Acute kidney injury (AKI) in the neonatal period is associated with worst outcomes as increased mortality and increased length of hospital stay. Very low birth weight (VLBW) newborns are at higher risk for developing several other conditions that are associated with worst outcomes. Understanding the risk factors for AKI may help to prevent this condition and improve neonatal care for this population.
MethodsThis retrospective cohort study included 155 very low birth weight newborns admitted between 2015 and 2017. The authors compared the newborns who developed neonatal AKI with the non-AKI group and analyzed the main risk factors for developing AKI in the population. The authors also performed an analysis of the main outcomes defined as the duration of mechanical ventilation, length of stay, and death.
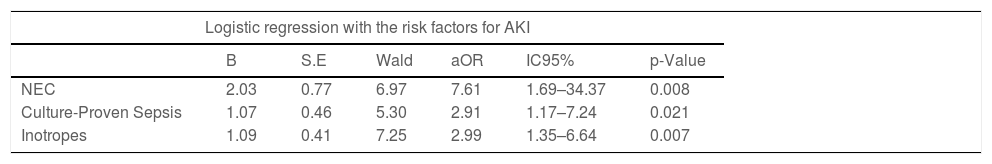
ResultsFrom the cohort, a total of 61 (39.4%) patients had AKI. The main risk factors associated with Neonatal AKI were necrotizing enterocolitis (aOR 7.61 [1.69 – 34.37]; p = 0.008), neonatal sepsis (aOR 2.91 [1.17 – 7.24], p = 0.021), and hemodynamic instability (aOR 2.99 [1.35 – 6.64]; p = 0.007). Neonatal AKI was also associated with an increase in the duration of mechanical ventilation in 9.4 days (p = 0.026) and in an increase in mortality 4 times (p = 0.009), after adjusting for the other variables.
ConclusionThe present results highlight the importance of minimizing sepsis and necrotizing enterocolitis, as well as the importance of identifying hemodynamic instability, to prevent AKI and diminish the burden of morbimortality in VLBW newborns.
The diagnosis of acute kidney injury (AKI) in the neonatal period is associated with increased mortality and increased length of hospital stay.1-4 Moreover, despite the different available definitions of neonatal AKI in the literature, the outcomes are the same, as shown by Chowdhary et al.,5 emphasizing the importance of this subject.
The assessment of kidney function in the newborn has physiological and clinical peculiarities that may hinder the diagnosis of AKI. After birth, the decrease in vascular resistance and the increase in renal blood flow determine an increase in the glomerular filtration rate and a decrease in basal serum creatinine, which are proportional to gestational age and days of life. Thus, the use of a single glomerular filtration rate determination is of little use for this population.6 Furthermore, very low birth weight (VLBW) newborns may present with non-oliguric AKI,7 making the diagnosis of AKI in this population more difficult. Novel biomarkers are being studied in the neonatal population, such as urinary neutrophil gelatinase-associated lipocalin (uNGAL) and the kidney injury molecule-1 (KIM-1); however, the studies analyzing them in very-low birth weight newborns are still scarce in the literature.8
Understanding the risk factors for AKI may help in the identification of newborns who should be monitored more closely through serial creatinine sampling, assessment of 24-hour urinary output and urine quality, serial blood pressure measurements, and avoidance of potential nephrotoxic medications, as proposed by Harer et al. in response to the 22nd Acute Disease Quality Initiative (ADQI).9
In a recent large and multicenter study, the incidence of AKI varied from 3 to 74% between the centers.1 This center-dependent incidence may explain the disparity of AKI-associated risk factors in this population across the different studies.2,4,7,10 The study of VLBW infants is of special interest since this group is more susceptible to the development of chronic kidney disease.11
The main objective of this study was to determine the incidence of AKI and its risk factors in VLBW (born with less than 1500g) newborns admitted to the Neonatal Intensive Care Unit (NICU). Secondly, the authors analyzed the association between AKI and duration of invasive mechanical ventilation, length of stay (LOS), and death.
MethodsPopulationIn this retrospective cohort study, the authors included all the newborns with less than 1500g at birth admitted to the NICU of the Instituto da Criança e do Adolescente, Hospital da Clínicas of the Faculty of Medicine of the University of São Paulo, Brazil, from 1st January 2015 to 31st December 2017. The exclusion criteria included major congenital malformations, congenital infections from the TORCHS group, chromosomal abnormalities, babies with less than 2 serum creatinine determinations, admissions after 5 days of life, and death before 7 days of life.
