The use of parenteral nutrition (PN) formulations that do not contain iodine can contribute to the deficiency of this mineral, potentially leading to hypothyroidism and, ultimately, neurocognitive impairments. This study aimed to evaluate TSH alterations in newborns receiving PN.
MethodsRetrospective study of neonatal intensive care unit patients receiving PN for > 15 days. Nutritional, anthropometric, and biochemical variables (TSH, T4, CRP) were analyzed. Hypothyroidism was defined by TSH > 10 mU/L.
ResultsTwo hundred newborns were evaluated [156 (78%) preterm, 31±5 weeks of gestational age, 112 (56%) with very or extremely low birth weight]. The median (IQR) hospital stay was 68 (42-110) days, PN duration was 31 (21-47) days, and 188 (94%) patients also received enteral nutrition. Overall, 143 (71.5%) newborns underwent at least one TSH measurement. The prevalence of hypothyroidism was 10.5%. The Median PN duration in this group was 51 (34-109) days. Among those with hypothyroidism, 10 received Lugol's solution and six levothyroxine. Thirteen patients received prophylactic Lugol's solution with a median PN duration of 63 (48-197) days. TSH levels correlated positively with PN duration (r = 0.19, p = .02).
ConclusionsThe present data suggest that changes in TSH and T4 levels are present in neonates receiving PN for > 15 days, suggesting this population may be at risk for developing hypothyroidism. Therefore, the authors suggest that TSH and T4 measurements should be included as routine in neonatal patients receiving PN for > 15 days if PN formulations are not supplemented with iodine, and that iodine supplementation be provided as necessary.
The mineral iodine has an integral role in the synthesis of thyroid hormones, such as thyroxine (T4) and tri-iodothyronine (T3).1 These hormones participate in the regulation of several metabolic pathways, including energy expenditure, body temperature, musculoskeletal growth and development, and cognition.1,2 Iodine deficiency in infants, especially in neonates, can contribute to neurological impairment and mortality.1,3-5
Daily iodine requirements in neonates are usually met by breastfeeding or enteral formulas, making hypothyroidism due to iodine deficiency uncommon in this population.6 Patients unable to feed are particularly at risk of iodine deficiency, especially those dependent on prolonged parenteral nutrition (PN).1,6-8 In the past, skin and catheter antisepsis were performed with povidone-iodine solutions which provided an iodine supply through transcutaneous absorption.1,6,7,9 Since these solutions were replaced by chlorhexidine, which does not contain iodine, supplementation has become necessary. International societies recommend that preterm newborns on PN should be offered a daily iodine intake of 1–10 mcg/kg/day.7,8
Iodine deficiency can be identified by alterations in thyroid-stimulating hormone (TSH) (mU/L), thyroglobulin (mcg/dL), and urinary iodine concentration (UIC), (mcg/L), and the presence of goiter on inspection or ultrasound.1 TSH level higher than 10 mU/L are diagnostic of hypothyroidism, which can be managed clinically with iodine and T4 supplementation.10
There are reports of iodine deficiency and hypothyroidism developing after prolonged PN in infants.11-15 The prevalence of hypothyroidism among children receiving prolonged PN (> 6 months) can be as high as 33%.11 There is not a cut-off point defined by the literature regarding to the risk of developing iodine deficiency and hypothyroidism in neonates receiving PN during a shorter period in the intensive care unit and there are no standardized recommendations concerning iodine monitoring or the optimal time to initiate supplementation. Therefore, considering the paucity of data regarding thyroid function in newborns receiving short-term PN, the aim of the present study was to evaluate the prevalence of thyroid abnormalities in newborns receiving PN (exclusively or not) for ≥15 days and identify related factors.
Materials and methodsThis retrospective study included newborns admitted to the Neonatal Intensive Care Unit of Hospital de Clínicas de Porto Alegre, a tertiary teaching hospital and referral center for high-risk pregnancies in Southern Brazil, between May 2015 and July 2020. The hospital has a dedicated Nutrition Support Team responsible for auditing all PN formulations prescribed in the hospital; newborns receiving PN were screened from the audit sheets. All those receiving PN (exclusively or not) for 15 days or more were eligible. All newborns were submitted to a neonatal screening test and patients with a positive screening test for congenital hypothyroidism and/or cervical or thyroidal malformations were excluded. After this initial screening, TSH dosages were at the discretion of the attending physician according to the time of parenteral nutrition and the patient's clinical condition.
