to determine the prevalence of pulmonary hemorrhage in newborns and evaluate the associated risk factors and outcomes.
Methodsthis was a retrospective case-control study involving 67 newborns who met the criteria for pulmonary hemorrhage. A control was selected for each case: the next-born child of the same gender, similar weight (± 200g) and gestational age (± 1 week), with no previous pulmonary hemorrhage and no malformation diagnosis. Factors previous to pulmonary hemorrhage onset, as well as aspects associated to the condition, were assessed.
Resultsthe prevalence was 6.7 for 1,000 live births, and the rates observed were: 8% among newborns <1,500g, and 11% among newborns <1,000g. Intubation in the delivery room (OR=7.16), SNAPPE II (OR=2.97), surfactant use (OR=3.7), and blood components used previously to pulmonary hemorrhage onset (OR=5.91) were associated with pulmonary hemorrhage. In the multivariate logistic regression model, only intubation in delivery room and previous use of blood components maintained the association. Children with pulmonary hemorrhage had higher mortality (OR=7.24). Among the survivors, the length of stay (p ≤ 0.01) and mechanical ventilation time were longer (OR=25.6), and oxygen use at 36 weeks of corrected age was higher (OR=7.67).
Conclusionspulmonary hemorrhage is more prevalent in premature newborns, and is associated with intubation in the delivery room and previous use of blood components, leading to high mortality and worse clinical evolution.
determinar a prevalência de hemorragia pulmonar entre os recém-nascidos internados no serviço e avaliar os fatores de risco e prognóstico associados.
Métodosestudo retrospectivo caso-controle com 67 recém-nascidos que preencheram os critérios pré-estabelecidos de hemorragia pulmonar. Para cada caso, foi selecionado um controle: a próxima criança nascida do mesmo sexo, com semelhantes peso (± 200g), idade gestacional (± 1 semana) e sem hemorragia pulmonar ou malformações. Foram estudados fatores prévios à ocorrência da hemorragia pulmonar e aspectos decorrentes do evento.
Resultadosa prevalência foi de 6,7 a cada 1.000 nascidos vivos, sendo de 8% entre os recém-nascidos menores que 1.500g e de 11% entre os recém-nascidos menores que 1.000g. A necessidade de intubação (IOT) em sala de parto (OR=7,16), uso de hemoderivados previamente à ocorrência de hemorragia pulmonar (OR=5,91), uso de surfactante (OR=3,7) e SNAPPE II ≥30 (OR=2,97) foram associados à hemorragia pulmonar. No modelo de regressão logística multivariado, a necessidade de IOT (OR=5,12) e uso de hemoderivados (OR=4,2) mantiveram essa associação. As crianças com hemorragia pulmonar apresentaram maior mortalidade (OR=7,24), e, entre as sobreviventes, maior tempo de internação (p ≤ 0,01), mais uso de oxigênio com 36 semanas (OR=7,67) e maior duração da ventilação mecânica (OR=35,6).
Conclusãoa hemorragia pulmonar é uma doença de maior prevalência em recém-nascidos pré-termos, e está associada à intubação em sala de parto e ao uso prévio de hemoderivados, acarretando maior mortalidade e pior evolução clínica das crianças.
Until the 1990s, pulmonary hemorrhage (PH) was more often diagnosed in threshold preterm newborns, mainly in cases of asphyxiation or those with serious diseases. Currently, it is more often described in extremely preterm newborns.1
Depending on the criteria used to define PH, prevalence rates reported in very low birth weight newborns range between 3% and 32%.2–5 In Brazil, two studies were retrieved: one was based on autopsies of newborns, with a prevalence of 34.5% of PH,6 and another was conducted in newborns weighing<1,500g, showing a prevalence of 9%.7
No Brazilian study has assessed the perinatal factors associated with PH. In international studies, the following are reported as risk factors for the occurrence of PH: intrapartum asphyxia, infection, persistent ductus arteriosus, need for resuscitation in the delivery room, low gestational age (GA) and birth weight, use of surfactant therapy, and lack antenatal corticosteroid use.1,8 Regarding prognosis, there are controversial data on neuromotor development; some studies have shown no impact,2,5 whereas others have demonstrated increased risk of cerebral palsy and cognitive disorders.9 Regarding mortality, studies show a rate between 38% and 57%.2,10,11
Considering the scarcity of more current Brazilian and international information about PH, this study aimed to determine the prevalence and associated risk factors and prognosis of newborns with PH.
