Changes in the epidemiology of respiratory infections during the restrictions imposed as a response to the coronavirus disease 2019 (COVID-19) pandemic have been reported elsewhere. The present study's aim was to describe the prevalence of a large array of respiratory pathogens in symptomatic children and adolescents during the pandemic in Southern Brazil.
MethodsHospitalized and outpatients aged 2 months to 18 years with signs and symptoms of acute COVID-19 were prospectively enrolled in the study from May to November 2020 in two hospitals in a large metropolitan area in a Brazilian city. All participants performed a real-time PCR panel assessing 20 respiratory pathogens (three bacteria and 17 viruses).
Results436 participants were included, with 45 of these hospitalized. Rhinovirus was the most prevalent pathogen (216/436) followed by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, 97/436), with a coinfection of these two viruses occurring in 31/436 participants. The remaining pathogens were found in 24 symptomatic participants (adenovirus, n = 6; Chlamydophila pneumoniae, n = 1; coronavirus NL63, n = 2; human enterovirus, n = 7; human metapneumovirus, n = 2; Mycoplasma pneumoniae, n = 6). Hospitalization was more common among infants (p = 0.004) and those with pathogens other than SARS-CoV-2 (p = 0.001).
ConclusionDuring the period of social distancing in response to COVID-19, the prevalence of most respiratory pathogens was unusually low. Rhinovirus remained as the main virus co-circulating with SARS-CoV-2. COVID-19 in symptomatic children was less associated with hospitalization than with other respiratory infections in children and adolescents.
The coronavirus disease 2019 (COVID-19) pandemic was the most important public health crisis of the 21st century. The efforts to contain the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission had a great impact on person-to-person interactions, including the widespread use of masks, hand hygiene, social distancing, and school closures. These changes provide a unique opportunity to improve the knowledge about the spreading of common community infectious agents and how restrictions impact communicable diseases.1
As respiratory pathogens such as viruses and bacteria are mainly transmitted through droplets, aerosols, and fomites, a decrease in the transmissions of infectious agents other than SARS-CoV-2 could be foreseen.2
The epidemiology of respiratory infections in Southern Brazil follows quite predictable seasons every year. Most respiratory viral infections have a peak in incidence around July, with a respiratory syncytial virus (RSV) as the most detected virus in children with clinically significant lower respiratory tract infections.3–5 In 2020, RSV and influenza detections and overall bronchiolitis diagnosis had an unprecedented decline, very different from what happened in previous usual seasons.6,7 Further, data from several countries also indicated a sharp decline in the detections of these pathogens in 2020.8
Although SARS-CoV-2 detections predominated during 2020 in most reports, many individuals tested negative. This can be a result of a lower threshold for testing during the pandemic and RT-PCR false-negative results for SARS-CoV-2.9,10 It could also be related to other respiratory pathogens, despite all restrictions mentioned above. Moreover, it is well established that COVID-19 in children and adolescents has been associated with a clinical course usually milder than in adults.11 From a pediatrics perspective, knowledge about other circulating respiratory pathogens other than SARS-CoV-2 is important, as these may be potentially more harmful, especially in young infants.12,13
The aim of the present study was to assess the epidemiology of common community-acquired respiratory pathogens in children who presented in outpatient clinics or emergency rooms with symptoms of lower respiratory symptoms in 2020.
Materials and methodsParticipants' selectionA cohort study was performed in two hospitals in Porto Alegre, Southern Brazil (Hospital Moinhos de Vento and Hospital Restinga e Extremo Sul). The first is a private tertiary hospital, and the second is a public hospital. Participants aged 2 months to 18 years were prospectively enrolled when seeking care at emergency rooms (ERs), outpatient clinics, or hospitalized in general wards or intensive care units (ICU) from May to later November 2020. The main inclusion criteria were the presence of at least one sign or symptom suggestive of COVID-19 (cough, fever, or sore throat). The key exclusion criterion was a failure in collecting a viable sample.
Study proceduresData collection and follow upAt enrollment in the study, after a properly signed consent was obtained, clinical and demographic data, comorbidities, and signs and symptoms suggestive of COVID-19 were collected, as well as biological samples, as specified in the pathogen detection kit. All participants were followed through phone calls up to 28 days from inclusion. The children's legal caregivers answered the questions regarding the hospitalization outcomes from the study inclusion in the ER (hospital admission, use of supplemental oxygen, admission to the ICU, use of invasive mechanical ventilation, and death). All data collection was performed in standardized questionnaires developed for this study in the Research Electronic Data Capture software (REDCap), and the interviews were performed by trained researchers.
