To identify and describe specific instruments to assess health-related quality of life (HRQoL) in children and adolescents with asthma.
Data sourceSearches were performed in the PubMed, Ovid, and LILACS databases using different combinations of key words (MeSH terms), selecting original articles on the development of specific HRQoL questionnaires, published in English, Portuguese, or Spanish, between 1990 and 2012.
Data synthesisA total of 15 instruments that met the inclusion criteria were identified. Most studies assessed reliability through internal consistency, reproducibility, and/or sensitivity to changes. Validity was assessed by comparison with healthy controls (discordant validity) or factorial analysis.
ConclusionsOf the 15 instruments, three are the most frequently used: Pediatric Asthma Quality of Life Questionnaire (PAQLQ), Pediatric Quality of Life Inventory 4.0 (PedsQL-Asthma), and Disability Kids (DISABKIDS). In general, these three tools have adequate psychometric characteristics and are practical to implement, but only PAQLQ has been culturally adapted to Brazil.
Identificar e descrever os instrumentos específicos que avaliam a QVRS de crianças e adolescentes com asma.
Fontes dos dadosRealizamos buscas nas bases de dados PubMed, Ovid e LILACS utilizando várias combinações de descritores (MeSH terms), selecionando artigos originais sobre desenvolvimento de questionários específicos de QVRS, publicados em inglês, português ou espanhol, entre 1990 e 2012.
Síntese dos dadosForam identificados 15 instrumentos que preencheram os critérios de inclusão. A maioria dos estudos avaliou confiabilidade mediante consistência interna e/ou reprodutibilidade e/ou sensibilidade às mudanças. A validade foi avaliada mediante a comparação com hígidos (validade discordante) ou análise fatorial.
ConclusõesDos 15 instrumentos, três são os mais utilizados, o PAQLQ, o PedsQL-Asthma e DISABKIDS. Em geral, estes três instrumentos possuem características psicométricas adequadas e são práticos de aplicar, mas apenas o PAQLQ completou a adaptação cultural para o Brasil.
Quality of life is one of the most important outcomes in the evaluation of patients with chronic diseases.1 Quality of life is defined by the WHO as “an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns”.2 In this context, health-related quality of life (HRQoL) can be considered as “(…)the impact that illness and/or their treatment may have on the lifestyle, psychological balance, and degree of well-being of patients, such as they are perceived, considered, and valued”.3 Thus, quality of life measures can provide information on how chronic diseases interfere in the social, emotional, and physical domains of the patient from his/her own perspective.. HRQoL evaluation is performed by using a questionnaire that can be generic or specific. Generic questionnaires assess HRQoL of an individual in general, while specific questionnaires assess the impact that certain diseases have on the subject's HRQoL.4
Asthma in children is a chronic disease with high prevalence and morbidity, resulting in significant personal, familial, and social consequences.5 In Brazil, childhood asthma shows prevalence with regional variations between 4.8% to 21.9%.6 The routine use of instruments to assess HRQoL is not yet widely disseminated in clinical practice. First, it is important to choose a instrument to assess HRQoL that fits the needs, either generic or specific. Furthermore, the instrument must be valid and reliable, and ideally, it must allow for the comparison of results with those performed in similar populations. It should also have an appropriately adapted (validated) version to the local cultural context, when it was created in a different language.7
The present article aimed to identify the specific instruments available to assess HRQoL in children and adolescents with asthma, analyzing its psychometric characteristics. It also aimed to identify, among these questionnaires, which had been linguistically and culturally adapted to Brazilian Portuguese.
MethodsResearch strategySearches were conducted in the PubMed, Ovid, and LILACS databases aiming to identify specific questionnaires that assess HRQoL in children and adolescents diagnosed with asthma. Filters for the age range 0-18 years and for articles published from 1990 to 2012 were included. The terms used in the search strategy and selection were: “Asthma” AND (“quality-of-life” OR “quality of life” OR “QoL” OR “health-related-quality-of-life” OR “health related quality of life” OR “HRQOL”) AND (“infant”[MeSH Terms] OR “child”[MeSH Terms] OR “adolescent” [MeSH Terms])).
