the years in light of the increased knowledge on the disease.20,21
The limitations can be grouped regarding a few factors:
- 1)
Heterogeneity: The AECC definition transforms multiple physiopathological processes and groups of very different patients into a single syndrome.22 The triggering mechanism of lung injury,23–25 the phase of the disease26, and the time of onset of pulmonary mechanical ventilation (PMV) greatly contribute to the question of heterogeneity.27,28 The practical implications of these problems are obvious, as a therapy administered to a group of patients with positive results may not have the same effect in another.
- 2)
Time of disease: The AECC definition excluded chronic respiratory failure and gave no explicit definition of “acute”, resulting in subjectivity.11
- 3)
Hypoxemia assessment: The AECC definition used the PaO2/FiO2 ratio to evaluate hypoxemia. However, there is inconsistency in the use of this ratio due to the effect of PEEP and FiO2.11,21,29–31 Although the ratio values to diagnose ALI (≤ 300) and ARDS (≤ 200) are explicit, it is necessary to consider that the index values vary considerably according to the FiO2 used, particularly with FiO2 < 0.5 and PaO2 > 100mmHg, or when the fraction of intrapulmonary shunt is low.30 Moreover, many patients who initially meet the criterion of AECC may show an increase in the PaO2/FiO2 ratio to > 200 after a short period of PEEP or an increase in FiO2.
Additionally, hypoxemia may be related to the presence of atelectasis, low cardiac output, and shunting of blood through the foramen ovale.32,33 There are no studies on the capacity of the PaO2/FiO2 ratio to predict structural changes in the alveolar-capillary membrane, as ARDS represents the common pathway of several events and disease.18 Several other factors interfere with PaO2/FiO2, whether or not there is pulmonary disease. For instance, in a given pulmonary condition, alterations in the cardiac output or in the arteriovenous difference of oxygen content result in an important modification of the ratio.34
The PaO2/FiO2 ratio is also influenced by the level of inspiratory pressure used during PMV. For this reason, many pediatricians introduced the use of mean airway pressure (Paw) and oxygenation index (OI),35 an index that takes Paw into account for its calculation. OI is obtained by the formula OI=Paw×FiO2×100/PaO2. This index has been associated with prognosis in adults and children by analyzing oxygenation. Compared with PaO2/FiO2, it has been demonstrated that when the ratio remains low, mortality increases in adults and children.36,37 Similarly to the definition of AECC, OI does not take into account the PEEP used, nor compliance and radiological data, and does not exclude heart disease.
- 4)
ALI category: In the AECC definition, all patients with PaO2/FiO2 < 300 are diagnosed as having ALI. There are problems in the classification of patients with a ratio of 201 to 300, as to whether they would be classified as ALI or ARDS.
- 5)
Chest radiological assessment: Although the AECC definition indicates as criteria the presence of bilateral infiltrates on the chest radiography in the frontal view, there is poor interobserver reliability in the interpretation of this examination, especially in younger children, and there is an agreement between intensivists and radiologists at random in only half of the cases.38,39 Additionally, pulmonary opacities can be changed by applying a higher Paw.
- 6)
Pulmonary capillary pressure: To differentiate inflammatory pulmonary edema from cardiogenic pulmonary edema, the definition of AECC requires PCP ≤ 18mmHg or absence of clinical evidence of left atrial hypertension. It must be considered, however, that increases in PCP and ARDS can coexist,40,41 and up to 50% of patients with ARDS have PCP > 18mmHg due to increases in intrathoracic pressure or volume overload;41,42 also, there is poor interobserver reliability in the measurement of PCP and the clinical evaluation of left atrial hypertension.43
- 7)
Risk factors: they were not formally included in the AECC definition.
As there was no agreement between the definitions developed to date, Ferguson et al.,44,45 in 2005, developed another clinical definition of ARDS, using the Delphi technique. This definition incorporated additional variables, such as the level of PEEP (PaO2/FiO2 ≤ 200 with PEEP ≥ 10cm H2O); a precise definition of acute onset (within 72hours); a subjective assessment of cardiac involvement (without clinical evidence of congestive heart failure); an objective assessment of cardiac involvement (PCP ≤ 18mmHg or ejection fraction ≥ 40%); assessment of pulmonary compliance (static compliance < 50cm H2O, with tidal volume of 8mL/kg); and quantification of radiological criteria for the disease in two or more quadrants.