The follow-up interviews were done at 7, 14 and 28 days from inclusion; up to ten phone call attempts were made at different times of the day, within three days of each follow-up date. The questions were asked retrospectively on the next scheduled interview when no contact was possible at D7 and D14. The losses were defined as no success in contacting within the 28-day follow-up.
Pathogen detectionAt inclusion, oropharyngeal and bilateral nasopharyngeal swab collection for SARS-CoV-2 detection was performed for all participants and analyzed through qualitative reverse transcription-polymerase chain reaction (RT-PCR) assay. RT-PCR assay was performed using PathTM 1-Step RT-qPCR Master Mix, CG (catalog number A15299, AppliedBiosystems, Frederick, Maryland, USA), and TaqManTM 2019-nCoV Assay Kit v1 (catalog number A47532, ThermoFisher Scientific, Pleasanton, California, EUA) in 10 µL total reaction, of which 5 µL were RNA, as described elsewhere.14,15
A second bilateral nasopharyngeal swab was collected at inclusion and stored at -80 °C and analyzed for all pathogens at the same time through the real-time PCR respiratory panel. The panel assessed the presence of a range of common community-acquired respiratory pathogens: three bacteria (Bordetella pertussis, Chlamydophila pneumoniae, Mycoplasma pneumoniae) and 17 viruses (adenovirus; bocavirus; coronavirus types HKU1, 229E, NL63, and OC43; influenza A virus types H1 and H3; influenza B virus; human enterovirus; human metapneumovirus; parainfluenza virus types 1, 2, and 3; RSV types A and B; and rhinovirus). Acid nucleic was extracted using MagMax™ Viral/Pathogenic Nucleic Acid Isolation (Applied Biosystems) in the KingFisher Duo Prime System platform (ThermoFisher, USA). All samples were quantified by NanoDrop™ Lite Spectrophotometer (ThermoFisher, Wilmington, Delaware, USA) and diluted between 0.5 and 2.0 ng/µL. RT-PCR assay was performed using the PathTM 1-Step RT-qPCR Master Mix CG (A15299, Applied Biosystems) and TaqMan® Microbial Assays-single tube assay (Applied Biosystems, Pleasanton, California, USA), in which the probe access code for the target pathogens are listed in Supplementary Table 1. TaqMan®Respiratory Tract Microbiota Amplification Control (A39178, ThermoFisher) was used for reaction control.
All the samples were analyzed in the Molecular Biology Laboratory at Hospital Moinhos de Vento. The SARS-CoV-2 results were available to participants and physicians within 48 h. However, the multiplex panels were analyzed after the end of the inclusions, and these data were not available for assistance purposes nor to the attending physicians.
Statistical analysisData normality assumptions were verified for continuous variables, and median values and interquartile ranges (IQR) were calculated. Percentages were used to describe categorical variables, and Pearson's Chi-square or Fisher's exact tests were used to evaluate the association between the pediatric hospitalized and outpatients in relation to the clinical information.
Descriptive analyses were performed considering: the proportion of detected pathogens according to age groups, the absolute frequency of the epidemiological weeks from May to November (dates always represent a Monday), and the absolute frequency of coinfections. The risk of hospitalization was assessed using a multivariable logistic regression model with adjustment for relevant covariates (institution at inclusion (public or private), categorial age (<5 years or ≥5 years), and the presence of asthma). The power estimation was performed using the observed values from the institutions at inclusion according to the groups (outpatients and hospitalized). The "ES.w2" function was used to calculate the effect size, and then "pwr.chisq.test" function with α = 0.05 (both from the "pwr" package) was applied. All data preprocessing and analyses were performed in R 3.5.0 statistical software (R Core Team, 2017, https://www.R-project.org).
Ethical approvalThe study was performed in accordance with Decree 466/12 of the National Health Council and the Good Clinical Practice Guidelines after approval by the Hospital Moinhos de Vento Institutional Review Board (IRB n° 4.637.933). Legal caregivers provided signed consent and authorization for their child's participation.