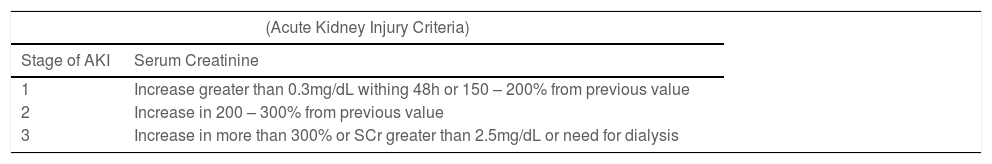
AKI definitionAKI definition was based on serum creatinine (SCr) determination (Table 1) according to the modified KDIGO criteria proposed by Koralkar et al.2 and lately by Jetton JG and Askenazi DJ.12 The urine output (UO) determinations were not included in the AKI definition in the present study.
In the center, the serum creatinine is measured by the colorimetric method of Jaffe, and the decision to collect serum creatinine depends on the attending neonatologist as there is no bundle related to screening for AKI.
Data collectionThe medical records of the included VLBW newborns were analyzed according to the following data: antenatal corticosteroids administration, type of delivery, gestational age (GA), birth weight (BW), gender, first and fifth minutes Apgar-score, and the presence of the following conditions traditionally associated with AKI: neonatal respiratory distress syndrome (RDS) (need of surfactant therapy), culture-proven neonatal sepsis, hemodynamically significant persistence of ductus arteriosus (PDA), necrotizing enterocolitis (NEC) (Bell´s Stage 2 or higher), hemodynamic instability defined as hypotension with the need of vasopressors or inotropes, use of vancomycin, amikacin, and ibuprofen, and need for dialysis. Small for gestational age at birth was defined as a birth weight of less than the 10th percentile at Fenton´s curve. The main outcome data were the duration of invasive mechanical ventilation, length of hospital stay, and death. The mother´s data included maternal conditions that could influence birth weight, such as diabetes mellitus, hypertension, and lupus.
Statistical analysesAll categorical variables were expressed as absolute values and percentage frequencies and were analyzed using Pearson´s chi-square test or Fisher´s exact test. All continuous variables were tested for normality with the Shapiro-Wilk test. Normally distributed variables were expressed as mean with the respective standard deviation (SD) and a two-sided Student´s t-test was performed to compare the means between the two groups. For non-normal distributions, the authors reported the medians, and interquartile-range and compared the groups using the Mann-Whitney U test.
Multivariate logistic regression was performed to identify the association between potential risk factors and AKI. Only the variables that were significantly different between the two groups (AKI vs non-AKI) were included in the model. To verify if they were highly related or not, before including them, the authors performed a diagnosis of multicollinearity. The Odds-Ratio was adjusted with a 95% confidence interval and only those with statistical significance were presented. A p-value less than 0.05 was considered significant.
All statistical analyses were performed using the IBM SPSS Statistics (v20.0) software for Windows.
Ethics approvalThis study was approved by the local ethics committee.
ResultsDuring the study period, there were 284 admissions of VLBW newborns. Of those, 75 newborns met exclusion criteria, of which 5 had TORCHS infections, 6 had chromosomal abnormalities, 26 had major congenital malformations, 34 died within 7 days of life, and 4 were admitted with more than 5 days of life. Fifty-four newborns had less than two SCr determinations and were, therefore, excluded. Of the remaining 155 newborns included in the study, a total of 61 (39.4%) patients had AKI.
