To analyze the prevalence of Mycobacterium leprae detection and the associated factors among social contacts in the school environment of multibacillary cases living in a hyperendemic municipality of the state of Mato Grosso.
MethodsCross-sectional study with 236 social contacts of multibacillary leprosy from public schools and residents in Cuiabá (Mato Grosso) in 2018. The sources of information were interviews and nasal swab tests for molecular analysis by polymerase chain reaction - PCR. For the prevalence ratio estimates, crude and adjusted analyses were performed using robust Poisson regression and their respective confidence intervals (95% CI). The ArcGIS 9.1 software was used for the geographic distribution analyses.
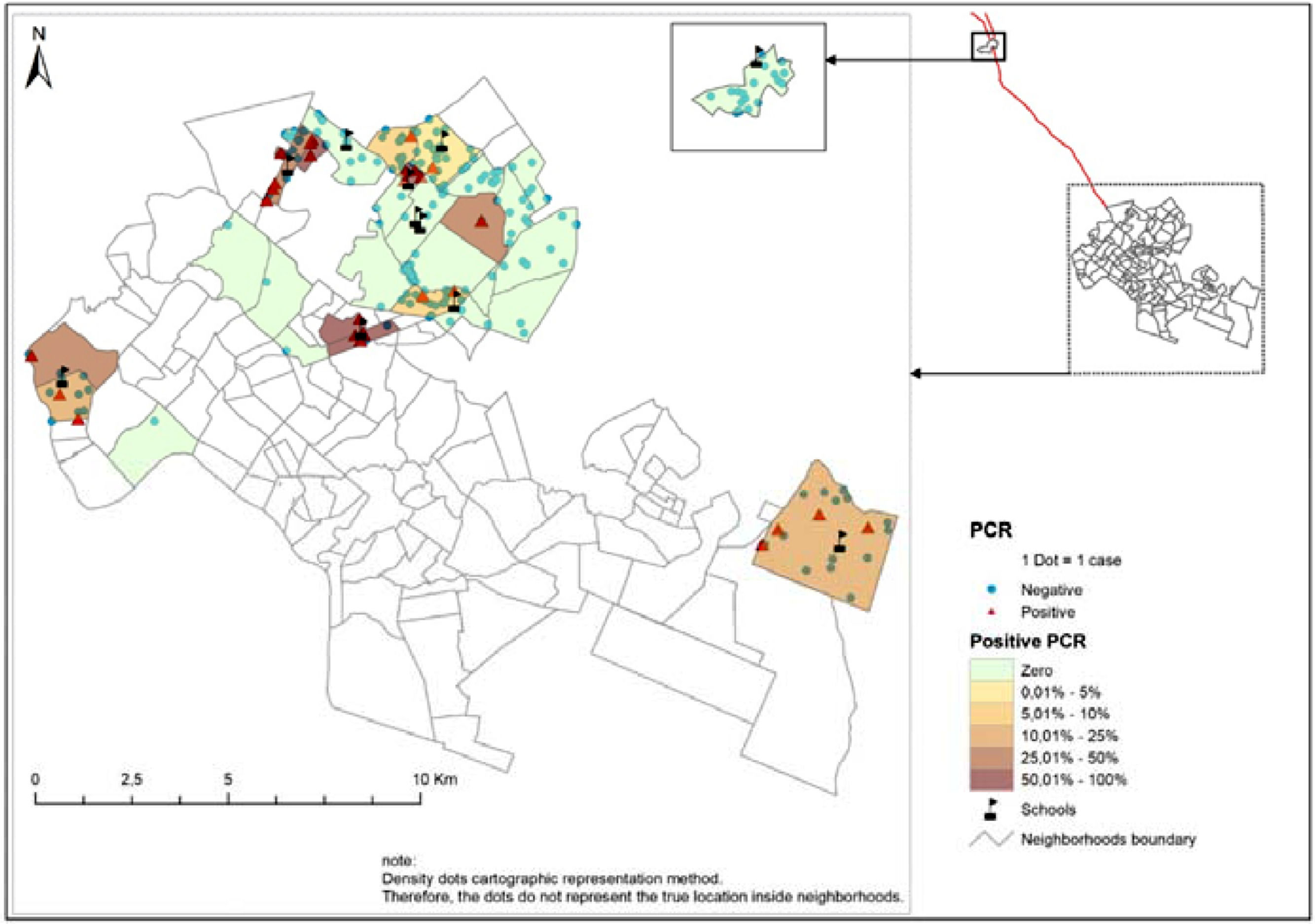
ResultsThe prevalence of detection of M. leprae in social contacts was 14%. A total of 63.6% of the schools surveyed had 5.1% to 50% of the social contacts of leprosy with positive PCR. The analysis of the geographic distribution in the neighborhoods showed a high prevalence of infection, being higher than 50% in some localities. The highest proportion of positive results occurred in the northern region of the city and from a precarious socioeconomic class.