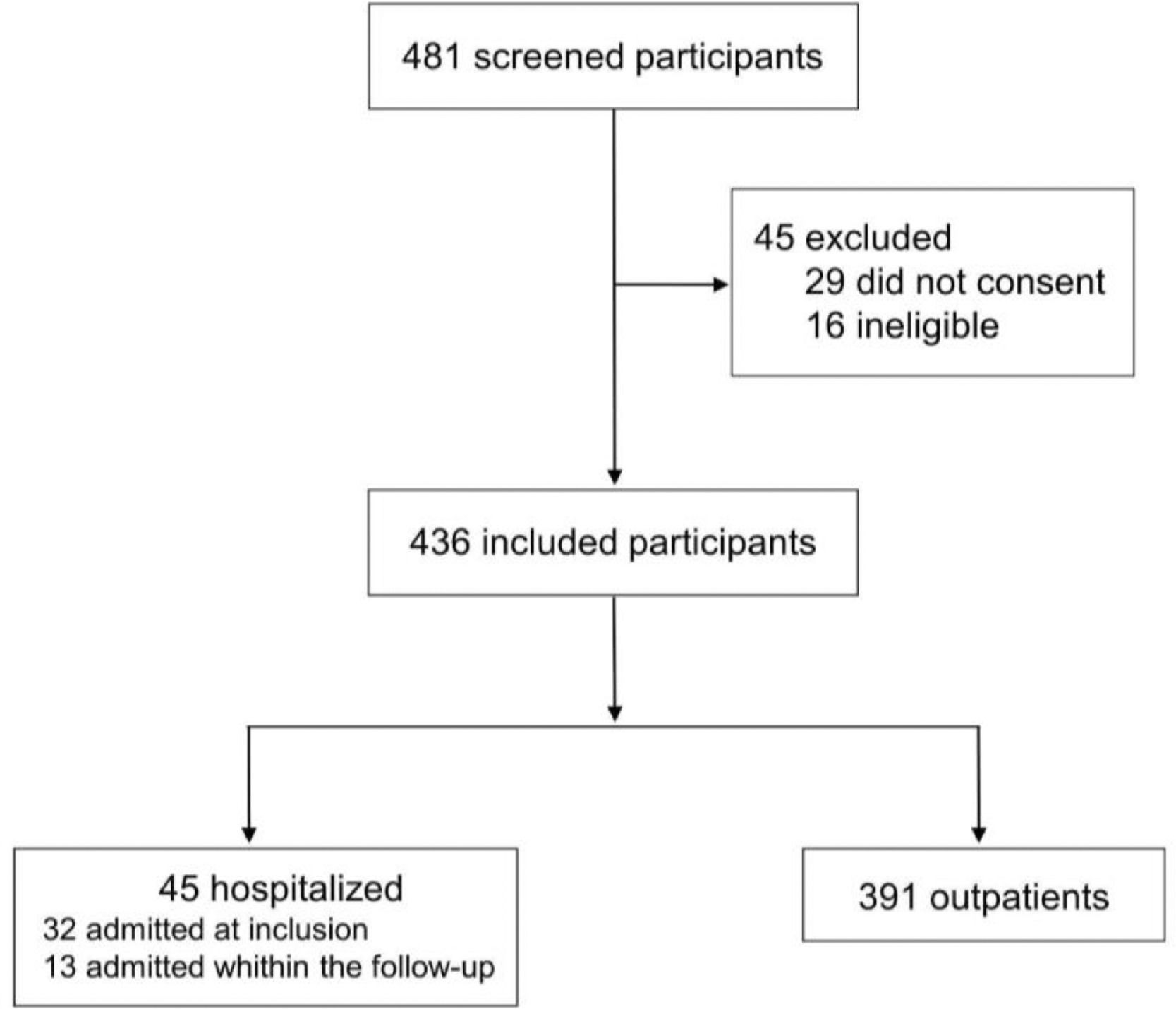
ResultsIn this study, 481 participants were screened, and 45 were excluded (29 for not consenting and 16 for not meeting inclusion criteria). A total of 436 children were included; of these, 45 (10.3%) subjects required hospitalization (32 were admitted at inclusion, and 13 were hospitalized within the 28-day follow-up period); 377 (86.5%) subjects were included as outpatients only, as shown in Figure 1. The success in the 28-days follow-up to identify hospitalization outcomes was obtained for 422 (96.8%) participants, with the remaining 14 (3.2%) considered lost to follow-up. Sixty-five legal caregivers answered the questions retroactively in the follow-up interview.