Patients and methodsAn observational, retrospective, case-control study was performed, assessing all children born at the Hospital das Clínicas de Ribeirão Preto (HCRMRP-USP) from January 1, 2005 to January 31, 2010, who had a diagnosis of PH. The study was approved by the Ethics Committee of HCFMRPU-SP (HCRP n. 3773/2010).
A total of 109 patients had their medical records reviewed, and the 67 who met the inclusion criteria were selected for study.
PH was defined as the presence of hemorrhagic fluid in the trachea, accompanied by significant clinical deterioration. For newborns not on ventilatory support, the need for endotracheal intubation and mechanical ventilation was defined as an important clinical deterioration, whereas for patients who were already on mechanical ventilation, it was defined as the need to increase inspiratory pressure or inspired oxygen fraction by at least 10%.
The next newborn admitted after the case was considered as control, providing it was of the same gender, of similar GA (± 1 week), and had a difference in birth weight compared to the case that did not exceed 200g.
Antenatal variables, including treatment with corticosteroids12 and maternal infection (maternal fever, foul amniotic fluid, and specific urinary complaints and/or laboratory diagnosis with maternal hemogram changes and/or bacterial growth in maternal cultures) were analyzed.
Regarding the children, the following data were recorded: gender, GA in weeks (New Ballard Score13 or calculated by ultrasonography), weight, birth weight adequacy in relation to GA according to Alexander's curve,14 Apgar score in the first and fifth minutes, need for intubation in the delivery room,15 SNAPPE II,16 (Score for Neonatal Acute Physiology, Perinatal Extension, Version II). Assessed in the first 24hours of life, use of surfactant therapy, number of surfactant doses (both pre-PH), time elapsed between birth and hemorrhagic episode and between the last surfactant dose and the hemorrhagic episode (for cases only), presence of patent ductus arteriosus before the onset of PH, use of volume expanders and total volume infused up to six hours before the hemorrhagic episode (for controls, they were verified six hours before the age of occurrence in the corresponding case), adequacy of the total volume given to the newborn calculated according to birth weight, and days of life.17
As prognostic variables, the need for oxygen at 28 days and at 36 weeks of corrected age, duration of mechanical ventilation and use of nasal CPAP, presence of perintraventricular hemorrhage (PIVH) according to the classification of Papile,18 type of outcome (discharge or death), and age of occurrence were assessed.
The statistical analysis was performed with the SAS/STAT® software 9.2 (NC, USA). Means, medians, and standard deviations were calculated for continuous variables, after evaluation of normal distribution: the groups were compared by Student's t-test. For categorical variables, the odds ratios (OR) and their confidence intervals (CI) were calculated. Data were adjusted in logistic regression models, quantifying the association between the dependent variable (PH) and the independent ones. Analyzing the 67 pairs (case/control) and considering the mean rate of intubation for infants born weighing<1,500g at the service as 55.9% (mean data of the last three years), it would be possible to detect differences with a minimum OR of 2.86 for a test with 80% power.
ResultsFrom January 1, 2005 to January 31, 2010, 9,983 children were born in HCFMRPU-SP. Of these, 109 newborns were identified with PH, of whom 67 met the previously proposed criteria for PH and were considered as cases. Thus, the prevalence of PH in newborns in HCFMRPU-SP was 6.7 per 1,000 births and among those whose birth weight was ≤ 1,500g was 8%, and 11% among those with birth weight ≤ 1,000g.
The prevalence of PH among hospitalized newborns was 3.62%. The mean (± SD) age at which PH occurred was 76±93hours, and the mean time from the last dose of surfactant was 54±93hours.