ConclusionThe results showed a high prevalence of detection of M. leprae among social contacts in areas with poor socioeconomic conditions. In these regions, there is a greater risk of infection and of getting sick.
Leprosy is a neglected tropical disease 1 and, in endemic areas, the risk of affecting children and adolescents increases considerably due to vulnerability and early exposure to the bacillus. It can cause physical deformities, impair their growth and development, and lead to suffering for the leprosy individual and the family.2-4
Worldwide, a total of 202.185 new leprosy cases were reported to the World Health Organization (WHO) in 2019, demonstrating a detection rate of 25.9 per million inhabitants, 7.4% of whom are children (<15 years of age).1 In 2019, the state of Mato Grosso (MT), located in the Midwest region of Brazil, among all Brazilian states, had the highest detection rate of new cases of leprosy in the general population (129.38 new cases/100.000 inhabitants). The capital Cuiabá recorded a rate of 50.45 new cases/100.000 inhabitants.1,5
Also, in the state of Mato Grosso, among children <15 years of age, the detection rate was 22.76/100.000 inhabitants, which is considered a parameter of hyperendemicity. With such indicators, they remain an aggravating factor for the control of the disease, indicating focuses of active transmission in the community; therefore, contact tracing of multibacillary cases is primarily a priority.4-7
Until recently, contact tracking was limited to household contacts. In contrast, studies suggest that social contacts are also considered risk groups for developing the disease and can behave as a reservoir for the infection, maintaining its transmissibility. Anyone with family relationships or not, such as neighbors, co-workers, schoolmates, among others, who have lived in close proximity with a case of leprosy without treatment, should be investigated.8-11
The leprosy diagnosis is essentially clinical due to the complexity of the disease and the inability of the bacillus to be cultured in vitro.12,13 However, in recent years, with advances in molecular biology, specific tools have been incorporated to assist in the detection of the bacillus, to early diagnosis, and the identification of those with subclinical infection.9,14,15
The Polymerase Chain Reaction (PCR) tests in field studies have shown that DNA-based PCR assays can be 100% specific, while the sensitivity ranges from 34 to 80% in patients with PB forms to greater than 90% in patients with multibacillary forms of the disease, being used in several types of clinical samples, to extract, amplify, and identifying the Mycobacterium leprae (M. leprae) DNA. Thus, it enables monitoring the bacillus, even at relatively low concentrations in the nasal mucosa.15,16 Studies used PCR to detect the bacillus in nasal swab samples.9,15,17 Some authors point out that bacillus transmission is not exclusively associated with leprosy without treatment, as healthy and asymptomatic contacts can carry M. leprae in the nasal mucosa.15,17,18
In areas considered endemic, the presence of infection in children suggests intense circulation and dissemination of the bacillus; thus, the identification of groups with a higher risk of developing leprosy becomes essential, especially in the children population considered more vulnerable to illness.3,6
In this regard, this study aims to analyze the prevalence of M. leprae detection and the associated factors among social contacts in the school environment of multibacillary cases living in the hyperendemic municipality of the state of Mato Grosso.