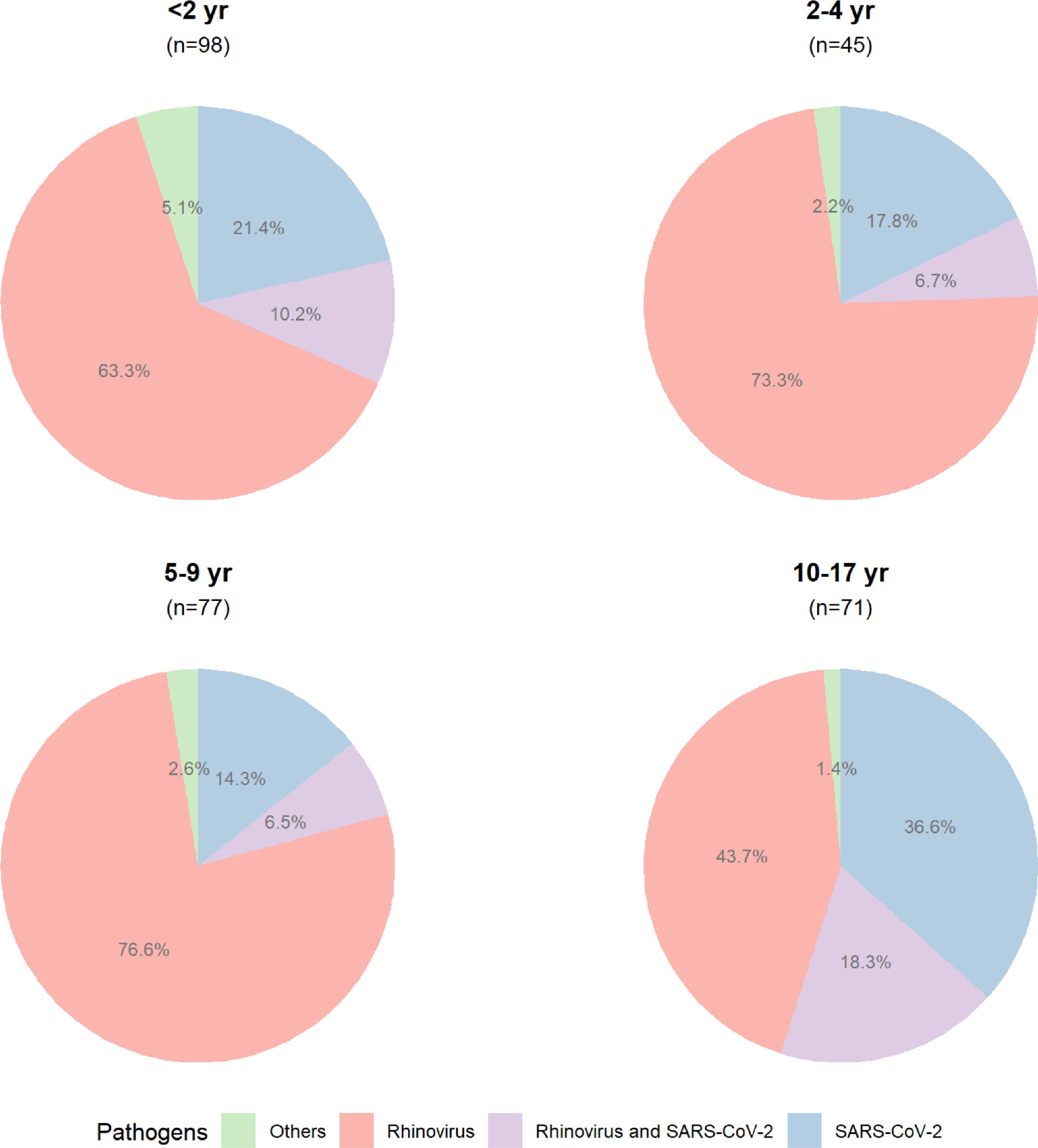
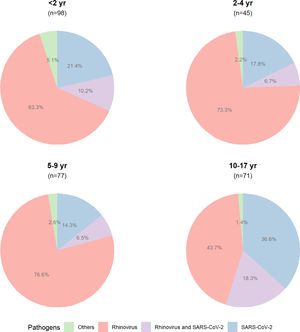
At baseline, the median age of the participants was 5.4 years (IQR, 2.0–10.2, range 0.2–17.3), 53.4% (233/436) were female, and the median days of symptoms onset to inclusion was 3.0 (IQR, 1.0–4.0, range 0.0–14.0), as shown in Supplementary Table 2. Almost half of the participants (49.5%, 216/436) were diagnosed positive for rhinovirus, followed by SARS-CoV-2 (22.2%, 97/436). The remaining pathogens were found in 24 participants (adenovirus, n = 6; Chlamydophila pneumoniae, n = 1; coronavirus NL63, n = 2; human enterovirus, n = 7; human metapneumovirus, n = 2; Mycoplasma pneumoniae, n = 6). The coinfection of rhinovirus and SARS-CoV-2 occurred in 7.1% (31/436) of participants. There was no detection of Bordetella pertussis, bocavirus; coronavirus types HKU1, 229E, and OC43; influenza A virus types H1 and H3; influenza B virus; parainfluenza virus types 1, 2, and 3; and RSV types A and B. The total number of tested pathogens and the frequency of detected ones are shown in Supplementary Table 3.