Demographic and clinical data were collected from medical charts and included age, sex, ethnicity, prenatal and birth information, and nutritional and anthropometric data. Gestational age was defined by the date of the last reliable menstruation or obstetric ultrasound before 12 weeks of gestation or, in the absence of these data, by neonatal clinical examination using the New Ballard or Capurro methods.16,17 Adequacy of weight for gestational age according to Alexander's classification (adequate, small, or large for gestational age), as well as neonatal complications associated with prematurity such as necrotizing enterocolitis and culture-proven sepsis, were recorded.18
Data regarding PN therapy (age at initiation, whether supplemental or exclusive, duration, indication) and iodine or levothyroxine supplementation (if indicated) were also reviewed. Nutrient targets (enteral or PN) were determined individually and prescribed by the NICU staff according to each patient's clinical condition. Serum levels of TSH (mU/L), free T4 (ng/dL), and C-reactive protein (CRP) (mg/L) were reviewed from patient records.
Two different biochemical methods were used to analyze TSH and free T4 during the study. TSH was determined by chemiluminescence microparticle immunoassay (reference range, 0.27–4.2 mU/L) from May 2015 to September 2019 and by electrochemiluminescence sandwich immunoassay (reference range, 0.35–4.94 mU/L) from October 2019 to July 2020. Free T4 was analyzed by a chemiluminescence microparticle immunoassay (reference range, 0.93–1.7 ng/dL) and by competitive electrochemiluminescence immunoassay (reference range, 0.7–1.48 ng/dL). TSH values were considered abnormal when > 4.20 or > 4.94 mU/L, according to the method used, hypothyroidism was diagnosed when TSH was > 10.0 mU/L and subclinical hypothyroidism was defined when TSH values were slightly increased and free T4 were within normal values. At least two TSH measurements were performed if the result was abnormal. Patients were divided into three groups according to PN duration (> 15, > 30 and > 45 days). Iodine supplementation was performed with inorganic iodine (Lugol's solution) as indicated by NICU staff, as was levothyroxine replacement.
Statistical analysisVariables were described as mean ± standard deviation, median (interquartile range), or absolute and relative frequency. Comparisons of means between two groups were performed with Student's T-test, and of medians with the Mann-Whitney U test; among three or more groups, means were compared by analysis of variance (ANOVA) with Bonferroni's post-hoc test, while medians were compared through the Kruskal–Wallis test. Associations between groups were assessed with the chi-square test. Spearman's rank correlation was used to analyze nonparametric variables and Pearson's correlation for parametric variables. Multiple logistic regression analyses were performed to identify risk factors associated with thyroid dysfunction.
All statistical analyses were performed in IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp, Armonk, NY). Statistical significance was accepted at p<.05. This study was approved by the Institutional Ethics Committee of Hospital de Clínicas de Porto Alegre and registered under number #2019-0389.
ResultsPatient characteristicsOf 605 newborns screened, 200 receiving PN for > 15 days were included. The most frequent exclusion criterion was PN duration < 15 days (Fig. S1). Of the included patients, 156 (78%) were preterm, 140 (70%) were delivered by cesarean section, 112 (56.0%) were classified as having extremely low or very low birth weight, and 66 (33%) were classified as small for gestational age (SGA). The mean maternal age was 26 ± 7 years, with a median of 2 (1-3) previous pregnancies. Maternal thyroid disease was reported in 10 patients; nine (4.5%) had hypothyroidism, all treated with levothyroxine, and one mother had untreated hyperthyroidism. Seven (3.5%) newborns underwent computed tomography with iodinated contrast. Seventeen (8.5%) had neonatal sepsis diagnosed through positive catheter blood cultures. Half of the sample had gastrointestinal tract complications during hospitalization; of these, 42 (21%) had enterocolitis (enteroviral or bacterial). Sociodemographic, clinical, and nutritional characteristics are detailed in Table 1.
Sociodemographic, clinical, and nutritional characteristics in newborns receiving PN >15 days (n = 200).
ELBW, extremely low birth weight; GA, gestational age; LBW, low birth weight; PN, parenteral nutrition; VLBW, very low birth weight.
Note: Data expressed as n (%), mean ± SD, or median (interquartile range).
*Patients can have more than one reason for PN.