Although it apparently solved the problems of the previous definitions, the same researchers reported that, although the Delphi definition is more specific than the AECC criterion, it was less sensitive when autopsy findings of diffuse alveolar damage were chosen as the gold standard for the diagnosis of ARDS.46
Accuracy of existing definitionsEsteban et al.,47 in 2004, performed a retrospective study to compare autopsy findings and clinical features of adults with a clinical diagnosis of ARDS, and found that the accuracy of AECC was only moderate (75% sensitivity and 84% specificity), working better in patients with extrapulmonary risk factors. The concordance between the AECC and Murray score was also studied, and was shown to be moderate.48
Moreover, three studies showed varying degrees of concordance between AECC and the ALI score.46,48,49
The Berlin DefinitionIn a study initiated in 2010, several members of the European Society of Intensive Care Medicine selected other professionals from Europe and the United States with the objective of reviewing the definition of ARDS.50 The discussion panel emphasized the applicability, reliability, validity (how physicians recognize the disease), and predictive capacity (capacity to predict response to treatment, prognosis, or both) of a new definition. It was also determined that any reviews of the definition should be compatible with the definition of AECC to facilitate the interpretation of previous studies. A pre-definition was established51 and empirically evaluated using meta-analysis of data from 4,188 patients with ARDS from four different centers and physiological data of 269 patients with ARDS from three single centers.
The pre-definition proposed three mutually exclusive categories of ARDS based on the degree of hypoxemia: mild ARDS (200mmHg < PaO2/FiO2 ≤ 300mmHg), moderate ARDS (100mmHg < PaO2/FiO2 ≤ 200mmHg), and severe ARDS (PaO2/FiO2 ≤ 100mmHg). Four auxiliary variables were proposed for the severe ARDS category: radiological severity; Csr (≤ 40mL/cmH2O); PEEP (≥ 10cm H2O); and corrected expired volume per minute (≥ 10 L/min). In the initial test of the proposal, the four auxiliary variables did not contribute to the validation of the predictive capacity of severe ARDS regarding mortality and were then removed from the final criteria of the definition. Thus, the final definition was once again submitted to discussion and refined, until its publication.
The literature was reviewed to identify studies that met the following criteria: 1) large prospective studies involving multiple centers, including consecutive patients or randomized studies, or prospective studies from a single center with radiological or physiological data of adult patients with ALI/ARDS according to the criteria of the AECC; 2) studies to collect data needed to apply both the draft of the Berlin definition, as well as the definition of AECC; and 3) the authors of these studies were invited to participate and share data.
The following variables were used in the analysis: hospital mortality or mortality at 90 days; number of days free of mechanical ventilation at 28 days after the ALI diagnosis; duration of mechanical ventilation in survivors, used as an indirect marker of the lung injury severity; and progression of ARDS severity at seven days, assessed using longitudinal data from patients. Radiologically, patients with more extensive involvement (three to four quadrants) were differentiated from those with mild lesions (two quadrants); static Crs complacency was calculated as tidal volume (in mL) divided by plateau pressure (cmH2O) subtracted from PEEP (cmH2O); the corrected expired volume per minute was calculated by the product of minute ventilation for PaCO2 divided by 40mmHg;52 the total weight of the lung was calculated based on computed tomographic images;53 and the intrapulmonary shunt fraction was calculated as previously reported54
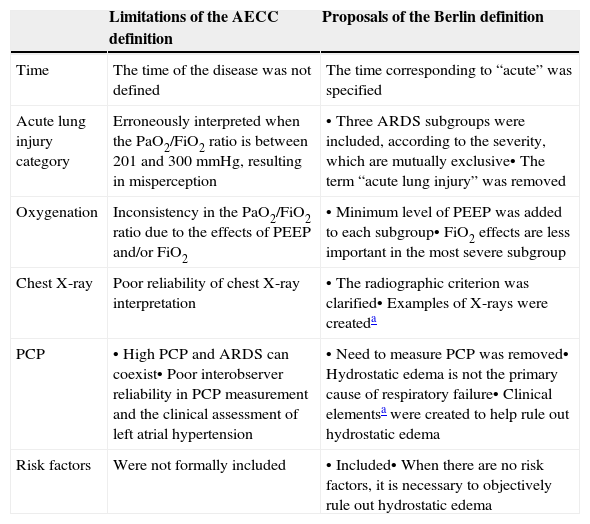
Proposals of the Berlin DefinitionTable 3 shows the main limitations of AECC and the use of the corresponding measure adopted by the Berlin consensus to overcome the difficulties. The Berlin definition is shown in Table 4.