All abstracts of the articles retrieved were read and, afterwards, all those that met the inclusion criteria were assessed in full. Directed manual searches from the list of tools in book chapters, congress abstracts, and sites of institutions linked to the validation of tools, such as the MAPI Research Institute or the website of the American Thoracic Society (ATS) (http://www.thoracic.org/), were also included. Finally, previous reviews on HRQoL tools in children were considered in the critical analysis.8–13
Inclusion and exclusion criteriaArticles that described the construction process of specific Instruments to assess HRQoL in children and adolescents (0-18 years) with asthma were included, as well as those describing the process of constructing a module specific for asthma in generic tools. The articles related to tools that assessed quality of life in families of children or adolescents with asthma were not included in the survey.
Data identificationThe articles resulting from the systematic search were verified and analyzed regarding the inclusion criteria and quality. Any discrepancy was resolved by consensus among the authors. The following characteristics were recorded all instruments. 1) number of items, number of domains, and which domains; 2) target age group, who answered the questionnaire, country of origin, year of publication, and number of subjects included; 3) cultural adaptation to Brazilian Portuguese; and 4) originally assessed psychometric properties.
Psychometric properties assessedValidityValidity is the capacity to measure that which is proposed to be measured. It is classically divided into three types: content, criterion, and construct; currently, construct validity assessment methods prevail.14 Two types of analysis are commonly used., One is construct representation, which uses the factorial analysis technique; the other is analysis per hypothesis, which uses the technique of concurrent and discriminant validation.4,15,16
ReliabilityReliability refers to the capacity of an instrument to achieve similar results when assessing subjects in different circumstances. It measures whether the instrument is free from random error, and is usually evaluated using measures of internal consistency as well as reproducibility or sensitivity to change.4,14,17
Once the instruments were identified, a new search in databases was performed to determine the number of articles that used each instrument since its publication year. Thus, by dividing the number of articles identified in the search by the number of years since the publication, the visibility index was obtained.
ResultsFrom a total of 2,301 articles obtained using the keywords “Asthma” and “Quality of Life”, with age limit between 0 and 18 years, 437 articles addressed HRQoL within the same age range. Of these, 162 used generic or specific instruments with specific modules to measure HRQoL in children and/or adolescents with asthma, as described in Table 1.
Number of articles per keyword and databases (English, Spanish, and Brazilian Portuguese).
| PubMed | Ovid | LILACS | |
|---|---|---|---|
| 1- ASTHMA AND QUALITY OF LIFEAge limit: (0 to 18 years) | 1,406 | 874 | 21 |
| 2- HEALTH-RELATED QUALITY OF LIFE AND ASTHMAAge limit: (0 to 18 years) | 284 | 141 | 12 |
| Total of specific instruments*: | 162 articles |
Of the total of 162 potential articles, 15 that assessed HRQoL of children and adolescents with asthma were identified; four of them were asthma-modules of generic instruments. Tables 2 and 3 show a summary of the characteristics of the 15 instruments evaluated.