COVID-19 was negatively associated with hospitalization in children and adolescents; there were 91 outpatients who were positive for SARS-CoV-2 compared to the only child that was admitted (24.1% vs. 2.2%, p = 0.001), as shown in Table 1. A greater proportion of children attended in the public institution were admitted, while in the private setting, most children were attended as outpatients rather than hospitalized (p < 0.001), with an estimated power of 100%. At the 28-day follow-up, 45 (10.3%) participants were hospitalized, especially those younger than five years (31/45, 68.9%) when compared with other age groups (p = 0.006). In a multivariable model, children younger than 5 years from a public hospital were independently associated with a greater risk for hospital admission (age: OR = 1.09, 95%CI 1.03–1.15, p = 0.002; public hospital: OR = 1.11, 95%CI 1.05–1.18, p < 0.001). The presence of asthma was not relevant for hospitalization in the cohort (OR = 1.03, 95%CI 0.96–1.12, p = 0.408). The interaction of age and public institution remained an important predictor of hospital admission (OR = 1.12, 95%CI 1.00–1.25, p = 0.047).
Clinical and demographic characteristics, and pathogen detection. (yr) years.
| Characteristics | Outpatients | Hospitalized | p value |
|---|---|---|---|
| (n = 377) | (n = 45) | ||
| Female sex, n (%) | 199 (52.8) | 25 (55.6) | 0.846b |
| Caucasian, n (%) | 273/375 (72.8) | 28/42 (66.7) | 0.509b |
| Institution at inclusion | |||
| Private, n (%) | 201 (53.3) | 10 (22.2) | < 0.001b |
| Public, n (%) | 176 (46.7) | 35 (77.8) | |
| Categorical age | |||
| < 2 yr, n (%) | 123 (32.6) | 19 (42.2) | 0.006b |
| 2-4 yr, n (%) | 48 (12.7) | 12 (26.7) | |
| 5-9 yr, n (%) | 102 (27.1) | 10 (22.2) | |
| 10-17 yr, n (%) | 104 (27.6) | 4 (8.9) | |
| Pathogens | |||
| Adenovirus | 6/375 (1.5) | 0 (0.0) | 1.000c |
| Chlamydophila pneumoniae | 0/375 (0.0) | 1 (2.2) | 0.107c |
| Coronavirus NL63 | 2/375 (0.5) | 0 (0.0) | 1.000c |
| Enterovirus | 5/375 (1.3) | 2 (4.4) | 0.167c |
| Metapneumovirus | 1/375 (0.3) | 1 (2.2) | 0.203c |
| Mycoplasma pneumoniae | 5/375 (1.3) | 1 (2.2) | 0.496c |
| Rhinovirus | 179/375 (47.7) | 27 (60.0) | 0.162b |
| SARS-CoV-2 | 91 (24.1) | 1 (2.2) | 0.001b |
| Coinfections | |||
| Rhinovirus and SARS-CoV-2 | 28 (7.4) | 1 (2.2) | 0.344c |
| SARS-CoV-2 and enterovirus | 1 (0.3) | 0 (0.0) | 1.000c |
| Rhinovirus and othersa | 12 (3.2) | 3 (6.7) | 0.208c |
| None pathogens | 128 (34.0) | 16 (35.6) | 0.962b |
| Underlying medical conditions | |||
| Asthma, n (%) | 55 (14.6) | 9 (20.0) | 0.461b |
| Diabetes mellitus, type 1, n (%) | 1 (0.3) | 0 (0.0) | 1.000c |
| Obesity, n (%) | 1/215 (0.5) | 0 (0.0) | 1.000c |
| Hypertension, n (%) | 1 (0.3) | 0 (0.0) | 1.000c |
Twenty-one (46.7%) children out of 45 required only supplemental oxygen, and four (8.9%) were admitted to ICU. The need for respiratory support from those children admitted was similar in both hospitals, public and private (19/35, 54.3% vs. 3/10, 30%, p = 0.284, respectively). The median of the onset of symptoms to hospital admission was 2.0 days (IQR, 1.0–5.2). For the whole cohort, there were no deaths detected, nor children needing invasive mechanical ventilation (Supplementary Table 2).