Limitations of the AECC ARDS definition and proposals of the Berlin definition to overcome these limitations.
| Limitations of the AECC definition | Proposals of the Berlin definition | |
|---|---|---|
| Time | The time of the disease was not defined | The time corresponding to “acute” was specified |
| Acute lung injury category | Erroneously interpreted when the PaO2/FiO2 ratio is between 201 and 300mmHg, resulting in misperception | • Three ARDS subgroups were included, according to the severity, which are mutually exclusive• The term “acute lung injury” was removed |
| Oxygenation | Inconsistency in the PaO2/FiO2 ratio due to the effects of PEEP and/or FiO2 | • Minimum level of PEEP was added to each subgroup• FiO2 effects are less important in the most severe subgroup |
| Chest X-ray | Poor reliability of chest X-ray interpretation | • The radiographic criterion was clarified• Examples of X-rays were createda |
| PCP | • High PCP and ARDS can coexist• Poor interobserver reliability in PCP measurement and the clinical assessment of left atrial hypertension | • Need to measure PCP was removed• Hydrostatic edema is not the primary cause of respiratory failure• Clinical elementsa were created to help rule out hydrostatic edema |
| Risk factors | Were not formally included | • Included• When there are no risk factors, it is necessary to objectively rule out hydrostatic edema |
AECC, American-European Consensus Conference; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen; PCP, pulmonary capillary pressure; PEEP, Positive end-expiratory pressure.
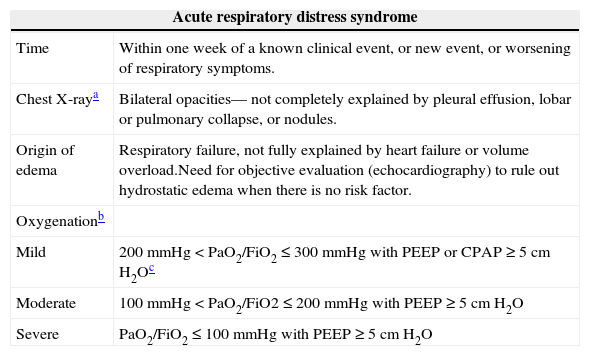
Berlin definition for acute respiratory distress syndrome.
| Acute respiratory distress syndrome | |
|---|---|
| Time | Within one week of a known clinical event, or new event, or worsening of respiratory symptoms. |
| Chest X-raya | Bilateral opacities— not completely explained by pleural effusion, lobar or pulmonary collapse, or nodules. |
| Origin of edema | Respiratory failure, not fully explained by heart failure or volume overload.Need for objective evaluation (echocardiography) to rule out hydrostatic edema when there is no risk factor. |
| Oxygenationb | |
| Mild | 200mmHg < PaO2/FiO2 ≤ 300mmHg with PEEP or CPAP ≥ 5cm H2Oc |
| Moderate | 100mmHg < PaO2/FiO2 ≤ 200mmHg with PEEP ≥ 5cm H2O |
| Severe | PaO2/FiO2 ≤ 100mmHg with PEEP ≥ 5cm H2O |
CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen; PEEP, Positive end-expiratory pressure.
The criteria for mild ARDS were met by 22% of patients (95% CI, 21%-24%), and these results were comparable with the diagnosis of ALI (not ARDS) of the AECC definition. 50% of patients (95% CI, 48%-51%) met the criteria for moderate ARDS and 28% (95% CI, 27%-30%) for severe ARDS. The mild, moderate, and severe stages of ARDS were significantly associated with increased mortality (27%, 95% CI, 24%-30%; 32%, 95% CI, 29%-34%; and 45%, 95% CI, 42%-48%, respectively, p<0.001). Compared to the AECC definition, the Berlin definition presented better predictive validity for mortality, with an area under the ROC curve (AUROC) of 0.577 (95% CI, 0.561-0.593) versus 0.536 (95% CI, 0.520-0.553, p<0.001). Moreover, the median number of days free of mechanical ventilation decreased significantly when comparing mild, moderate, and severe ARDS.50
Limitations of the Berlin DefinitionObviously, studies aimed to establish diagnostic and definition criteria for a given disease are not free of difficulties that are inherent to the biological area.