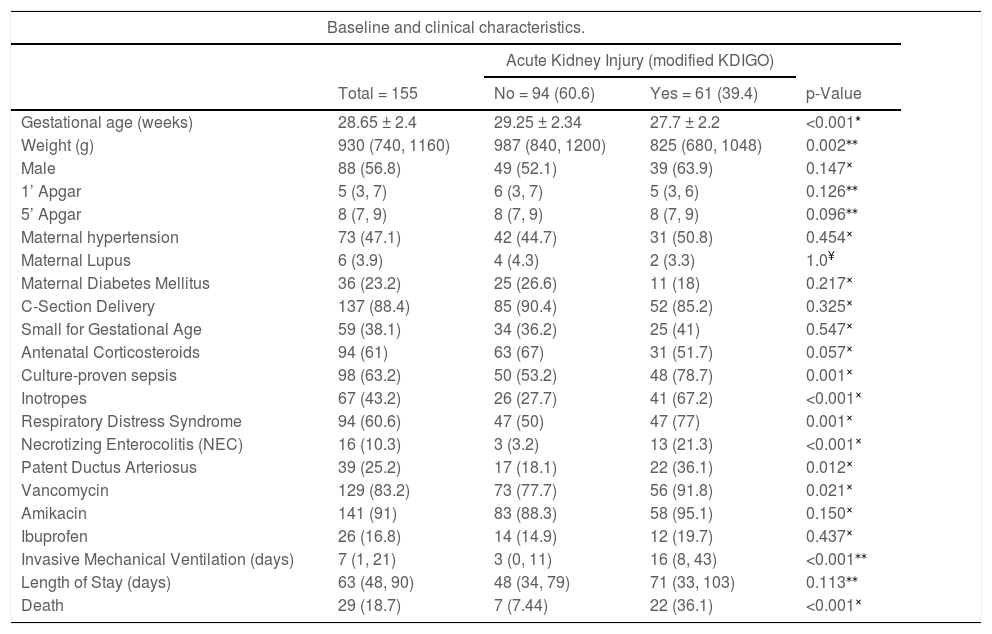
Table 2 summarizes the baseline and clinical characteristics of the study participants and the differences between the groups. Of note, 33 (54%) patients had AKI stage 1, 18 (29%) patients had AKI stage 2 and the remaining 16% had AKI stage 3, of which three received peritoneal dialysis (PD). The mortality rate increased according to the stage of AKI (30.3%, 38.9%, and 50%, respectively).
Demographic and clinical characteristics of the entire cohort, non-AKI group and AKI group.
| Baseline and clinical characteristics. | ||||
|---|---|---|---|---|
| Acute Kidney Injury (modified KDIGO) | ||||
| Total = 155 | No = 94 (60.6) | Yes = 61 (39.4) | p-Value | |
| Gestational age (weeks) | 28.65 ± 2.4 | 29.25 ± 2.34 | 27.7 ± 2.2 | <0.001* |
| Weight (g) | 930 (740, 1160) | 987 (840, 1200) | 825 (680, 1048) | 0.002⁎⁎ |
| Male | 88 (56.8) | 49 (52.1) | 39 (63.9) | 0.147× |
| 1’ Apgar | 5 (3, 7) | 6 (3, 7) | 5 (3, 6) | 0.126⁎⁎ |
| 5’ Apgar | 8 (7, 9) | 8 (7, 9) | 8 (7, 9) | 0.096⁎⁎ |
| Maternal hypertension | 73 (47.1) | 42 (44.7) | 31 (50.8) | 0.454× |
| Maternal Lupus | 6 (3.9) | 4 (4.3) | 2 (3.3) | 1.0¥ |
| Maternal Diabetes Mellitus | 36 (23.2) | 25 (26.6) | 11 (18) | 0.217× |
| C-Section Delivery | 137 (88.4) | 85 (90.4) | 52 (85.2) | 0.325× |
| Small for Gestational Age | 59 (38.1) | 34 (36.2) | 25 (41) | 0.547× |
| Antenatal Corticosteroids | 94 (61) | 63 (67) | 31 (51.7) | 0.057× |
| Culture-proven sepsis | 98 (63.2) | 50 (53.2) | 48 (78.7) | 0.001× |
| Inotropes | 67 (43.2) | 26 (27.7) | 41 (67.2) | <0.001× |
| Respiratory Distress Syndrome | 94 (60.6) | 47 (50) | 47 (77) | 0.001× |
| Necrotizing Enterocolitis (NEC) | 16 (10.3) | 3 (3.2) | 13 (21.3) | <0.001× |
| Patent Ductus Arteriosus | 39 (25.2) | 17 (18.1) | 22 (36.1) | 0.012× |
| Vancomycin | 129 (83.2) | 73 (77.7) | 56 (91.8) | 0.021× |
| Amikacin | 141 (91) | 83 (88.3) | 58 (95.1) | 0.150× |
| Ibuprofen | 26 (16.8) | 14 (14.9) | 12 (19.7) | 0.437× |
| Invasive Mechanical Ventilation (days) | 7 (1, 21) | 3 (0, 11) | 16 (8, 43) | <0.001⁎⁎ |
| Length of Stay (days) | 63 (48, 90) | 48 (34, 79) | 71 (33, 103) | 0.113⁎⁎ |
| Death | 29 (18.7) | 7 (7.44) | 22 (36.1) | <0.001× |
Shapiro-Wilk test was performed for all continuous variables: Only gestational age had normal distribution.