PN was initiated mainly due to prematurity (n = 142, 71.0%) and malformation (n = 47, 23.5%), of which 21 (44.7%) were gastroschises, 14 (23.5%) atresias, six (12.8%) omphaloceles, four (8.5%) VACTERL associations, and two (4.2%) due to other causes. The median age at PN initiation was 1 (1-2) day of life, although two patients started PN at 20 and 27 days of life. The median duration of PN was 31 (21-47) days. PN was supplemental in nearly all cases (n = 193, 96.5%); 35 (17.5%) received colostrum therapy, 188 (94%) were breastfed, and 190 (95%) received infant formula.
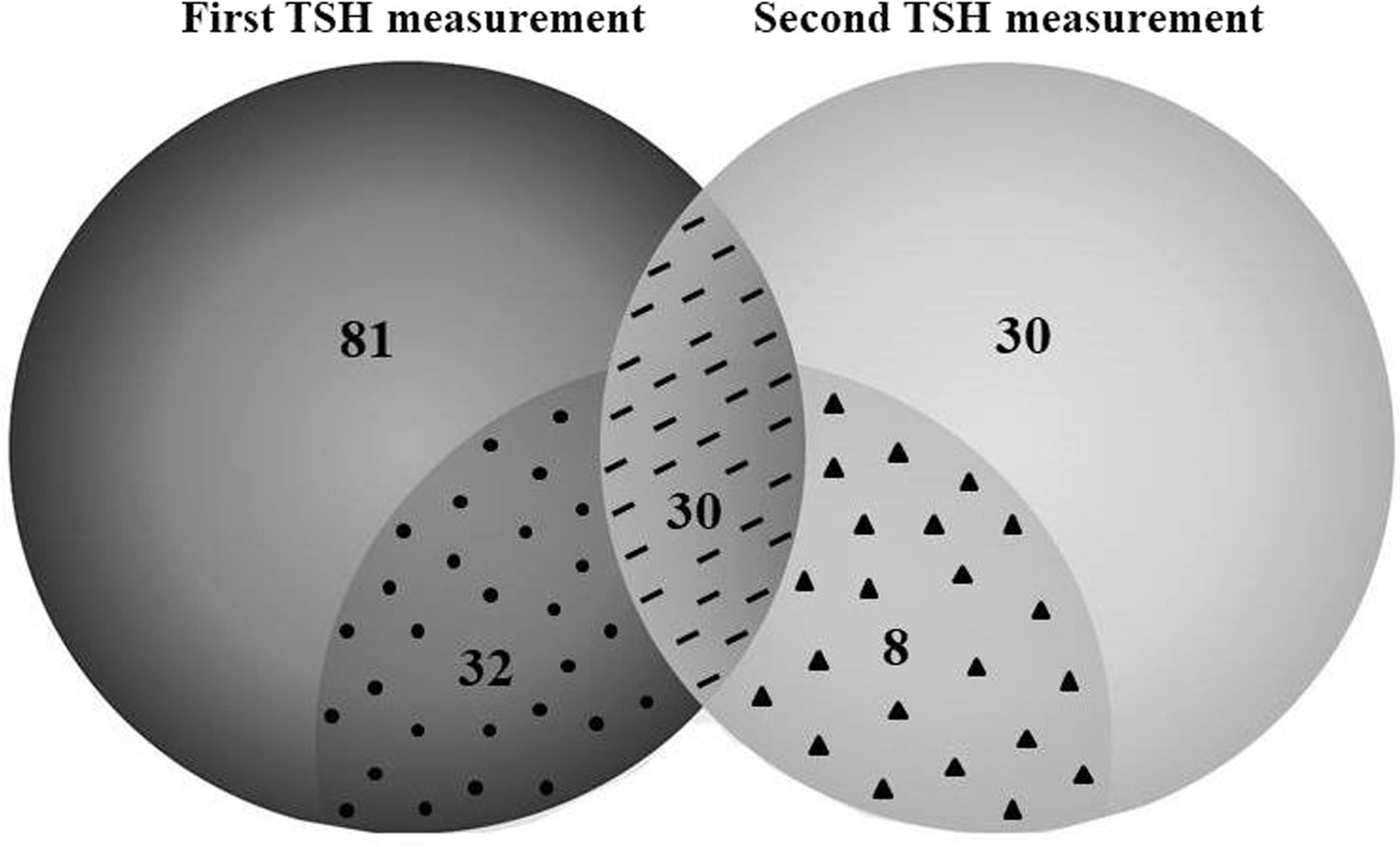
Thyroid function testsMore than 70% of infants (n = 143) had at least one TSH measurement during PN support, and approximately one-third (n = 68) underwent repeated thyroid function tests (Table S1, figure 1). The median time between two TSH measurements was 21 (10–43) days. TSH alterations were confirmed in 70 (48.9%) patients, and the prevalence of hypothyroidism was 10.5%. In addition, subclinical hypothyroidism was present in 10.5% of patients.