Thus, the definition shown here has limitations explained by the authors,50 namely:
- 1)
The capacity of the Berlin definition was statistically superior when compared to the definition of AECC; however, the difference was small and would not have clinical significance if the Berlin definition had been designed only as a clinical prediction tool, which did not occur.
- 2)
The results cannot be generalized. However, the authors used data from a large population of patients, including clinical trials, academic centers, and community patients. Children were not included.
- 3)
Data loss: data regarding Crs and PEEP was not obtained from some patients. However, this does not appear to be important, given the strength of the data for the sensitivity of analyses that excluded individual groups.
- 4)
Difficulties in the analysis of auxiliary variables: the auxiliary variables did not identify subgroups at higher risk, as the number of quadrants in the chest X-ray cannot be reliably measured, PEEP was not used as a predictor, and Csr and the corrected expired volume per minute were not accurately measured. In the Berlin definition test, both PEEP and Csr were evaluated as they are used in practice and not as pre-specified elements.
- 5)
The study did not aim to develop a prognostic model of ARDS. Also, the crossing-over of ARDS categories, considering the definitions of AECC and Berlin, made comparison difficult. It is possible that the prognosis and the relative proportion of patients within each ARDS category would be different if the epidemiology of the syndrome evolves due to changes in clinical practice or risk factors.
The importance of announcing a new definition of ARDS for pediatric intensivists/emergency physicians/neonatologists is justified in itself. That is, from the experience of applying other definitions, and, consequently of their improvement, the new definition discloses another method to diagnose, stratify the severity, apply therapeutic strategies, and more accurately establish the prognosis of a disease as serious as ARDS. As the Berlin consensus was initially presented at a congress that did not necessarily include pediatricians, the need for a wider dissemination of this new definition to the pediatric intensive care area is obvious.
The definition of the Berlin consensus has important differences when compared to the definitions published to date, a characteristic that promptly results in practical aspects, including:
- 1)
The condition previously called “acute lung injury”, considered a minor and not clinically significant condition, was excluded and reclassified as mild ARDS. The study that applied the Berlin consensus50 showed an alarming fact: almost a quarter of patients, considering the previous definition, were diagnosed as ALI and not as mild ARDS (PaO2/FiO2 between 200 and 300mmHg). It is noteworthy that the mortality in patients with mild ARDS was 27%. Certainly, patients at this stage of disease severity should be treated promptly, following established protocols of noninvasive mechanical ventilation and lung-protective invasive mechanical ventilation with PEEP, and deserve attention from the health teams regarding monitoring and stricter therapeutic clinical intervention. It is also noteworthy that patients with PaO2/FiO2 between 200 and 300mmHg had no established diagnosis of ARDS or ALI;
- 2)
More adequate use of available therapeutic interventions. Thus, noninvasive mechanical ventilation using positive pressure is indicated in mild cases of ARDS, as well as the use of low to moderate PEEP (6 to 9 cmH2O) and mechanical ventilation with low tidal volumes (5-7mL/kg). In cases of moderate ARDS, in addition to the principles of lung-protective ventilation, it may be necessary to use high PEEP (≥ 10cm H2O). In severe ARDS, other therapies, some of which adjunctive, can be introduced (high-frequency oscillatory ventilation, inhaled nitric oxide, prone position, neuromuscular blockers, and extracorporeal membrane oxygenation).
- 3)
Better prognostic evaluation of the child, so that family members and guardians can receive information on the child's condition that is closer to reality, regarding the perspective of clinical evolution;
- 4)
Another important aspect for researchers is that future studies should necessarily group the patients according to the new classification of the syndrome, in order to obtain comparable data. Pediatricians working in clinical research or basic research need to validate the new data in children as soon as possible.
ARDS is a serious disease that constitutes an ongoing diagnostic and therapeutic challenge. The evolution of definitions used to describe the disease clearly shows that studies are needed to validate the current definitions, especially in pediatrics, where data are much scarcer.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank all Brazilian pediatric intensivists that have been strenuously devoted to the study and care of critically-ill children, especially those affected by acute respiratory failure.
Please cite this article as: Fioretto JR, de Carvalho WB. Temporal evolution of acute respiratory distress syndrome definitions. J Pediatr (Rio J). 2013;89:523–530.