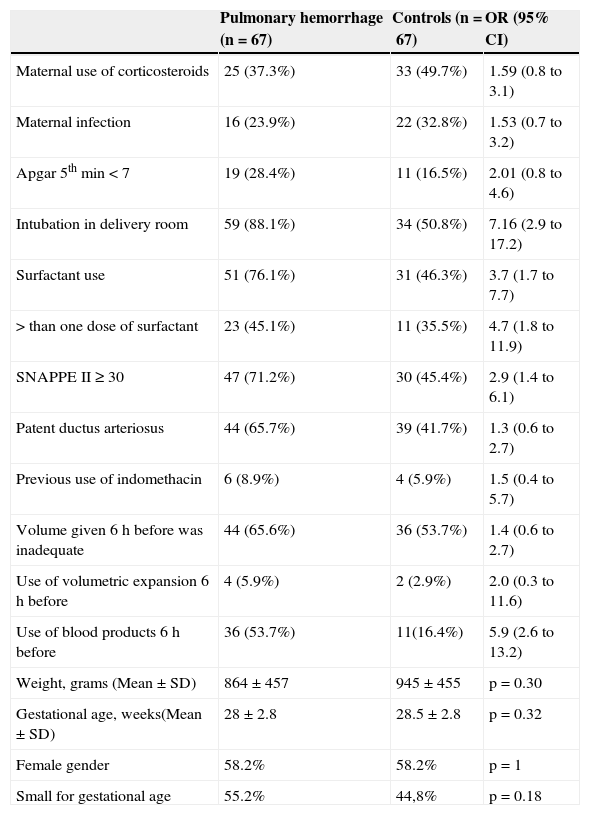
The main neonatal and postnatal characteristics of the children studied are described in Table 1. The analysis of antenatal factors (use of corticosteroids before delivery and maternal infection) showed no difference between groups. Regarding the birth conditions, newborns who had PH were more often intubated in the delivery room (88.1% vs. 50.8%). When analyzing the use of surfactant, newborns who had PH received surfactants more frequently than the control group (76.1% vs. 46.3%), and the total number of doses was greater for the group with PH.
Neonatal and postnatal characteristics of the children studied.
| Pulmonary hemorrhage (n=67) | Controls (n=67) | OR (95% CI) | |
|---|---|---|---|
| Maternal use of corticosteroids | 25 (37.3%) | 33 (49.7%) | 1.59 (0.8 to 3.1) |
| Maternal infection | 16 (23.9%) | 22 (32.8%) | 1.53 (0.7 to 3.2) |
| Apgar 5th min<7 | 19 (28.4%) | 11 (16.5%) | 2.01 (0.8 to 4.6) |
| Intubation in delivery room | 59 (88.1%) | 34 (50.8%) | 7.16 (2.9 to 17.2) |
| Surfactant use | 51 (76.1%) | 31 (46.3%) | 3.7 (1.7 to 7.7) |
| >than one dose of surfactant | 23 (45.1%) | 11 (35.5%) | 4.7 (1.8 to 11.9) |
| SNAPPE II ≥ 30 | 47 (71.2%) | 30 (45.4%) | 2.9 (1.4 to 6.1) |
| Patent ductus arteriosus | 44 (65.7%) | 39 (41.7%) | 1.3 (0.6 to 2.7) |
| Previous use of indomethacin | 6 (8.9%) | 4 (5.9%) | 1.5 (0.4 to 5.7) |
| Volume given 6 h before was inadequate | 44 (65.6%) | 36 (53.7%) | 1.4 (0.6 to 2.7) |
| Use of volumetric expansion 6 h before | 4 (5.9%) | 2 (2.9%) | 2.0 (0.3 to 11.6) |
| Use of blood products 6 h before | 36 (53.7%) | 11(16.4%) | 5.9 (2.6 to 13.2) |
| Weight, grams (Mean±SD) | 864±457 | 945±455 | p=0.30 |
| Gestational age, weeks(Mean±SD) | 28±2.8 | 28.5±2.8 | p=0.32 |
| Female gender | 58.2% | 58.2% | p=1 |
| Small for gestational age | 55.2% | 44,8% | p=0.18 |
OR, odds ratio; CI, confidence interval. SD, standard deviation.