The median time from onset of symptoms to enrollment for outpatients and hospitalized was similar (3.0 (2.0–4.0) vs. 2.0 (1.0–4.0) days; p = 0.269), as was the use of azithromycin at inclusion (3.4%, 13/377 vs. 2.2%, 1/45; p = 1.000), respectively. In contrast, the use of antibiotics other than azithromycin (2.1%, 8/377 vs. 11.1%, 5/45; p = 0.007) was higher in hospitalized children at inclusion. Outpatients were more immunized for influenza than hospitalized participants, 62.9% (246/377) vs. 35.6% (16/45); p < 0.001, respectively.
The most commonly reported symptoms for outpatients and hospitalized were related to the respiratory tract (cough, coryza, stuffy nose or sputum production) and these were present in 88.6% (333/376) vs. 88.9% (40/45); discomfort (myalgia, malaise, fever or chills) in 84.5% (317/375) vs. 95.6% (43/45); and to olfactory-gustatory disorders (dysgeusia, anosmia or appetite loss) in 58.2% (195/335) vs. 64.3% (27/42), similar in both groups, respectively. Headache (55.2%, 180/326 vs. 21.6%, 8/29; p < 0.001) and sore throat (43.6%, 147/337 vs. 20.5%, 8/39; p = 0.009) were more associated with outpatients. Hospitalized participants presented more symptoms as dyspnea (31.5%, 117/372 vs. 65.1%, 28/43; p < 0.001), nausea (26.0%, 94/362 vs. 44.2%, 19/43; p = 0.019) and vomiting (20.2%, 76/377 vs. 42.2%, 19/45; p = 0.002) than outpatients. The percentage of diarrhea (25.6%, 96/375 vs. 31.1%, 14/45), conjunctivitis (20.1%, 75/374 vs. 8.9%, 4/45) and skin rash (7.5%, 28/375 vs. 6.8%, 3/44) did not differ between the outpatients and hospitalized participants, respectively, as described.
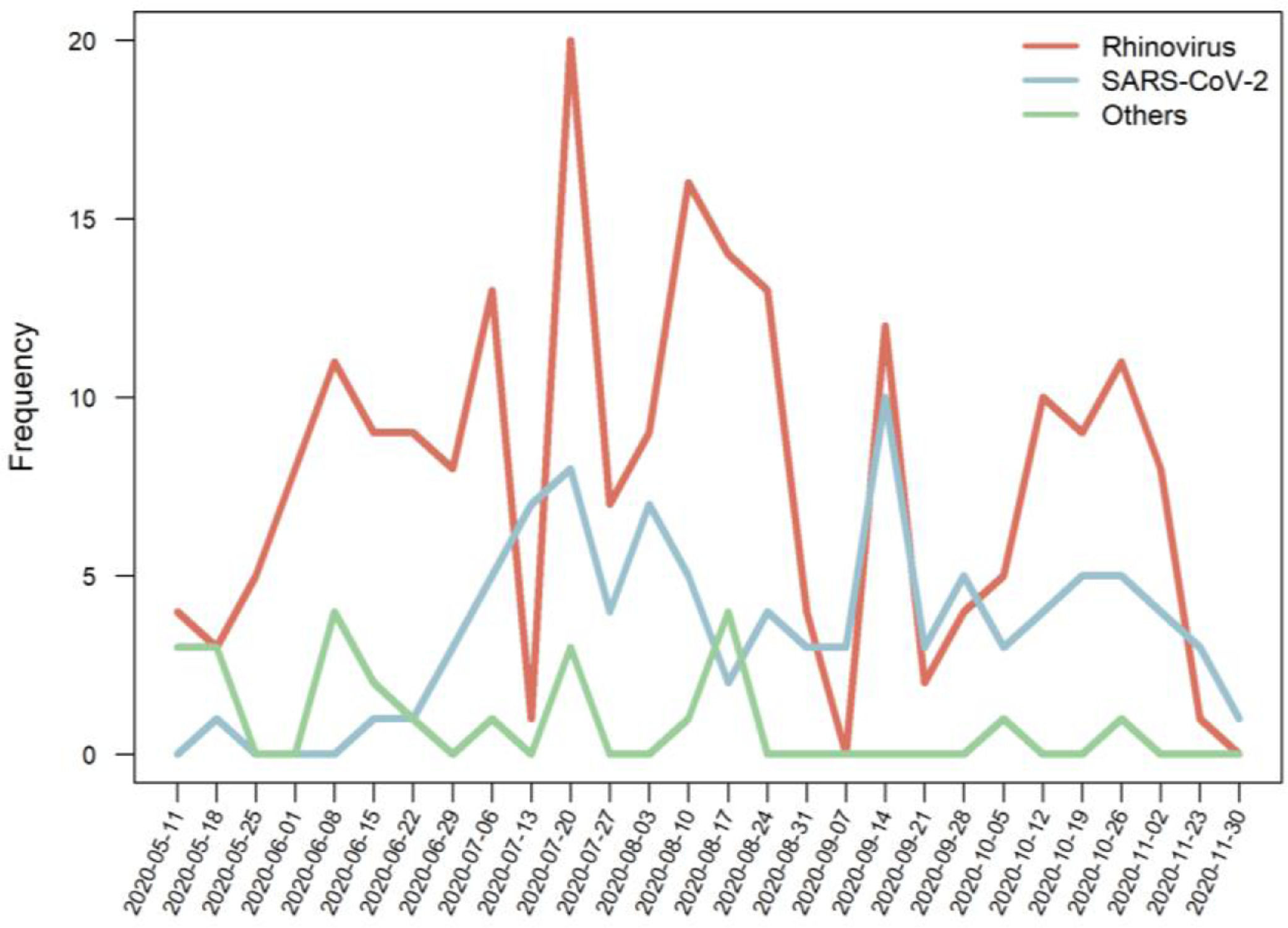
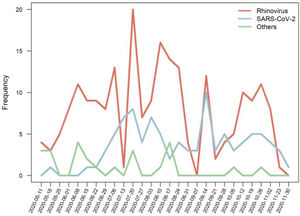
The proportion of detected pathogens, according to the age group, is shown in Figure 2, and the frequencies of detected pathogens are in Figure 3.