MethodsStudy designCross-sectional study conducted with social contacts of leprosy from public educational institutions in Cuiabá - Mato Grosso in 2018. This study was approved by the Research Ethics Committee of the Mato Grosso State Health Department (CEP/SES/MT; protocol number 491.444).
Study areaThe municipality of Cuiabá has a territorial extension of 3,362.8 km², human population density of 163.88 people per sq. km of land area, a Human Development Index (HDI) corresponding to 0.785, and a population of 551,098 inhabitants, among which 126,425 (22.90%) consist of people under 15 years of age.19
The urban area of the municipality has 115 districts distributed in urban and rural areas (North Region, 10 districts; South Region, 34 districts; East Region, 49 districts; West Region, 24 districts).20
Eligibility criteriaThe study population consisted of social contacts aged from 5 to 14 years old, coming from 11 public educational institutions, residing in 48 neighborhoods located in urban and rural regions.
Social contacts were selected from all cases of multibacillary leprosy in children under 15 years of age identified in the registry of the National System of Notifiable Diseases of the state of Mato Grosso (SINAN/MT), in the period of July 2016 to December 2017.
Social contact was defined as a participant under the age of 15 who stayed in a school environment for an average of 4 hours a day with confirmed cases of leprosy reported on SINAN/MT, those who did not show symptoms and signs suggestive of the disease following an evaluation by specialist physicians, and those who collected a laboratory sample of a nasal swab to investigate M. leprae.
A total of 238 social contacts were included in the study. Of these, 2 contacts were excluded (one for confirming the diagnosis of leprosy during the dermato-neurological examination and another for not performing the nasal swab collection). In the end, 236 social contacts were analyzed (n = 33 with positive PCR; n = 203 with negative PCR).
Study variables and data sourceThe outcome variable was the result of the detection of M. leprae by nasal swab (contact with positive and negative PCR). The exposure variables studied were demographic/socioeconomic (age group, sex, region of residence, condition of residence and economic class) and epidemiological/immunological (vaccinal scar by Bacillus Calmette-Guérin (BCG), presence of a lesion, sits close to the case, contact with a case of leprosy out of school, number of people in the household, case of leprosy in the family).
The classification of socioeconomic status was carried out using the criterion of the Brazilian Association of Research Companies, whose function is to estimate the purchasing power of families, with a points system based on the possession of household items and the level of education of the householder.21
After signing the free and informed consent form (by parents/guardians of the study participants) and assent form (children), the information was obtained through interviews with the application of a semi-structured questionnaire and the results of a laboratory test using a nasal swab (PCR).
Nasal swab sample procedureNasal swab samples were collected from the nasal cavities of all social contacts, using a sterile swab with a plastic rod and a specific cotton tip to collect clinical samples. We attempted to collect the nasopharyngeal rub with a 360° rotating procedure and later stored it in a Styrofoam box at a temperature from 2°C to 8°C. All collected samples were stored (-20°C) and sent, according to standards established by the Brazilian Health Regulatory Agency, to the Lauro de Souza Lima Institute, in the municipality of Bauru – state of São Paulo - for analysis by PCR technique. The PCR analysis: the result ≤37 (CT - Cycle Threshold) was used as a positivity parameter for nasal swab PCR. Bacillus DNA samples extracted from the nasal swab were determined using the TaqMan system by qPCR. Specific primers were used: MLRLEP - F: 5´- GCA GTA TCG TGT TAG TGA A - 3´ and MLRLEP - R: 5´- CGC CGA CGG CCG GAT CAT CGA - 3´.