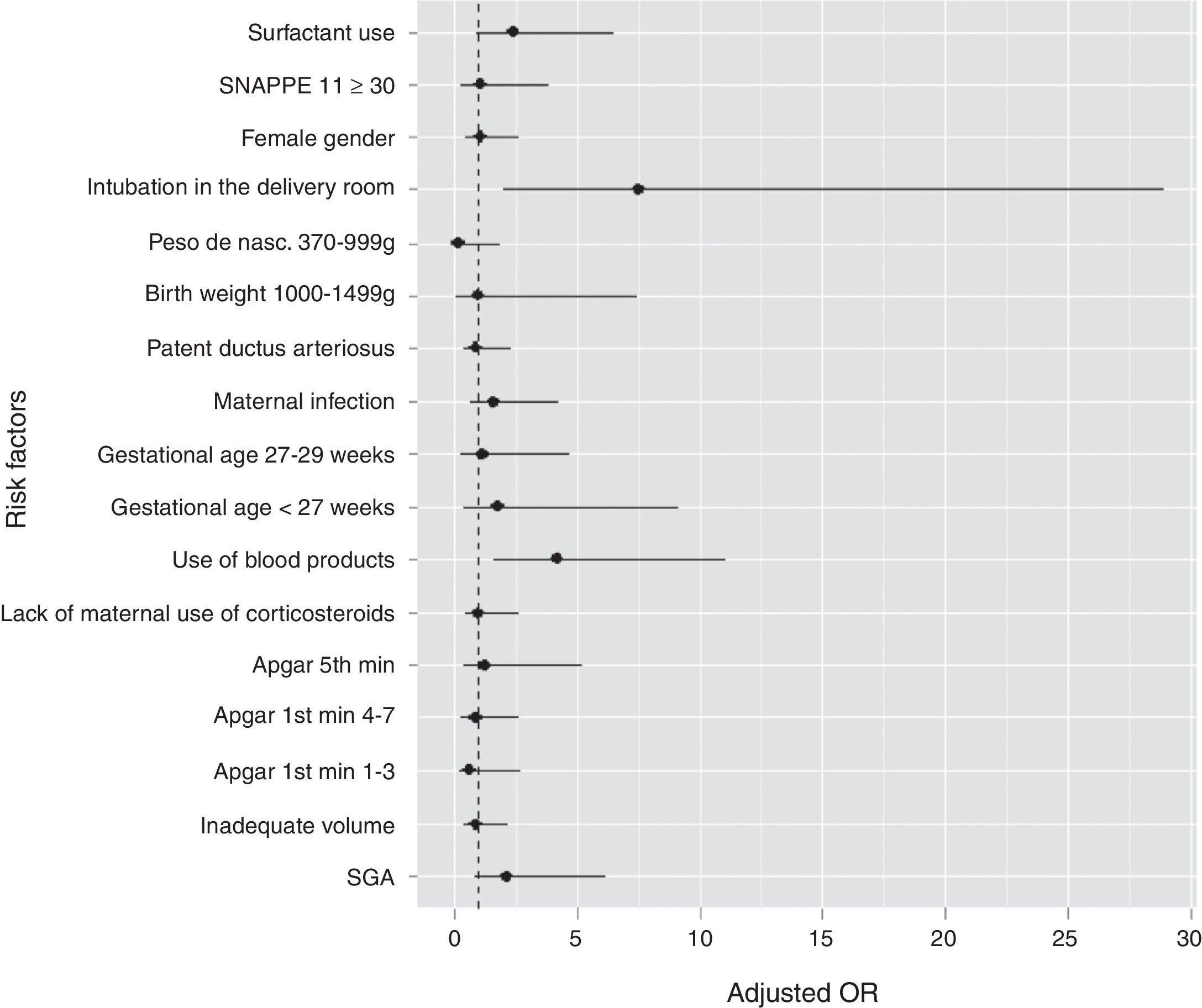
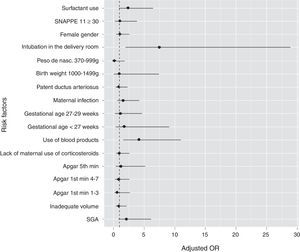
Newborns who had PH showed a higher proportion of SNAPPE II ≥ 30. The use of blood products (plasma and packed red blood cells) six hours before the episode was more frequent in the group with PH (53.7% vs 16.4%). Fig. 1 graphically shows the OR values of all studied variables.