TSH measurements in newborns receiving prolonged parenteral nutrition during hospitalization.
Patients in whom thyroid function was not evaluated had an older gestational age at birth, higher rates of normal birth weight, shorter length of hospital stay, and received PN support for fewer days than patients for whom thyroid function tests were performed (Table S2).
Patients who developed hypothyroidism received PN for longer (51 days, IQR: 34–109) than those who remained euthyroid (36 days, IQR: 21–47) (p < 0.01). This group also presented higher CRP levels at the second measurement, with a mean of 24.7 (IQR: 3.9–42.3) mg/L versus 2.8 (IQR: 0.7–17.8; p < 0.05) mg/L. Detailed data comparing patients with and without hypothyroidism are reported in Table 2. Absolute mortality was higher in the hypothyroid group compared to the normal TSH group (26.7% vs. 7.8%; p < 0.05). However, when adjusted for gestational age, there was no difference in mortality (RR 1.75; 95% CI 0.49 to 6.23).
Comparisons between newborns receiving PN >15 days according to TSH groups: Hypothyroidism versus normal TSH.
| Variables | Hypothyroidism (n = 15) | Normal TSH(n = 128) | p |
|---|---|---|---|
| Age at PN initiation, daysa | 1 (1–2) | 1 (1–1) | .41 |
| Sex, maleb | 9 (60.0) | 73 (57.0) | .83 |
| Cesarean deliveryb | 11 (73.3) | 91 (71.1) | .86 |
| GA, weeksc | 29 ± 5 | 29 ± 4 | .72 |
| GA classification, pretermb | 13 (86.7) | 120 (93.8) | .31 |
| Apgar, 1-minutea | 5 (2–6) | 5 (3–8) | .18 |
| Apgar, 5-minutea | 7 (6–8) | 8 (6–9) | .16 |
| Birth weight, ga | 990 (650–1900) | 1038 (778–1535) | .53 |
| Birth weight classificationb | |||
| ELBW | 8 (53.3) | 61 (47.7) | .24 |
| VLBW | 3 (20.0) | 33 (25.8) | |
| LBW | 1 (6.7) | 25 (19.5) | |
| Normal | 3 (20.0) | 9 (7.0) | |
| Length, cmc | 36.5 ± 6.3 | 36.8 ± 5.3 | .83 |
| Head circumference, cmc | 26.4 ± 4.4 | 26.2 ± 3.6 | .83 |
| Thoracic circumference, cmc | 22.6 ± 4.5 | 23.4 ± 4.2 | .51 |
| Abdominal circumference, cmc | 21.0 ± 5.0 | 21.8 ± 4.5 | .58 |
| First CRP, mg/La | 20.9 (1.5–54.4) | 11.5 (1.1–20.1) | .35 |
| Second CRP, mg/La | 24.7 (3.9–42.3) | 2.8 (0.7–17.8) | <.05 |
| Supplemental PNb | 15 (100.0) | 127 (99.2) | .73 |
| PN duration, daysa | 51 (34–109) | 36 (21–47) | <.01 |
| Length of hospital stay, daysa | 114 (75–152) | 87 (62–117) | .19 |
CRP, C-reactive protein, ELBW, extremely low birth weight; GA, gestational age; LBW, low birth weight; PN, parenteral nutrition; TSH, thyroid-stimulating hormone; VLBW, very low birth weight.
Note: Data expressed as n (%), mean ± SD, or median (interquartile range), and p<.05 is considered statistically.
Ten patients with hypothyroidism received iodine supplementation (Lugol's solution) and six were treated with levothyroxine. Of note, only 23 patients in the whole sample (11.5%) received iodine supplementation, of whom 13 had normal TSH measurements (Table S3). No differences in clinical, anthropometric, or nutritional variables were observed between patients who received iodine supplementation and those who did not.
When patients were stratified by PN duration (15-30, 30-45 and > 45 days), birth weight and gestational age were higher and the length of hospital stay was shorter in the first group. Table 3 describes comparisons among PN groups related to PN duration. A weak correlation was observed between TSH and PN duration (r = 0.19, p = .02).
