This study aimed to evaluate gestational weight gain and birth weight in women with gestational diabetes mellitus of two Brazilian cohorts enrolled three decades apart.
MethodsThe authors compared data of 2362 women from the Lifestyle INtervention for Diabetes Prevention After Pregnancy study (LINDA-Brasil, 2014–2017) to those of 359 women from the Estudo Brasileiro de Diabetes Gestacional study (EBDG, 1991–1995). Gestational weight gain was classified by the 2009 Institute of Medicine criteria; large and small for gestational age newborns, by the Intergrowth-21st chart. Differences in birth weight means between pregestational BMI and gestational weight gain categories were evaluated by ANOVA; the associations of gestational weight gain and birth weight, through multivariable Poisson regression.
ResultsIn LINDA-Brasil, women presented higher pregestational body mass index (30.3±6.5 vs. 24.6±4.4kg/m2) and were frequently obese (46.4 vs. 11.1%) compared to those of the EBDG. In the EBDG, gestational weight gain was larger (11.3±6.1 vs. 9.2±7.6kg) and rates of small for gestational age higher (7.5 vs. 4.5%) compared to LINDA-Brasil. In LINDA-Brasil, excessive gestational weight gain was associated to macrosomia (adjusted relative risk [aRR]: 1.59, 95% CI 1.08–2.35) and large for gestational age (aRR: 1.40; 95% CI 1.05–1.86); less gain increased the risk of low birth weight (aRR: 1.66; 95% CI 1.05–2.62) and small for gestational age (aRR: 1.79; 95% CI 1.03–3.11). These associations were similar in the EBDG, although not statistically significant.
ConclusionsImprovements in gestational weight gain and rates of small for gestational age occurred over time in gestational diabetes mellitus pregnancies, accompanied by a worsening in maternal weight profile. This highlights the nutritional transition during this period and the importance of avoiding excessive gestational weight gain as well as promoting adequate weight before conception.
Low and middle-income countries have faced a rapid transformation due to nutrition transition, resulting from urbanization, change in technologies that led to a decrease in physical activity and consumption of high calorie foods, increasing noncommunicable diseases including obesity and type 2 diabetes.1 Brazil is among the countries with the highest prevalence of overweight, and had the third largest increase in the absolute number of obese people in the last 30 years (20 million).2 From 1974 to 2013, the prevalence of overweight among women rose from 10.9% to 59.8%; the prevalence of obesity rose from 2.9 to 25.2%, with high rates in childbearing ages.3,4
Maternal overweight and obesity can lead to several complications, including gestational diabetes mellitus (GDM).5 GDM, defined as hyperglycemia detected during pregnancy, complicates 3% to 25% of pregnancies and is associated with adverse pregnancy outcomes,6 and increased risk of future maternal type 2 diabetes mellitus.7 Pregestational body mass index (BMI) is an important predictor of GDM.8
Patterns of adequate gestational weight gain (GWG) in women with GDM are not established and inadequate weight gain, either more or less than those recommended by the Institute of Medicine (IOM), is commonly described.9 A major consequence of inadequate GWG, whether excessive or insufficient, is upon birth weight. Excessive GWG increases the risk of macrosomia (or large for gestational age), whereas insufficient GWG increases the risk of small for gestational age (or low birth weight). Besides the influences of pregestational BMI and GWG, the presence of gestational hyperglycemia may further influence birth weight adequacy,10 mainly as delivery of big newborns.
Given the ongoing obesity epidemic and the established relationship between obesity and gestational diabetes and its complications, it is remarkable that only a few studies have examined the evolution of the patterns of GWG in women with GDM over the last three decades, particularly in low- or middle-income countries, where most of the burden lies. Thus, the aim was to describe how maternal pregestational weight and GWG profiles influenced birth weight in two GDM cohorts in Brazil, one before and the other during the ongoing nutritional transition.
MethodsStudy populationTwo multicenter Brazilian cohorts of women with GDM were analyzed within a temporal interval of almost 30 years. Below, the two cohort studies are described.
Cohort 1: The recruitment cohort for the Lifestyle Intervention for Diabetes Prevention After Pregnancy-Brazil (LINDA-Brasil, 2014–2017).