After performing multivariate logistic regression with the variables of interest, only necrotizing enterocolitis (aOR 7.61 [1.69 – 34.37]; p = 0.008), neonatal sepsis (aOR 2.91 [1.17 – 7.24], p = 0.021), and hemodynamic instability (aOR 2.99 [1.35 – 6.64]; p = 0.007), were statistically relevant to the model (Table 3). Linear regression was performed for the continuous variable mechanical ventilation and included AKI, neonatal sepsis, use of inotropes, NEC, PDA, birthweight, and gestational age as independent variables, as shown in Table 4. In the regression model, neonatal AKI was associated with an increase in the duration of mechanical ventilation in 9.4 days (p = 0.026). Table 4 also shows the results of a logistic regression analysis using the same variables from the linear regression which confirms the association of neonatal AKI to mortality (aOR 4.5 (1.46 – 14.01); p = 0.009) after adjusting for the other variables.
Logistic regression was performed with AKI as outcome.
Sex, Weight, RDS, PDA, and Vancomycin were also included with no statistical significance. Logistic regression model = [X² (8) = 46.610; p < 0.001; R² Cox & Snell = 0.26; R² Nagelkerke = 0.352].
aOR = adjusted odds ratio.
Logistic regression was performed for analysis of death and linear regression was performed for analysis of days of invasive mechanical ventilation and length of stay. Were included in the models: AKI, gestational age, weight, culture-proven sepsis, necrotizing enterocolitis, and PDA. We reported the values corresponding to the AKI factor only.
The present study is one of the few studies in Brazil that evaluated the rate of AKI and its risk factors in VLBW infants, based on the modified KDIGO criteria.2,12 In the cohort, the rate of AKI in VLBW newborns was 39.4%, similar to that observed by other authors.13 Jetton et al., in 2017, conducted a multicenter study that included 2022 newborns with an overall rate of neonatal AKI of 29.92%. However, in the subgroup of preterm infants younger than 29 weeks, the incidence was 48%.1 These authors used the urinary output as a diagnostic criterion of AKI, which could explain the higher rate compared to the present study. In studies that have not considered the urinary output in the definition of AKI, the frequency of AKI ranged from 18% to 39.8% of the cases.2,13
The present data shows that VLBW infants who developed AKI remained in mechanical ventilation for longer periods and presented 4.5 times higher mortality, after controlling for other confounding variables. These results highlight the importance of knowing the risk factors to prevent AKI. The increase in mortality in patients with AKI has been reported in other studies in the neonatal population, regardless of the AKI definition, gestational age, birth weight, and associated pathologies1,3,4,10,14 as well as the longer need for mechanical ventilation.4,14,15
Regarding the risk factors, in the AKI group, the patients were born with lower gestational age (27.7 vs 29.25 weeks, p < 0.001), and lower weight (825 vs 987 grams, p 0.002) when compared with the non-AKI group. Moreover, the AKI group had a higher rate of neonatal RDS (p 0.001), culture-proven neonatal sepsis (p 0.001), the persistence of ductus arteriosus (p 0.012), necrotizing enterocolitis (p < 0.001), hemodynamic instability with the need of inotropes or vasopressors (p < 0.001), and use of vancomycin (p 0.021). After logistic regression analysis, however, only necrotizing enterocolitis, hemodynamic instability, and neonatal sepsis showed significance as risk factors for developing AKI. To exclude a possible association between these variables, a multicollinearity diagnosis was performed before including them in the multivariate analysis.