Comparisons between newborns receiving PN >15 days according to the duration of PN: 15-30 days, 31-45 days, >45 days.
| Variables | PN >15 days (n = 97) | PN >30 days (n = 53) | PN >45 days (n = 50) | p |
|---|---|---|---|---|
| GA, weeksa | 33 ± 5 | 30 ± 5 | 29 ± 4 | <.001 |
| Birth weight, gb | 1650 (950–2698) | 1045 (703–2340) | 1060 (789–1721) | <.01 |
| Length of hospital stay, daysb | 46 (33–69) | 85 (51–126) | 112 (85–156) | <.001 |
| Age at PN initiation, daysb | 1 (1–2) | 1 (1–2) | 1 (1–1) | .02 |
| Supplemental PNc | 92 (94.8) | 51 (96.2) | 50 (100.0) | .27 |
| First TSH, mU/Lb | 3.10 (2.44–4.96) | 4.13 (2.24–6.13) | 4.89 (2.03–7.71) | .14 |
| Second TSH, mU/Lb | 4.17 (2.51–5.69) | 4.19 (2.70–5.79) | 5.79 (3.35–10.41) | .10 |
| First fT4, ng/dLa | 1.20 ± 0.22 | 1.22 ± 0.45 | 1.18 ± 0.46 | .89 |
| Second fT4, ng/dLa | 1.14 ± 0.30 | 1.25 ± 0.29 | 1.20 ± 0.50 | .74 |
CRP, C-reactive protein; GA, gestational age; PN, parenteral nutrition; TSH, thyroid-stimulating hormone; fT4, free thyroxine.
Note: Data expressed as n (%), mean ± SD, or median (interquartile range), and p<.05 is considered statistically.
In the present study's sample, consisting mainly of preterm infants receiving supplemental PN for > 15 days, 48.9% of patients had increased TSH levels, and the overall prevalence of hypothyroidism was 10.5%. There was a trend toward higher TSH levels with increasing duration of PN time, although the number of TSH measurements reduced over time. As in other institutions, iodine is not usually added to the PN preparations, which facilitates the development of deficiency.
Iodine is not always added in PN solutions, which could favor the occurrence of thyroid alterations.6,7 In the past, povidone-iodine solutions were used in the skin to disinfect the insertion sites of PN catheters, contributing to the maintenance of iodine levels, and making deficiency unlikely. However, these solutions were replaced by chlorhexidine, which does not contain iodine, contributing to the deficiency of this nutrient. After this replacement, several studies have demonstrated the occurrence of iodine deficiency in infants receiving prolonged PN.11–14
Considering this high probability of developing nutritional deficiencies, the addition of micronutrients, including iodine, in PN solutions is essential. Nutrient provided intravenously has a higher bioavailability, however little is known about how much metabolism may be affected by this route of administration. For this reason, Guidelines were developed in order to better guide health professionals regarding the recommendations for micronutrient supplementation in patients receiving PN.7,8
On the other hand, enteral nutrition formulations are added with iodine, contributing to avoiding this micronutrient deficiency. Breast milk contains iodine, however, its content varies according to the lactating's iodine intake, ranging from 18 to 1153 mcg/L, depending if the lactating is iodine-deficient or not.19 Also, the lactating woman's iodine status can influence children's iodine status, whereas lactating women with iodine deficiency (breast milk content < 100 mcg/L) contributed to a reduced urinary iodine content in infants (< 100 mcg/L).19
It is important to highlight that infants born before 32 weeks of gestational age who are SGA can be considered at risk for thyroid dysfunction, as are those with low and very low birth weight; the prevalence of transient hypothyroxinemia among preterm infants ranges from 35% to 85%.3,5,20,21 This condition, called “transient hypothyroxinemia of prematurity”, can occur due to various factors, including immaturity of the hypothalamic-pituitary-thyroid axis, cessation of hormone supply through the placenta after birth, the limited ability of the thyroid to produce hormones, iodine deficiency, and increased demand for thyroid hormone due to non-thyroidal illness.3,22 This condition lasts about 2–3 weeks and, although self-limited, can affect neurodevelopment.3
Gastrointestinal complications, present in half of the present study's patients, are also important risk factors for the development of nutritional deficiencies, including iodine deficiency, as described in a case report of six patients (age 5 months to 12 years) receiving PN, most with concomitant enteral nutrition, who developed hypothyroidism during the treatment of short bowel syndrome.23 Several other case reports reinforce the association of prolonged exclusive PN and thyroid dysfunction.12–14