Data processing and analysisFor data management and analysis, the software SPPS 20 (Statistical Package for the Social Sciences) was used, and for geographic analysis, ArcGIS 9.1 (ESRI, Redmond, California). Data consistency was assessed using the Validate application (Microsoft®, Power Apps, Validate, WA, USA).
Descriptive analysis was performed with absolute (n) and relative (%) frequencies, measures of central tendency, and variability (mean, standard deviation, minimum and maximum). Nasal swab PCR was calculated by dividing the number of positives by the number of the population tested and multiplied by 100 for proportional presentation.
Prevalence ratios (PR) were calculated to investigate the existence of associations between the independent variables and the detection of M. leprae infection. The adjusted prevalence ratios were obtained using Poisson's multiple regression analysis with robust variance, considering the independent variables that obtained a p-value ≤ 0.20 in the bivariate analysis (region of residence, condition of residence, and number of people in the household). For the final analysis, a significance level of 5% (p<0.05) was accepted.22
The cartographic representation was carried out using the methodology of point density in the areas, whose punctual representation ensures the confidentiality of records and allows visual analysis of the geographical distribution of the places from where social contacts come and the proportion of positive and negative PCR in the studied neighborhoods.21
ResultsThe prevalence of detection of M. leprae infection among the investigated social contacts was 14%. In 63.6% of the schools that participated in the study, contacts had positive PCR tests from 5.1% to 50%. The analysis of the geographic distribution in the neighborhoods showed a high prevalence of infection with proportions above 50% in some places; the highest prevalence was in the northern region of the city (42.4%) (Fig 1).
Regarding the demographic/socioeconomic characteristics of social contacts, the mean age was 11.5 years old (SD=±2.41; minimum 5 years old; maximum 14 years old), with 54.2% being female. Among these infected children, the most prevalent age group, 72.7%, was from 10 years old to 14 years old. A higher prevalence was identified among social contacts with positive PCR (66.7%), those belonging to the socioeconomic classes C/D/E concerning the higher economic class. For epidemiological/immunological factors, homogeneity was observed among the investigated variables. That is, there were no statistically significant differences among the predictor variables (Table 1).
Bivariate detection's analysis of the Mycobacterium leprae among social contacts, according to demographic/socioeconomic, epidemiological and immunological variables.
| Demographic/ socioeconomic variables | Positive PCR (n = 33) | Negative PCR (n = 203) | PR Crude | 95% CI | p value | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Age range | |||||||
| 10 – 14 years old | 24 | 72.7 | 157 | 77.3 | 0.81 | 0.40-1.64 | 0.561 |
| 5 – 9 years old | 09 | 27.3 | 46 | 22.7 | 1 | ||
| Sex | |||||||
| Male | 17 | 51.5 | 91 | 44.8 | 1.25 | 0.66-2.37 | 0.474 |
| Female | 16 | 48.5 | 112 | 55.2 | 1 | ||
| Region of residence | |||||||
| Othersa | 28 | 84.8 | 192 | 94.6 | 0.40 | 0.18-0.91 | 0.039 |
| West | 05 | 15.1 | 11 | 5.4 | 1 | ||
| Residence condition | |||||||