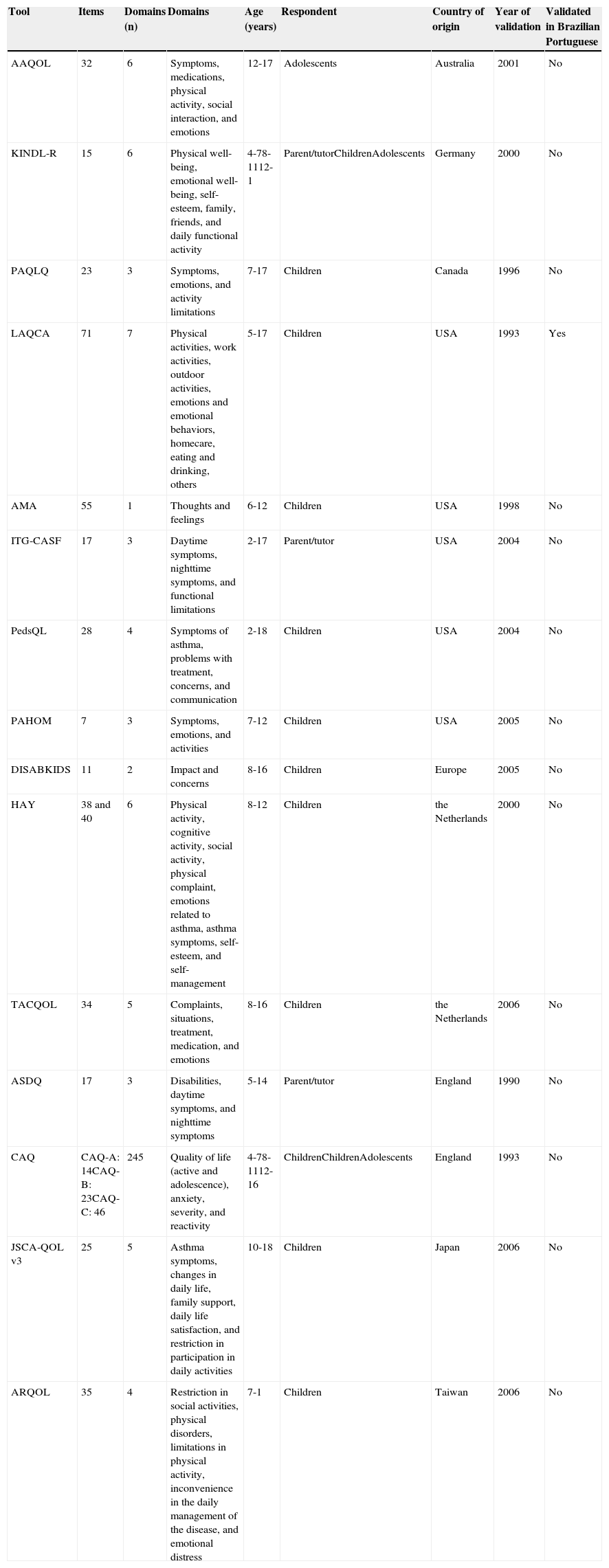
General characteristics of specific instruments to assess quality of life in children and adolescents with asthma.
| Tool | Items | Domains (n) | Domains | Age (years) | Respondent | Country of origin | Year of validation | Validated in Brazilian Portuguese |
|---|---|---|---|---|---|---|---|---|
| AAQOL | 32 | 6 | Symptoms, medications, physical activity, social interaction, and emotions | 12-17 | Adolescents | Australia | 2001 | No |
| KINDL-R | 15 | 6 | Physical well-being, emotional well-being, self-esteem, family, friends, and daily functional activity | 4-78-1112-1 | Parent/tutorChildrenAdolescents | Germany | 2000 | No |
| PAQLQ | 23 | 3 | Symptoms, emotions, and activity limitations | 7-17 | Children | Canada | 1996 | No |
| LAQCA | 71 | 7 | Physical activities, work activities, outdoor activities, emotions and emotional behaviors, homecare, eating and drinking, others | 5-17 | Children | USA | 1993 | Yes |
| AMA | 55 | 1 | Thoughts and feelings | 6-12 | Children | USA | 1998 | No |
| ITG-CASF | 17 | 3 | Daytime symptoms, nighttime symptoms, and functional limitations | 2-17 | Parent/tutor | USA | 2004 | No |
| PedsQL | 28 | 4 | Symptoms of asthma, problems with treatment, concerns, and communication | 2-18 | Children | USA | 2004 | No |
| PAHOM | 7 | 3 | Symptoms, emotions, and activities | 7-12 | Children | USA | 2005 | No |
| DISABKIDS | 11 | 2 | Impact and concerns | 8-16 | Children | Europe | 2005 | No |
| HAY | 38 and 40 | 6 | Physical activity, cognitive activity, social activity, physical complaint, emotions related to asthma, asthma symptoms, self-esteem, and self-management | 8-12 | Children | the Netherlands | 2000 | No |
| TACQOL | 34 | 5 | Complaints, situations, treatment, medication, and emotions | 8-16 | Children | the Netherlands | 2006 | No |