The frequencies of coinfection by other pathogens (adenovirus, Chlamydophila pneumoniae, coronavirus NL63, enterovirus, metapneumovirus, Mycoplasma pneumoniae) and rhinovirus, and SARS-CoV-2 are shown in Supplementary Fig. 1.
DiscussionDuring the period of restrictions in response to COVID-19, rhinovirus was the most detected respiratory pathogen in children and adolescents in the present study, followed by SARS-CoV-2. Respiratory infections other than COVID-19 were more associated with hospitalizations.
Despite wide variations in the seasonality of respiratory pathogens compared to different settings, both temperate and tropical areas usually have quite predictable epidemiologies throughout the year in southern Brazil.3,5 Moreover, RSV is usually the most prevalent virus among children with lower respiratory tract infections, sharing an important burden with rhinovirus, metapneumovirus, influenza, and adenovirus.16,17 However, social restrictions in response to COVID-19 pandemic led to important changes in people interactions, and changes in the epidemiology of communicable diseases have been reported in many countries. Of note, the incidence of many of the viruses mentioned above, such as RSV and influenza, had a sharp decline during the period of more rigid restrictions6,7,18,19 throughout the world. It is well recognized in the locale that RSV is the most prevalent virus in the winter season.4 Restrictions started in March 2020, and included social distancing, hand hygiene measures, mandatory use of masks, restrictions on commerce activities, and the closure of all schools. The present data highlight the occurrence of completely unusual epidemiology during the COVID-19 pandemic, with a significant presence of the rhinovirus and SARS-CoV-2 across all pediatric age groups.