VLBW newborns have glomerular underdevelopment, due to immaturity possibly associated with severe intrauterine growth restriction, and may be more susceptible to the development of kidney damage.6,11 Lower birth weight and lower gestational age are risk factors for developing AKI reported by several authors.3,10,14,16 In the present study, which focused only on VLBW newborns, these variables have not shown statistical relevance while the rates of enterocolitis, hemodynamic instability, and sepsis were high (10.3%, 43.2%, and 63.2%, respectively).
Necrotizing enterocolitis may be associated with neonatal AKI in almost 50% of the cases, particularly in newborns with low gestational age.17,18 The increase in abdominal pressure leading to a decrease in renal perfusion pressure is a possibly involved mechanism,19 as well as the release of bacterial toxins with renal tubular necrosis, and the administration of broad-spectrum antibiotics with a potential nephrotoxic effect.6,20 An association between sepsis and the development of AKI was also observed, in agreement with other similar studies.7,21 Very low birth weight and most premature newborns often have a greater need for invasive devices (umbilical catheters, peripherally inserted central catheter) and, consequently, a greater risk of nosocomial infection.22 Sepsis may lead to target organ damage, secondary to the inflammatory process, with the release of inflammatory cytokines, increased activity of leukocyte cells, and cell damage.23
In the past few years, several studies have been published focusing on risk factors and outcomes related to neonatal AKI. However, only a few focused on VLBW newborns, with different criteria to define AKI among them.2,3,7,13-15 In developing countries, such as Brazil, it is of great importance to know the risk factors for AKI in VLBW newborns, to improve its diagnosis and management in the NICU to minimize sequelae. Moreover, a wide variation in the attainment of serum creatinine in different centers resulted in an incidence of AKI varying from 3 to 74% in a multicenter study,1 enlightening the importance of knowing the local incidence and associated risk factors.
The exclusion of 54 (25%) newborns from the original cohort, who had less than two SCr determinations, may be considered a major limitation of the present study, as it may have impacted the rate of AKI, when compared to similar studies that used the same AKI definition. The inadequate frequency of SCr sampling appears to be a common obstacle to the diagnosis of AKI in newborns. In the AWAKEN study, the number of patients with less than two SCr determinations was 561 (28%).1 In the same study, they found that some centers had a median of one SCr determination by the patient. Additionally, the rate of AKI was higher in the centers with more SCr attainment. In the study by Stojanovic et al., 148 (38%) newborns were excluded from the initial cohort for the same reason.4 Furthermore, a recent study found that less than 50% of pediatricians and neonatologists were aware of the AKIN and KDIGO criteria to define AKI in this age group,24 which may explain the low frequency of serial SCr attainment in the AKI - susceptible newborns.
Another limitation that can be cited is the small sample size of the cohort secondary to its nature as a single-center study. This fact may have led to missing other risk factors that are less common. The authors also chose to exclude infants with major risk factors as malformations, to try to identify the acquired conditions related to neonatal AKI.
In the present study, AKI diagnosis was performed exclusively with SCr determination. The urinary output (UOP) was not included because of the difficulties related to its estimation. It is not the practice in the NICU to routinely use an indwelling urinary catheter, and the use of diaper weight to quantify the urinary output may be associated with measurement bias. Moreover, there is no consensus about the cutoff to define oliguria and risk of AKI in this population25 and VLBW newborns often present with non-oliguric acute kidney injury, as observed by Daga et al., in a study of 115 VLBWs with AKI, in which none of them had oliguria.7
To our best knowledge, the present study is the largest single-center study in Latin America that analyzed the risk factors associated with neonatal AKI in the VLBW population. The present findings are consistent with the existing literature. The authors hope the present study can enhance the awareness of neonatologists and pediatric intensivists about the importance of serial SCr determination in the follow-up of susceptible newborns for the prevention and early diagnosis of neonatal AKI as it is related to the worst outcomes in these populations.
The incidence of neonatal AKI, based on serial SCr evaluation, proved to be 39.4% among VLBW newborns with at least two SCr determinations. Neonatal AKI was associated with a 4-times increase in mortality and a longer duration of invasive mechanical ventilation.
In the cohort, after adjusting for potential confounders in the logistic regression, necrotizing enterocolitis, neonatal sepsis, and hemodynamic instability were associated with neonatal AKI. The present results highlight the importance of minimizing sepsis and necrotizing enterocolitis, as well as the importance of identifying hemodynamic instability, to prevent AKI and diminish the burden of morbimortality in VLBW newborns.