| Rented/donated | 14 | 42.4 | 46 | 22.7 | 2.16 | 1.16-4.04 | 0.015 |
| Own | 19 | 57.6 | 157 | 77.3 | 1 | ||
| Economic classb | |||||||
| C/D/E | 22 | 66.7 | 134 | 67.3 | 0.97 | 0.49-1.90 | 0.939 |
| A/B | 11 | 33.3 | 65 | 32.7 | 1 | ||
| Epidemiological/ immunological variables | Positive PCR (n = 33) | Negative PCR (n = 203) | PR Crude | 95% CI | p-value | ||
| n | % | n | % | ||||
| BCG scar | |||||||
| Absent | 2 | 6.1 | 10 | 4.9 | 1.20 | 0.32-4.44 | 0.677 |
| Present | 31 | 93.9 | 193 | 95.1 | 1 | ||
| Presented lesion | |||||||
| Yes | 8 | 24.2 | 35 | 17.2 | 1.43 | 0.69-2.96 | 0.334 |
| No | 25 | 75.8 | 168 | 82.8 | 1 | ||
| Sits Close to the case | |||||||
| Yes | 07 | 21.2 | 37 | 18.2 | 1.17 | 0.54-2.53 | 0.682 |
| No | 26 | 78.8 | 166 | 81.8 | 1 | ||
| Contact outside school | |||||||
| Yes | 2 | 6.1 | 06 | 3.0 | 1.83 | 0.53-6.38 | 0.310 |
| No | 31 | 93.9 | 197 | 97.0 | 1 | ||
| Number of people in the household | |||||||
| ≥ 5 | 12 | 36.4 | 103 | 50.7 | 0.60 | 0.31-1.16 | 0.125 |
| From 1 to 4 | 21 | 63.6 | 100 | 49.3 | 1 | ||
| Family leprosy case | |||||||
| Yes | 5 | 15.2 | 38 | 18.8 | 0.80 | 0.32-1.95 | 0.622 |
| No | 28 | 84.8 | 165 | 81.2 | 1 | ||
PR, prevalence ratio; 95% CI, 95% confidence interval.
In the bivariate analysis, there was a statistically significant difference between the variable's region of residence and condition of residence (PR Crude: 0.40; 95% CI: 0.18-0.91; p-value: 0.039); (PR Crude: 2.16; 95% CI: 1.16-4.04; p-value: 0.015) respectively (Table 1). Table 2 shows the cohabitation variables (region of residence, condition of residence and number of people in the household) that were included in the final model and after the adjustments were not shown as factors associated with M. leprae infection among the investigated social contacts (PR Adjust: 1.09; 95% CI: 0.96-1.24; p-value: 0.164, PR Adjust: 0.94; 95% CI: 0.88-1.00; p: 0.069, PR Adjust: 1.03; 95% CI: 0.98-1.08; p-value: 0.229, respectively).
Analysis of the final model for the detection of Mycobacterium leprae among social contacts, according to cohabitation variables.
| Cohabitation variables | PR Crude | PR Adjusted | 95% CI | P valueb |
|---|---|---|---|---|
| Region of residence | ||||
| Othersa | 0.40 | 1.09 | 0.96-1.24 | 0.164 |
| West | 1 | 1 | ||
| Condition of residence | ||||
| Rented/donated | 2.16 | 0.94 | 0.88-1.00 | 0.069 |
| Own | 1 | 1 | ||
| Number of people in the household | ||||
| ≥ 5 | 0.60 | 1.03 | 0.98-1.08 | 0.229 |
| From 1 to 4 | 1 | 1 |
PR, prevalence ratio; 95% CI, 95% confidence interval.
This study is a pioneer in the investigation of molecular detection of M. leprae using PCR in school social contacts. The objective was to analyze the prevalence of detection of bacillus infection and the associated factors between social contacts in the school environment of multibacillary cases residing in the midwest of Brazil. The results indicated a prevalence of infection among contacts of 14%, with most children investigated being girls and with a higher prevalence of infection between the ages of 10 and 14 years and from an unfavorable socioeconomic class. Among the schools surveyed, 63.6% had contacts with positive PCR. The analysis of the spatial distribution in the neighborhoods showed a high prevalence of infection (over 50%) in administrative areas of the city. The analysis of the investigated exposure factors did not indicate any association with the infection.