| ASDQ | 17 | 3 | Disabilities, daytime symptoms, and nighttime symptoms | 5-14 | Parent/tutor | England | 1990 | No |
| CAQ | CAQ-A: 14CAQ-B: 23CAQ-C: 46 | 245 | Quality of life (active and adolescence), anxiety, severity, and reactivity | 4-78-1112-16 | ChildrenChildrenAdolescents | England | 1993 | No |
| JSCA-QOL v3 | 25 | 5 | Asthma symptoms, changes in daily life, family support, daily life satisfaction, and restriction in participation in daily activities | 10-18 | Children | Japan | 2006 | No |
| ARQOL | 35 | 4 | Restriction in social activities, physical disorders, limitations in physical activity, inconvenience in the daily management of the disease, and emotional distress | 7-1 | Children | Taiwan | 2006 | No |
*Developed and validated in cooperation with the following countries: Austria, France, Germany, Greece, the Netherlands, Sweden, and England.
AAQOL, Adolescent Asthma Quality of Life Questionnaire; AMA, About My Asthma; ARQOL, Asthma Related Quality of Life; ASDQ, Asthma Symptoms and Disability Questionnaire; CAQ, Childhood Asthma Questionnaires; DISABKIDS, Disability Kids; HAY, How Are You; ITG-CASF, Integrated Therapeutics Group Child Asthma Short Form; KINDL-R, Kinder Lebensqualität Fragebogen; LAQCA, Life Activities Questionnaire for Childhood Asthma; PAHOM, Pediatric Asthma Health Outcome Measure; PAQLQ, Paediatric Asthma Quality of Life Questionnaire; PedsQL, Pediatric Quality of Life Inventory 4.0; JSCA-QOL, Quality of Life Questionnaire for Japanese School-aged Children with Asthma; TACQOL-Asthma, TNO-AZL Questionnaires for Children's Health-Related Quality of Life.
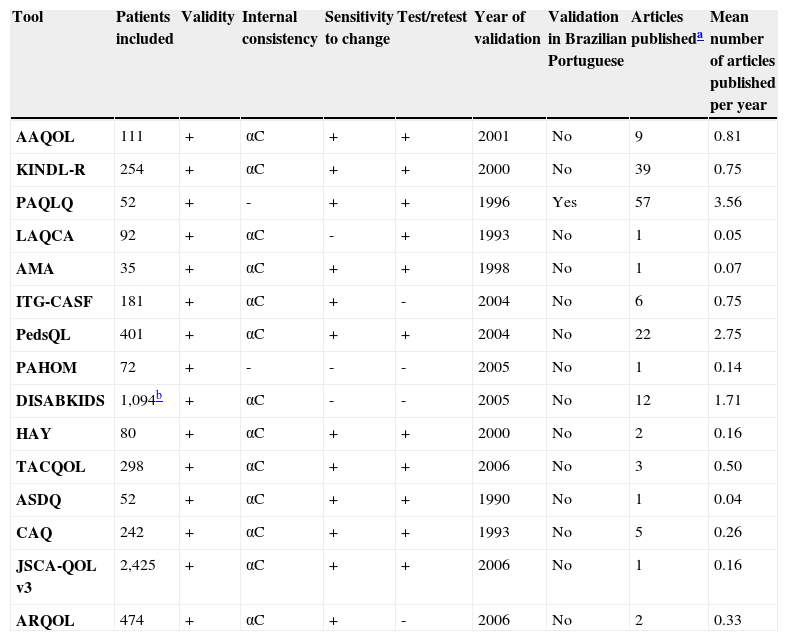
Characteristics of validation and publication of specific tools to assess quality of life in children and adolescents with asthma.