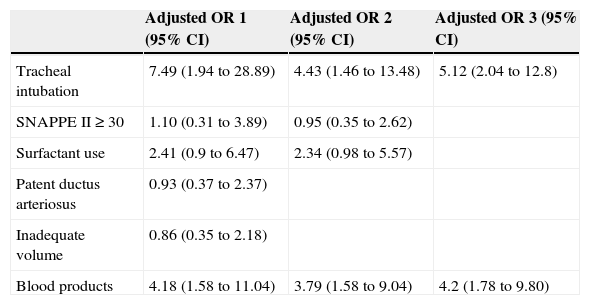
Table 2 shows the adjustment, by logistic regression, of the variables associated with PH in the univariate analysis, in which only the need for tracheal intubation and use of blood products maintained the association. When adjusting the model using variables with the highest association, considering that two of them, SNAPPE II ≥ 30 and surfactant use, had not shown significance in the first analysis, both variables maintained the association.
Multivariate analysis. Odds ratio (OR) adjusted for factors associated with hemorrhage.
| Adjusted OR 1 (95% CI) | Adjusted OR 2 (95% CI) | Adjusted OR 3 (95% CI) | |
|---|---|---|---|
| Tracheal intubation | 7.49 (1.94 to 28.89) | 4.43 (1.46 to 13.48) | 5.12 (2.04 to 12.8) |
| SNAPPE II ≥ 30 | 1.10 (0.31 to 3.89) | 0.95 (0.35 to 2.62) | |
| Surfactant use | 2.41 (0.9 to 6.47) | 2.34 (0.98 to 5.57) | |
| Patent ductus arteriosus | 0.93 (0.37 to 2.37) | ||
| Inadequate volume | 0.86 (0.35 to 2.18) | ||
| Blood products | 4.18 (1.58 to 11.04) | 3.79 (1.58 to 9.04) | 4.2 (1.78 to 9.80) |
CI, confidence interval.
Adjusted OR 1: all significant variables in the univariate analysis.
Adjusted OR 2 and 3: variables with the highest association in the previous model.
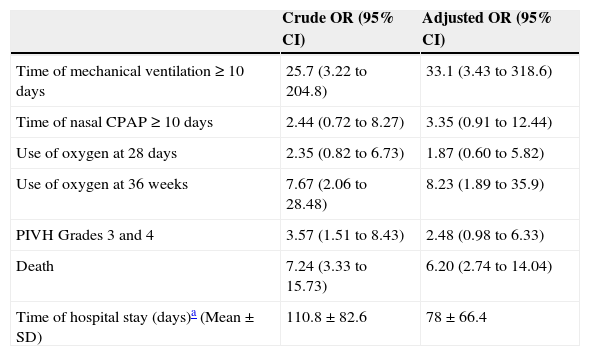
When analyzing the clinical outcome in the two groups (Table 3), the group with PH needed longer time of mechanical ventilation than controls, and also showed greater frequency of oxygen use at 36 weeks of corrected age. There was an association between PH and evolution with perintraventricular bleeding, especially in the most severe cases. Patients who had PH died more often than those in the control group (65% vs. 21%), and those who survived had a longer mean hospital stay than controls.
Variables associated with the prognosis of studied children. Crude OR of prognostic variables and adjusted OR for use of antenatal corticosteroids and SNAPPE II.
| Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Time of mechanical ventilation ≥ 10 days | 25.7 (3.22 to 204.8) | 33.1 (3.43 to 318.6) |
| Time of nasal CPAP ≥ 10 days | 2.44 (0.72 to 8.27) | 3.35 (0.91 to 12.44) |
| Use of oxygen at 28 days | 2.35 (0.82 to 6.73) | 1.87 (0.60 to 5.82) |
| Use of oxygen at 36 weeks | 7.67 (2.06 to 28.48) | 8.23 (1.89 to 35.9) |
| PIVH Grades 3 and 4 | 3.57 (1.51 to 8.43) | 2.48 (0.98 to 6.33) |
| Death | 7.24 (3.33 to 15.73) | 6.20 (2.74 to 14.04) |
| Time of hospital stay (days)a (Mean±SD) | 110.8±82.6 | 78±66.4 |
PIVH, peri-intraventricular hemorrhage; SD, standard deviation.