As for the profile of contacts, the average age was 11.5 years, with a higher proportion among females. There was a higher prevalence of infection among children aged between 10 and 14 years old and living in the northern region of the city. Surveillance in the child population is extremely important for disease control, as the presence of cases in these individuals signals adults in the contagious form without treatment. The difference between sexes and age should be observed at the planning of educational strategies, mainly aimed at contacts that extend beyond the intra-household contact.3,4
The overall prevalence of 14% with a positive PCR result among social contacts is considered high because the population studied is concentrated on children. However, infectivity does not represent an active disease but evidences the presence of the bacillus in the nasal mucosa, which presupposes a greater risk of disease development, contributing to the continuation of the endemic.23,24
However, the prevalence of infection distributed by neighborhoods reaches a level above 50% among contacts from these locations. Social determinants are intrinsic factors in the onset of the disease, and the large population in these areas favors the transmission of the disease. There is also the issue of exposures and re-exposures to the bacillus that social contacts are subject to when living with multibacillary patients who go beyond the school environment and which may justify the persistence of hyperendemicity in the State.6,23,24
It is noteworthy that in 63.6% of the schools surveyed, the prevalence of positive PCR results was 5% to 50% distributed in 48 districts located in the city's administrative areas. This shows the need to expand epidemiological surveillance actions in these geographic areas with a view to including strategies aimed at the active and systematic detection of leprosy cases and identification of household, neighborhood and social contacts that are more susceptible to M. leprae. The prioritization of school environments and locations with a higher concentration of infection detection as educational spaces for disease prevention can contribute to reducing the incidence of leprosy cases, especially in hyperendemic areas.4,6,8,24
The investigated exposure factors that could be indicated as associated with infection among social contacts, which were the region of residence, condition of residence, and a number of people living in the household, did not remain as predictors of the outcome after adjustments. In this study, other unexplored intervening factors related to the qualitative approach could justify such results.
The leprosy-specific infection markers used in this study are essential for detecting the infection. They can be combined with a clinical assessment to monitor patients and identify exposed individuals who are at higher risk of developing the condition.15,18
PCR has been useful in detecting small amounts of bacillus present in tissues. Its quick and specific, and sensitive identification of the microorganism is important when dealing with microorganisms such as M. leprae, whose cultivation cannot be carried out in a culture medium. The existence of healthy carriers or subclinical infection with the presence of bacilli in the nasal secretion indicates its high infectivity, especially in children. The difficulty in finding alcohol-acid-resistant bacilli through histopathological methods in the early stages of the disease makes the PCR technique a safe tool. There is a need for greater investments by public managers, especially to invest in innovative techniques. Therefore, screening contacts is an important strategy for detecting new cases and interrupting disease transmission, as observed in this study.25
The present study study showed relevant information. However, it may have some limitations. Care related to the process of selection of participants and analysis were considered to minimize biases typical of cross-sectional studies, which do not allow inferring time causality, and some answers could be limited to knowledge of the information collected by children/parents and guardians. All multibacillary cases were identified to select social contacts to ensure greater representation. The social contacts arising from these cases allowed the performance of an important analysis of the entire population investigated. The scarcity of studies on the subject for comparison purposes was also a point that should be considered.
The findings showed a high prevalence of detection of M. leprae infection among social contacts. The spatial distribution of social contacts with the infection, when related to the other variables, suggests that in areas with precarious socioeconomic conditions, there is a greater risk of transmission, and the living conditions in which the individual is inserted can favor the acquisition of the disease.
Thus, it is necessary that health professionals encourage and collaborate with health education on leprosy in schools, with the adoption of active learning methods so that students reflect on their own well-being and, consequently, adopt practices that contribute to the dissemination of this subject outside the school environment, mainly in the state of Mato Grosso, as it is a hyperendemic region for leprosy.
It should be noted that the present study is innovative, and the findings evidenced are important to contribute to scientific knowledge and corroborate with more effective strategies to control the disease.
Investment in future studies with the expansion of other issues related to accessibility, educational approach, and those related to the environment that could be related to possible associated factors investigated in this more vulnerable group is required.
FundingThis research is funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through Process Number 435239/2018-0 and Programa de Apoio à Pós-Graduação (PROAP).