| Tool | Patients included | Validity | Internal consistency | Sensitivity to change | Test/retest | Year of validation | Validation in Brazilian Portuguese | Articles publisheda | Mean number of articles published per year |
|---|---|---|---|---|---|---|---|---|---|
| AAQOL | 111 | + | αC | + | + | 2001 | No | 9 | 0.81 |
| KINDL-R | 254 | + | αC | + | + | 2000 | No | 39 | 0.75 |
| PAQLQ | 52 | + | - | + | + | 1996 | Yes | 57 | 3.56 |
| LAQCA | 92 | + | αC | - | + | 1993 | No | 1 | 0.05 |
| AMA | 35 | + | αC | + | + | 1998 | No | 1 | 0.07 |
| ITG-CASF | 181 | + | αC | + | - | 2004 | No | 6 | 0.75 |
| PedsQL | 401 | + | αC | + | + | 2004 | No | 22 | 2.75 |
| PAHOM | 72 | + | - | - | - | 2005 | No | 1 | 0.14 |
| DISABKIDS | 1,094b | + | αC | - | - | 2005 | No | 12 | 1.71 |
| HAY | 80 | + | αC | + | + | 2000 | No | 2 | 0.16 |
| TACQOL | 298 | + | αC | + | + | 2006 | No | 3 | 0.50 |
| ASDQ | 52 | + | αC | + | + | 1990 | No | 1 | 0.04 |
| CAQ | 242 | + | αC | + | + | 1993 | No | 5 | 0.26 |
| JSCA-QOL v3 | 2,425 | + | αC | + | + | 2006 | No | 1 | 0.16 |
| ARQOL | 474 | + | αC | + | - | 2006 | No | 2 | 0.33 |
AAQOL, Adolescent Asthma Quality of Life Questionnaire; AMA, About My Asthma; ARQOL, Asthma Related Quality of Life; ASDQ, Asthma Symptoms and Disability Questionnaire; CAQ, Childhood Asthma Questionnaires; DISABKIDS, Disability Kids; HAY, How Are You; ITG-CASF, Integrated Therapeutics Group Child Asthma Short Form; KINDL-R, Kinder Lebensqualität Fragebogen; LAQCA, Life Activities Questionnaire for Childhood Asthma; PAHOM, Pediatric Asthma Health Outcome Measure; PAQLQ, Paediatric Asthma Quality of Life Questionnaire; PedsQL, Pediatric Quality of Life Inventory 4.0; JSCA-QOL, Quality of Life Questionnaire for Japanese School-aged Children with Asthma; TACQOL-Asthma, TNO-AZL Questionnaires for Children's Health-Related Quality of Life; αC, Cronbach's alpha coefficient.
Developed in Australia in 2001 to evaluate the HRQoL of adolescents with asthma (age range: 12-17 years). It is a multidimensional, self-administered questionnaire that estimates the impact of asthma on the physical, emotional, and social areas.18,19 The final version contains 32 items divided into five domains: symptoms, medications, physical activity, emotions, and social interaction. There is also a sixth domain (positive effects) that does not count in the total score of the tool, but provides complementary information.18 To validate the instrument, 111 subjects from three pediatric asthma outpatient clinics participated. The construct validity was studied by comparing it with the Pediatric Asthma Quality of Life Questionnaire (PAQLQ), regarding internal consistency and reproducibility.18 The visibility index of the tool was 0.81 articles/year.
About My Asthma (AMA)Developed in the U.S. in 1998 to assess the stressors that affect HRQoL of children and adolescents with asthma aged between 6 and 12 years. The AMA is a self-administered questionnaire, adapted from another tool, previously developed to estimate stress levels in children with cancer.20 It consists of 55 items, with which participants can classify their thoughts and feelings in a four-point scale. The intensity of the asthma stressors is reflected by total score, which ranges from 1 to 55 points.20 Construct validity, concurrent validity, and internal consistency of the tool were studied in these questionnaires.20 The visibility index of the tool was 0.07 articles/year.