When the OR for these variables was adjusted for antenatal corticosteroid use and SNAPPE II, duration of mechanical ventilation, use of oxygen at 36 weeks of age, and the death outcome remained significant.
DiscussionSeveral studies aiming to define factors associated with the occurrence of PH and physiopathological mechanisms involved with it can be retrieved in the literature.2–5,9–11,19,20 However, most clinical studies are old; therefore, there is a possibility that new therapies can modify the profile of affected patients, as well as factors most often associated with the disease. No studies performed in Brazil or in developing countries assessing the factors associated with PH were retrieved.
In the present study, 92.4% of newborns who had PH were born weighing less than 1,500g, and 77.6% had less than 29 weeks of GA, a clear indication that PH is associated with prematurity and its complications.
Among prenatal factors, the use of antenatal corticosteroids has been identified as an important protective factor against the occurrence of PH,19 especially in pregnant women with GA between 24 and 26 weeks. This effect is attributed to the increased production of surfactant and possible structural changes in fetal pulmonary vessels. In the present study, only 37.3% of children who had PH and 49.7% of children in the control group received antenatal corticosteroids, lower values than those reported by the Brazilian Network on Neonatal Research,12 which reduced the analyzed sample size, making it difficult to demonstrate the possible protective effect of the drug.
Maternal chorioamnionitis could lead to an acceleration of lung maturation in preterm infants, thus exerting a protective effect on the incidence and severity of respiratory distress syndrome of the newborn.21 However, this effect is accompanied by an increased susceptibility of the newborn lung to postnatal insults, possibly through the action of released inflammatory factors.21 The balance between the two effects could lead to PH. In the present study, no association was found between maternal infection and PH.
The mean time for the occurrence of PH was 76hours after birth and 54hours after the last surfactant dose. It is, therefore, a relatively early event. These findings had been reported in previous studies, in which the mean age of occurrence of PH varied between 40 h4,5,19 and 72h.10
An association was found between the need for intubation in the delivery room and the presence of PH, similar to the finding of Berger et al.19 This association remained even when analyzed in a multivariate logistic regression model. Other studies found no such association.5,22 The need for intubation and ventilation with positive pressure and oxygen, is not only an indicator of neonatal hypoxia, pulmonary disease, or extreme prematurity, but can also lead to excessive alveolar distension, causing stress damage to the alveolar capillaries, thereby contributing to the genesis of PH.19 Despite this association, when the Apgar values at the first and fifth minutes were analyzed, no association with PH was observed. Other case-control studies also did not find this association.5,10,22
SNAPPE II,16 which is an excellent predictor of neonatal survival,23 was used to evaluate the association between clinical severity of the newborn within the first hours of life and risk of PH. An association was found between SNAPPE II ≥ 30 and PH in the univariate analysis, but the association was not maintained when analyzed in a multivariate logistic regression model. Only the study by Berger et al.19 had assessed this association, finding an increased risk of PH in patients with SNAPPE II ≥ 24.