Interestingly, rhinovirus showed a decline in the detection after restriction measures but an earlier relapse or even a non-decline in some settings, mainly through surveillance studies.1,20,21 Rhinovirus has been reported as a major pathogen in both upper and lower respiratory tract infections in childhood.17 The reasons for the persistence of rhinovirus in the community is not completely understood. The increased frequency of rhinoviruses compared to SARS-Cov2 and other viruses may be due to the fact that rhinovirus is a non-enveloped RNA virus, which may increase its resistance and be detected in environments for a long time.21,22 Nonetheless, studies assessing the survival of respiratory viruses on surfaces have not shown striking differences among pathogens.23
Although many reports focus on the low circulation of RSV and influenza, the decline in detections of other pathogens such as adenovirus, Bordetella pertussis and Mycoplasma pneumoniae is also important to address.1 Although some reports described the coinfections between SARS-CoV-2 and some of these pathogens, most findings suggest that the occurrence is relatively low,24 possibly due to the similarity in transmission routes of these respiratory pathogens.6
In line with most reports, COVID-19 in children was usually a mild disease in the present study. The detection of SARS-CoV-2 in the pediatric population was less frequent among hospitalized children compared to other agents, including rhinovirus. Although respiratory viruses are the main drivers of lower respiratory infection in children, especially in young infants, COVID-19 in this population has been associated with a milder clinical course.11 The reasons for such differences are not fully understood, although lower expression of ACE2 receptors and a more efficient innate immune response have been raised as possible mechanisms.25
Another main finding of the present study is that the pediatric population, especially the children under 5 years, who attended at a public hospital (thus, of lower socio-economic stratum) presented a significantly higher risk of hospitalization. These results are in accordance with the findings on other respiratory viruses such as RSV, in which a great burden for morbidity and mortality is observed in low-middle income countries.26 Inequalities in health care are a reality in poor neighborhoods, even in developed countries.27 Therefore, more and better information in this regard enables policy changes.
The present study has some limitations worth mentioning. First, similar data from the previous seasons are not available for comparison. However, compared to any data collected before 2020 from the same region, the differences are striking, with a large predominance of RSV, as mentioned above. Second, the inclusion criteria of the present study were quite loose, which allows for the speculation that some participants with other agents or even with non-infectious diseases could have been included. As all participants were tested just once and false-negative results can occur, some individuals might be misclassified, especially for SARS-CoV-2.9,10 As one group of children was recruited when they were already hospitalized, comparison with outpatients in relation to severity can be subject to some bias. Also, detection of rhinovirus, as well as other respiratory viruses, does not necessarily mean a causal relationship and may reflect only a bystander due to prolonged shedding.28,29 However, as individuals enrolled were acutely ill, it should not be the case for most of them. Finally, rhinovirus was not subtyped, and the impact of potentially virulent strains, such as type C, cannot be assessed.30 Despite the limitations mentioned above, this study provides important information about the epidemiology of respiratory viruses in periods of social distancing.
ConclusionThe period of social distancing during the COVID-19 pandemic led to important changes in the prevalence of most respiratory pathogens. Rhinovirus was the main circulating virus and must be considered in differential diagnosis with COVID-19 in children and adolescents.
COVIDa study groupAmanda Paz Santos, Fernanda Lutz Tolves, Shirlei Villanova Ribeiro.
FundingThis work was supported by the Brazilian Ministry of Health through the Institutional Development Program of the Brazilian National Health System (PROADI-SUS) in collaboration with Hospital Moinhos de Vento.
The authors thank the Scientific Committee of the Núcleo de Apoio à Pesquisa (NAP) of Moinhos de Vento Hospital for technical-scientific consultancy. We thank the assistance team, laboratory team, and local staff from Hospital Moinhos de Vento and from Hospital Restinga e Extremo Sul. We also thank the support from the coordinators and the staff of the Brazilian National Immunization Program and the Coordination of Surveillance of Respiratory Transmitted Diseases and Chronic Conditions from the Brazilian Ministry of Health. We thank Adriane Isabel Rohden, Camila Dietrich, Charles Francisco Ferreira, Elvira Alicia Aparicio Cordero, Jaina da Costa Pereira, and Thainá Dias Luft for their contributions.