Asthma-Related Quality of Life (ARQOL)A self-administered questionnaire developed in Taiwan in 2006 to evaluate the HRQoL in children and adolescents with asthma, aged 7-13 years, which assesses the impact of asthma.21 The final version contains 35 items divided into five domains: restrictions to social life, physical disturbances from signs and symptoms, physical activity limitation, difficulties in the daily management of the disease, and emotional distress. The tool validation involved 251 children with asthma recruited from three medical centers and 223 children from six elementary schools. Psychometric properties assessed were construct validity through concurrent validity, and internal consistency by reproducibility.21 The visibility index of the tool was 0.33 articles/year.
Asthma Symptoms and Disability Questionnaire (ASDQ)Developed in England in 1990 to evaluate HRQoL of children and adolescents aged 5 to 14 years. The ASDQ is a questionnaire designed to be answered by the parent/tutor of the child/adolescent (proxy).22 The final version contains 17 items divided into three domains: disability, daytime symptoms, and nighttime symptoms.22 Construct validity and internal reliability were the psychometric properties studied.22 The visibility index of the tool was 0.04 articles/year.
Childhood Asthma Questionnaires (CAQ)Developed in 1993 in England to assess HRQoL and the level of stress the disease causes. The tool consists of three versions, depending on the age of the subjects: CAQ-A for children 4 to 7 years; CAQ-B for 8 to 11 years; and CAQ-C for 12 to 16 years. Over 200 asthmatic children participated in the study for its validation, as well as a similar number of non-asthmatic individuals.23,24 The internal consistency and reproducibility were evaluated in the three questionnaires.18 The visibility index of the tool was 0.26 articles/year.
Disability Kids (DISABKIDS)A generic tool, developed in collaboration among seven European countries (Austria, France, Germany, Greece, the Netherlands, Sweden, and England), which assesses HRQoL of children and adolescents aged 4 to 7 years and 8 to 16 years, with specific versions for the two age groups. The focus of the DISABKIDS project lies in seven chronic conditions: asthma, juvenile rheumatoid arthritis, epilepsy, cerebral palsy, diabetes mellitus, atopic dermatitis, and cystic fibrosis.25,26 The DISABKIDS asthma module consists of 11 items and two domains: the impact domain (six items) relating to restrictions and symptoms, and the concerns domain (five items), about fears related to asthma. Both DISABKIDS are based on self-reported and by-proxy measurements.25,26 The visibility index of the tool was 1.71 articles/year.
How Are You (HAY)Developed in the Netherlands in 2000, it evaluates the HRQoL of asthmatic children aged 8 to 12 years. HAY is self-administered, and can also be answered by proxy. It contains three dimensions: physical activities, social activities, and self-management.27 The questionnaire consists of a general section and a specific section for asthma. The generic section can be answered either by asthmatic or healthy children and includes four domains: physical activity, cognitive activities, social activities, and physical complaints. Regarding the specific section, it includes the following domains: symptoms of asthma, self-management, asthma-related emotions, and self-concept.27 A total of 228 Dutch children with asthma participated in tool validation. Reproducibility and sensitivity to change was evaluated in a subgroup of 80 children with asthma. In order to test reproducibility, the researchers applied test-retest reliability.27 The visibility index of the tool was 0.16 articles/year.
Integrated Therapeutics Group Child Asthma Short Form (ITG-CASF)A tool developed in the United States in 2000 to evaluate HRQoL of children and adolescents with asthma, aged 2 to 17 years. It is a questionnaire answered by proxy, containing 17 items, aiming to investigate daytime and nighttime asthma symptoms and their functional limitations. Reliability was analyzed to validate the tool, with the participation of 181 children with asthma.28 The visibility index of the tool was 0.75 articles/year.