Other studies have reported conflicting data regarding the association between PN and hypothyroidism development. A cross-sectional study with a small sample size analyzed children (age < 17 years) receiving chronic PN (for >6 months) and showed a higher prevalence (85%) of iodine deficiency identified by urine samples (urinary iodine concentration: < 100 mcg/L), of which 55% had severe iodine deficiency. Furthermore, 33% had acquired hypothyroidism (TSH > 4 mcgIU/L).11 Although the median duration of PN was 30 (IQR: 17–40) months, much longer than in the present study, almost one-third of patients were on exclusive PN, versus only 3.5% in the sample used.11 Otherwise, no association was found between the duration of PN and iodine deficiency or hypothyroidism.11
On the other hand, a cohort study that included 32 pediatric patients who received PN for ≥ 6 months demonstrated no TSH alterations or iodine deficiency; however, patients only received on average 50 to 58% of their total caloric intake from PN.24 It is important to highlight that there was no difference in serum iodine concentrations according to energy intake from PN.24 Another study analyzed 15 children on prolonged PN (14–84 weeks) with iodine supplements and found that UIC remained below the normal range despite supplementation.25
Maternal levels of iodine also contribute to thyroid dysfunction in newborns. In a prospective observational study evaluating mothers and infants, the frequency of iodine deficiency among mothers at the moment of delivery was 64%, represented by serum iodine levels of 98 mcg/L (reference value: > 150 mcg/L).4 Extremely low gestational age newborns on exclusive PN were iodine-deficient, as demonstrated by a low UIC of 73 (34–163) mcg/L, when compared to those not on exclusive PN (p = .02).4 An interesting result of this study is that, at 1 month of age, infants admitted to the neonatal intensive care unit had increased thyroglobulin levels, and after starting enteral nutrition, these levels improved.4
The presence of acute illness and inflammation can also influence thyroid responses to the environment, as observed in the euthyroid sick syndrome, which is characterized by normal TSH levels, but low T3 and T4 serum concentrations.2 In the present study, the authors had a 14.8% prevalence of low T4 with normal TSH. Considering critical illness can contribute to thyroid dysfunction, the authors analyzed CRP levels, but no statistically significant association was identified. Low CRP serum levels (< 10 mg/L) in the presence of negative blood cultures can be considered a good marker of an absence of infection in newborns.26–28
In addition, iodine deficiency can also be observed in individuals of other age groups. A study that evaluated 18,864 Brazilian children aged between 6 and 14 years regarding urinary iodine concentration demonstrated that deficiency was present as mild (6.9%), moderate (2.6%), and severe (0.6%) among this group.29
To our knowledge, this is the first study to evaluate the prevalence of TSH abnormalities in newborns receiving PN for >15 days, as well as to show that TSH alterations are present earlier than previously thought (median 43 days of life).11–15 The main limitations of this study are related to its retrospective design and a selection bias, considering not all patients had thyroid function analyzed, and iodine content was not assessed in mothers or newborns. Considering the high prevalence of hypothyroidism identified in the sample and the contribution of hypothyroidism to impairments in neurocognitive development and growth, iodine supplements–which are readily available and easy to use–must be incorporated into routine care.
The literature suggests that iodine status (as it pertains to the supply of this nutrient) can be varied in this population. Monitoring iodine status, especially in populations that are prone to developing deficiency, is important and will help prevent possible neurocognitive and growth impairments. The present study's data strongly recommends thyroid tests to monitor thyroid function in newborns on PN, as early on iodine content was not assessed in mothers or newborns. s. In addition, iodine supplementation should be implemented as needed to prevent deficiency.
Newborns receiving PN for more than 15 days may be at risk of developing hypothyroidism, considering iodine is not commonly added to PN solutions and its supplementation is not necessarily part of routine care. Monitoring thyroid function as a surrogate outcome of iodine deficiency in this population will contribute to preventing potential adverse neurocognitive outcomes and growth impairment. Large adequately powered prospective studies controlling for confounders are needed to confirm these findings.
FundingThis study was supported by Fundo de Incentivo a Pesquisa e Eventos (FIPE) – Hospital de Clínicas de Porto Alegre (HCPA) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
The authors thank Fundo de Incentivo a Pesquisa e Eventos (FIPE)–Hospital de Clínicas de Porto Alegre (HCPA) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).