This is an ongoing randomized clinical trial aiming to investigate the effectiveness of a lifestyle intervention program implemented after a pregnancy complicated by GDM in delaying or preventing the development of type 2 diabetes.11 The recruitment cohort is enrolling women with prior GDM in high-risk prenatal care services of the Brazilian public health service, the Sistema Único de Saúde (SUS), in three Brazilian cities (Porto Alegre, State of Rio Grande do Sul; Curitiba, State of Paraná; Fortaleza, State of Ceará). At enrollment, women answered a structured questionnaire including demographic and socioeconomic data such as age, self-reported skin color, years of schooling, smoking, parity, prepregnancy weight, and height. Postpartum follow-up was performed through telephone interviews, and included retrieving information on pregnancy outcomes (such as maternal weight at each prenatal appointment, pregnancy complications) and delivery (maternal weight, mode of delivery – vaginal or cesarean section –, maternal complications), as well as data on the newborns (weight, gestational age at birth, gender, Apgar scores, and complications). The detailed methodology is described elsewhere11; the study protocol was registered on December 23, 2014 in the ClinicalTrials.gov site, identifier NCT02327286.
Cohort 2: The Estudo Brasileiro de Diabetes Gestacional (EBDG, 1991–1995).
The EBDG was a study conducted between May 1991 and August 1995, aiming to estimate the prevalence of GDM, according to common diagnostic criteria used at that time, in women of the public health system; another aim was to define diagnostic glycemic thresholds for GDM in the Brazilian pregnant population. It was conducted in capitals of six States in Brazil (Porto Alegre, State of Rio Grande do Sul; São Paulo, State of São Paulo; Rio de Janeiro, State of Rio de Janeiro; Salvador, State of Bahia; Fortaleza, State of Ceará; Manaus, State of Amazonas). All pregnant women attended in those clinics were included; they answered a standardized questionnaire. Demographic characteristics included age, self-reported skin color, years of schooling, smoking, parity, prepregnancy weight; height and weight were measured in duplicate during the interview. Data about pregnancy evolution (such as maternal weight gain and pregnancy complications), delivery (mode of delivery – vaginal or cesarean section –, maternal weight at delivery) and immediate postpartum (such as weight, gestational age, gender, Apgar scores, as well as maternal and newborn complications) were collected from the medical records.12
Definition of outcomesPregestational BMI was defined as the weight in kg divided by the square of the height in meters. In the LINDA-Brasil study, BMI was calculated using the self-reported pregestational weight in kg and the height in meters recorded in the chart or the self-reported height. In the EBDG study, BMI was calculated using the self-reported pregestational weight in kg and the mean of two height measurements, in meters. Pregestational nutritional status was classified as underweight (<18.5kg/m2), normal (≥18.5 and <25kg/m2), overweight (≥25 and <30kg/m2), or obesity (≥30kg/m2).13
Total GWG was calculated by subtracting pregestational weight from the last measured weight, which was self-reported or collected from the prenatal chart in the LINDA-Brasil study; and obtained from the prenatal chart, in EBDG. Adequate total GWG was classified according to the IOM recommendation: 12.5–18kg for underweight women; 11.5–16kg for normal BMI women; 7–11.5kg for overweight women, and 5–9kg for obese women.13
Gestational age at delivery was estimated by the first echography before the 20th gestational week or by the reported date of the last menstrual period. In cases with relevant discrepancies of gestational age (more than 2 weeks), individualized analyses were performed.
In LINDA-Brasil, GDM was defined as having a label of GDM in the prenatal chart, irrespective of the diagnostic criteria employed to define hyperglycemia (most were IADPSG/2013 World Health Organization [WHO] criteria).14 In EBDG, GDM was defined as fasting plasma glycemia≥110mg/dL and/or 2-h post 75-g glucose oral load≥140mg/dL (1999 WHO criteria).15
Newborn weight adequacy was classified according to the Intergrowth-21st chart,16 which adjusts birth weight to gestational age and newborn gender. Newborns with birth weight below the 10th percentile were considered as small for gestational age (SGA); and above the 90th percentile, as large for gestational age (LGA). Macrosomia was defined as birth weight≥4000g; low weight, as weight≤2500g.17
Ethical aspectsLINDA-Brasil was approved by the ethic committee of the three centers (Plataforma Brasil No. 12-0097), and all participants signed informed consents. The EBDG study was approved by the ethics committees of the six centers (Project No. 90-058), and patients gave their oral informed consent to participate.