Kinder Lebensqualität Fragebogen (KINDL-R)A generic tool developed in Germany in 2000 to evaluate HRQoL of children and adolescents. The KINDL-R is a self administered questionnaire, which can also be answered by proxy,29 containing three versions: Kiddy-Kindl (4 to 7 years old), Kid-Kindl (8 to 11 years old), and Kiddo-Kindl (12 to 16 years old). In addition to the generic versions, the KINDL-R has six independent modules to assess quality of life of children with specific diseases: asthma, diabetes, epilepsy, neurodermatitis, oncology, and spina bifida.30
The generic KINDL-R questionnaire consists of 24 items, associated with six dimensions: physical well-being, emotional well-being, self-esteem, family, friends, and daily functional status (school or nursery/kindergarten). The sub-scales of these six dimensions can be combined to produce a total score. The KINDL-asthma module has 15 items. Content validity and internal consistency were evaluated.30 The visibility index of the tool was 0.75 articles/year.
Life Activities Questionnaire for Childhood Asthma (LAQCA)Developed in the U.S. in 1993, it evaluates the quality of life of children and adolescents with asthma (aged 5 to 17 years). The LAQCA is a self-administered questionnaire. The process of development and validation of the questionnaire consisted of two steps. In total, 92 children/adolescents participated in the process.31 The final version of the tool uses 71 items, divided into seven parts: physical activities (20 items), work activities (4 items), outdoor activities (16 items), emotions and emotional behaviors (6 items), home care (11 items), eating and drinking (5 items), and miscellaneous (9 items). The internal consistency and reproducibility of the questionnaire were assessed with an independent sample of 46 asthmatic children.31 The visibility index of the tool was 0.05 articles/year.
Pediatric Asthma Health Outcome Measure (PAHOM)A tool developed in the United States in 2005 to assess HRQoL in children with asthma, aged 5 to 12 years. The PAHOM consists of 71 items divided into seven dimensions: absence of symptoms, moderate respiratory problems, severe respiratory problems, emotional absence, presence of emotional problems, lack of activity, and presence of activity problems. Unlike most current health outcome measures, PAHOM provides a calendar as a visual aid to help the children remember their health status during the last seven days.32 The visibility index of the tool was 0.14 articles/year.
Pediatric Asthma Quality Of Life Questionnaire (PAQLQ)Developed in Canada in 1996 to evaluate HRQoL of children and adolescents aged 7 to 17 years, it meets the following criteria: reflects areas of function that are important for children with asthma, including both emotional and physical functions, is reproducible when the clinical status is stable, and is sensitive to changes that are important to the patient.33,34 The tool has 23 items divided in three domains (activity limitation, symptoms, emotional function). In the activity limitation domain, three of the items are ‘individualized’. At the first visit, the patient is asked to identify three physical activities or sports he/she practices and considers important; these activities are maintained individualized for each patient throughout the follow-up. Currently, the activities are standardized: 1) physical activity, 2) activities with animals, 3) activities with friends and family. Construct validity, reproducibility, and sensitivity to change were evaluated; minimal important difference was estimated at 0.5 points.33,34 The visibility index of the tool was 3.56 articles/year.
Pediatric Quality of Life Inventory 4.0 (PedsQL)A generic tool developed in the U.S. in 1987, designed to measure health-related quality of life in children and adolescents aged 2 to 18 years. It has 23 items divided into five domains: physical functional status, emotional functional status, social functional status, and school functional status. The fifth domain is the psychosocial, consisting of the sum of the domains, except for the physical. There are versions for three age ranges, which are self-administered from 5 years of age, and it also has versions that can be answered by proxy. PedsQL 4.0 was designed to be used independently or together with modules separated from the questionnaire and designed for specific diseases, including asthma. PedsQL 3.0-Asthma (asthma module) has 28 multidimensional items that encompass asthma symptoms, treatment problems, concerns, and communication.35 To validate the PedsQL 4.0 (generic module), 730 healthy children/adolescents aged 2 to 18 years participated in the study.31 In the validation of the PedsQL 3.0 (asthma module), 529 families of asthmatic children aged 2 to 16 years participated in the study.35 Construct validity was evaluated through discordant validity and internal consistency.35 The visibility index of the tool was 2.75 articles/year.