The use of surfactant also showed an association, including greater risk as the number of doses increased. However, this association was not maintained when analyzed in a multivariate logistic regression model. Although the meta-analysis performed 20 years ago by Raju and Langenberg3 demonstrated that the use of surfactant increased the risk of PH by approximately 50%, other studies in which a similar multivariate analysis was performed showed no association.19,24
Kluckow et al.25 reported that newborns with PH used more albumin than those without it (83% vs. 44%, p=0.02). This was the only study that correlated the use of a blood product with the PH episode. In the present study, previous use of blood products (plasma or packed red blood cells) showed a difference between groups, maintaining the association even when adjusted in a multivariate logistic regression model. The use of blood products prior to the PH episode could have caused a sudden increase in blood volume, leading to a stress injury of the capillary wall, with passage of fluid and plasma proteins, which can also lead to left ventricular failure, contributing to an increase in pulmonary capillary pressure, as already proposed by Cole et al.20
In 2011, Polglase et al.26 demonstrated that immediately after an intravenous volume overload, lambs had an increase in pulmonary blood flow and left ventricular ejection volume; 50% of them had PH. The elevation in pulmonary capillary pressure can lead to alveolar capillary wall injury, causing pulmonary edema due to increased permeability with passage of proteins.27,28 In the present study, there was no difference between the volumes infused 24h before the onset of hemorrhage in cases and controls. No difference was observed, either, regarding patients who received volume expansion with saline solution prior to the episode; however, the number of patients was very small in both groups (four of them with PH and two controls).
Regarding the presence of patent ductus arteriosus (PDA), Klucow and Evans25 performed echocardiograms in newborns before the PH episode, showing that they had significant PDA and increased pulmonary blood flow when compared to newborns who did not have PH. Some studies10,22 have shown this association; however, other case-control studies did not find it.2,4,5,11,19 In the present study, this association was not found; however, it should be noted that this was a retrospective analysis and there was no systematic evaluation of echocardiograms in all cases, which reduced the impact of this analysis.
Regarding the prognostic variables, this study found a high mortality rate among children that had PH (75.8% vs. 30.3%), with a seven-fold higher risk of death when compared to controls. Other studies have reported mortality rates of 47-91%.2,4,10 A Brazilian study showed that PH was an independent risk factor of death in newborns with GA<32 weeks.29
Children with PH who survived had a higher mean of mechanical ventilation than controls. This difference could be explained by the fact that children who had PH had a more severe evolution, requiring more aggressive mechanical ventilation, whereas the controls were able to keep good levels of oxygenation and ventilation, with less aggressive mechanical ventilation (nCPAP).
The use of oxygen at 36 weeks of corrected age can be used as an indicator of lung lesion severity. In the present study, an association was observed between PH and oxygen use; this association remained even when the value was corrected in logistic regression. In the literature, only one study found this association.22
The combination of PH with perintraventricular hemorrhage (PIVH) was analyzed by Pandit et al.,2 who found a three-fold higher risk of PIVH grades III and IV in children who had PH, similar to the initial univariate analysis of the present study. However, when the results were corrected for use of antenatal maternal corticosteroids and SNAPPE II, it became non-significant, demonstrating that these variables could also be associated with the etiology of PIVH.
The occurrence of PH significantly increases the risk of death in newborns and its effects are very detrimental even when the child survives, which can be inferred by the increased duration of oxygen use and hospitalization in patients who had PH.
The present study has limitations, as it is a case-control retrospective study. The relative rarity of PH complicates the design of a prospective study, as there were only 67 cases that met the criteria for PH in a five-year period, which limited the assessment of clinical outcomes due to the small sample size. Nevertheless, the sample size led to a 99.9% power to detect the difference found in the two variables that were associated with PH. As the study period increases, there is risk of the effect from changes in therapeutic approaches that occur very quickly. Thus, the results should be interpreted according to these limitations.
Another aspect to be considered as a limitation of this retrospective study was the incapacity to consistently and accurately obtain data on mechanical ventilation between possible variables associated with PH, as ventilation with high tidal volumes is associated with volutrauma, which may play an important role in the physiopathology of PH, considering that the need for intubation and ventilation in the delivery room with a self-inflating balloon performed at the time, without PEEP (Positive end-expiratory pressure) and with no control of peak inspiratory pressure, could lead to overdistention, facilitating pulmonary injury.
The results of this study indicate that PH is a more prevalent disease in newborn preterm infants weighing less than 1,000g at birth, and is associated with intubation in the delivery room and previous use of blood products, resulting in higher mortality and worse clinical outcome in these children.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Ferreira CH, Carmona F, Martinez FE. Prevalence, risk factors and outcomes associated with pulmonary hemorrhage in newborns. J Pediatr (Rio J). 2014;90:316–22.