Statistic analysisAbsolute and relative frequencies or mean and standard deviation of main variables were calculated. Differences in means of birth weight between pregestational BMI groups and GWG categories were evaluated using the ANOVA test. The relative risk was calculated for macrosomia, low birth weight, SGA, and LGA. All variables with p<0.25 in univariable analyses were included in multivariable models, performed with Poisson regression with robust estimates. Models were adjusted for the following covariates: age, self-declared race/color group, schooling, smoking, parity, pregestational BMI, fasting and 2-h glycemia, total gestational weight gain, and gestational age at delivery. Multicollinearity among variables was explored through correlation matrix; variance inflation factor values >5 indicated multicollinearity. When not specified, the significance level of 0.05 was considered. Estimated confidence intervals were set at 95%. Analyses were performed with SPSS software, v.18.
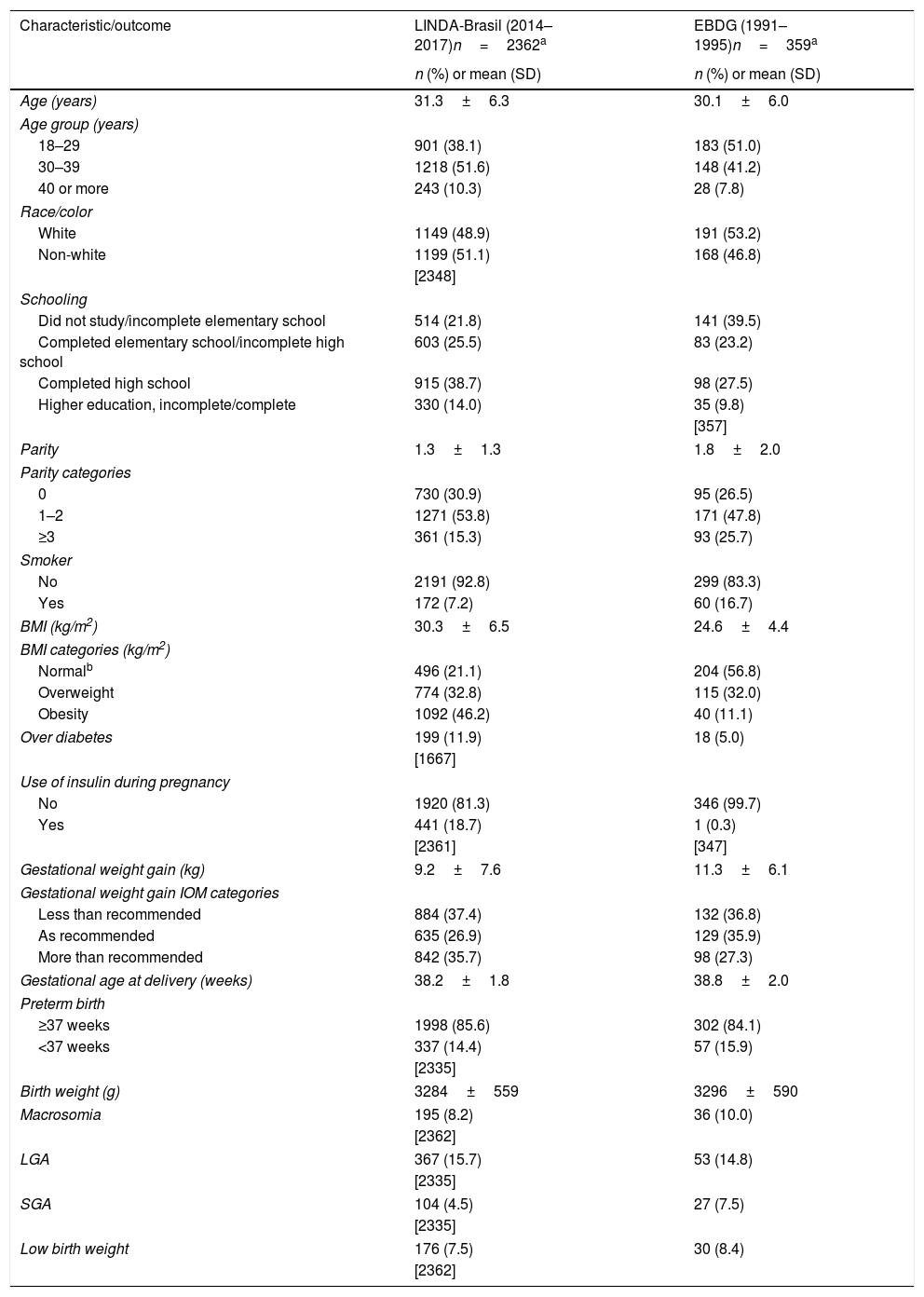
ResultsThis study included women of LINDA-Brasil with data collected between January 2014 and July 2017 (n=2362); and women of EBDG reaching diagnosis of gestational diabetes (n=359). As shown in Table 1, maternal age and schooling were higher, and parity and smoking rates, lower, in participants of the LINDA-Brasil (2014–2017) study, compared to the EBDG (1991–1995) cohort. In LINDA-Brasil, women presented higher pregestational BMI (30.3±6.5kg/m2vs. 24.6±4.4kg/m2) and were frequently obese (46.4% vs. 11.1%) compared to those of EBDG. In EBDG, higher GWG (11.3±6.1 vs. 9.2±7.6kg) was found, but women in the LINDA-Brasil cohort had excessive GWG more frequently than women in the earlier cohort. Mean gestational age at delivery and birth weight were similar between the two cohorts, but SGA rates were higher in EBDG.