Quality of Life Questionnaire for Japanese School-aged Children with Asthma (JSCA-QOL v3)A tool developed in Japan in 2006 to evaluate HRQoL of Japanese children/adolescents with asthma aged between 10 and 18 years.36 It is a self-administered questionnaire, and the latest version includes 25 items divided into five domains: asthma crisis, changes in daily life, family support, satisfaction with daily life, restriction to participate in daily activities, and a summary scale. To validate the instrument, 2,425 Japanese children with asthma participated in the study. Validity was assessed by factorial analysis, reliability was studied by internal consistency, and reproducibility by test-retest.36 The visibility index of the tool was 0.16 articles/year.
TNO-AZL Questionnaires for Children's Health-Related Quality of Life (TACQOL-Asthma)A tool developed in the Netherlands in 2006 that evaluates HRQoL of children and adolescents (8-16 years). TACQOL-Asthma can be used independently or in combination with the generic TACQOL (developed and validated in 1995).7,37 The TACQOL-Asthma questionnaire was adapted in a pilot study of 72 subjects and was subsequently validated with the participation of 298 patients, where the items were tested for internal consistency, reliability, and content validity.7,37 The visibility index of the tool was 0.50 articles/year.
DiscussionA total of 15 specific HRQoL questionnaires specific for asthma in children and adolescents were identified. Of these, the three tools with the highest visibility (publications/year since its publication) were PAQLQ, PedsQL-Asthma, and DISABKIDS.
In the last 20 years there has been a progressive increase in the use of HRQoL tools in intervention and impact studies of asthma in children and adolescents. However, in the last five years, there have been no publications on the development of new questionnaires,12 but the number of cultural adaptations of instrument that already exist has shown a considerable increase. Both situations can be explained; firstly, the development of HRQoL questionnaires is a complex and time-consuming task;7 secondly, in the study of asthma, it is very important to be able to compare results between populations, and this is only possible when comparable tools are used.7,14 However, differently from objective measures such as pulmonary function, it is thought that results of HRQoL are not readily comparable among the different tools.7,14 Thus, tools with greater dissemination, such as PAQLQ, PedsQL-Asthma, and DISABKIDS, have versions and cultural adaptations in several languages, contributing even more to their prevalence in literature.
Few data are available on the integration of these instrument in clinical practice and strategies necessary for the best use of these tools in the long-term monitoring of children.
In Brazil, PAQLQ is the only tool with complete validation (cultural adaptation). The linguistic validation was performed in 2001, and only recently was the cultural validation of this version completed, showing good psychometric properties.38
Regarding the limitations of this systematic review, it is noteworthy that original articles published in languages other than English, Spanish and Portuguese were not included in the search.
However, among the tools identified, some were found to have been originally developed in languages other than English, the language in which the results were published. Considering that English is the predominant language in health sciences, it is believed that few tools were out of the present systematic review. As a possible expression of this situation, a recent study assessed the HRQoL tools available in Latin American countries between 2000-2010, for children and adolescents.13 Of 31 tools, among specific and generic, only PAQLQ was available and had had cultural adaptations in several countries.
In conclusion, there are many specific questionnaires to assess HRQoL of children and adolescents diagnosed with asthma. Of these, the three most frequently used are the PAQLQ, the PedsQL-Asthma, and DISABDKIDS, while other questionnaires have had few publications, suggesting limited use. Only one tool has been validated in Brazil. The choice of an HRQoL instrument requires attention regarding its original psychometric properties, but also requires the feasibility study of its adaptation, through consideration the cultural elements present in its creation.4,14
FundingCNPq, CAPES, FAPERGS.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Roncada C, Mattiello R, Pitrez PM, Sarria EE. Specific instruments to assess quality of life in children and adolescents with asthma. J Pediatr (Rio J). 2013;89:217–25.