Characteristics and outcomes of pregnancy in women with gestational diabetes mellitus in two cohorts: LINDA-Brasil and EBDG.
| Characteristic/outcome | LINDA-Brasil (2014–2017)n=2362a | EBDG (1991–1995)n=359a |
|---|---|---|
| n (%) or mean (SD) | n (%) or mean (SD) | |
| Age (years) | 31.3±6.3 | 30.1±6.0 |
| Age group (years) | ||
| 18–29 | 901 (38.1) | 183 (51.0) |
| 30–39 | 1218 (51.6) | 148 (41.2) |
| 40 or more | 243 (10.3) | 28 (7.8) |
| Race/color | ||
| White | 1149 (48.9) | 191 (53.2) |
| Non-white | 1199 (51.1) | 168 (46.8) |
| [2348] | ||
| Schooling | ||
| Did not study/incomplete elementary school | 514 (21.8) | 141 (39.5) |
| Completed elementary school/incomplete high school | 603 (25.5) | 83 (23.2) |
| Completed high school | 915 (38.7) | 98 (27.5) |
| Higher education, incomplete/complete | 330 (14.0) | 35 (9.8) |
| [357] | ||
| Parity | 1.3±1.3 | 1.8±2.0 |
| Parity categories | ||
| 0 | 730 (30.9) | 95 (26.5) |
| 1–2 | 1271 (53.8) | 171 (47.8) |
| ≥3 | 361 (15.3) | 93 (25.7) |
| Smoker | ||
| No | 2191 (92.8) | 299 (83.3) |
| Yes | 172 (7.2) | 60 (16.7) |
| BMI (kg/m2) | 30.3±6.5 | 24.6±4.4 |
| BMI categories (kg/m2) | ||
| Normalb | 496 (21.1) | 204 (56.8) |
| Overweight | 774 (32.8) | 115 (32.0) |
| Obesity | 1092 (46.2) | 40 (11.1) |
| Over diabetes | 199 (11.9) | 18 (5.0) |
| [1667] | ||
| Use of insulin during pregnancy | ||
| No | 1920 (81.3) | 346 (99.7) |
| Yes | 441 (18.7) | 1 (0.3) |
| [2361] | [347] | |
| Gestational weight gain (kg) | 9.2±7.6 | 11.3±6.1 |
| Gestational weight gain IOM categories | ||
| Less than recommended | 884 (37.4) | 132 (36.8) |
| As recommended | 635 (26.9) | 129 (35.9) |
| More than recommended | 842 (35.7) | 98 (27.3) |
| Gestational age at delivery (weeks) | 38.2±1.8 | 38.8±2.0 |
| Preterm birth | ||
| ≥37 weeks | 1998 (85.6) | 302 (84.1) |
| <37 weeks | 337 (14.4) | 57 (15.9) |
| [2335] | ||
| Birth weight (g) | 3284±559 | 3296±590 |
| Macrosomia | 195 (8.2) | 36 (10.0) |
| [2362] | ||
| LGA | 367 (15.7) | 53 (14.8) |
| [2335] | ||
| SGA | 104 (4.5) | 27 (7.5) |
| [2335] | ||
| Low birth weight | 176 (7.5) | 30 (8.4) |
| [2362] | ||
LINDA-Brasil, Lifestyle Intervention for Diabetes Prevention After Pregnancy-Brasil; EBDG, Estudo Brasileiro de Diabetes Gestacional; SD, standard deviation; BMI, body mass index; IOM, Institute of Medicine; LGA, large for gestational age; SGA, small for gestational age (Intergrowth-21st curves).
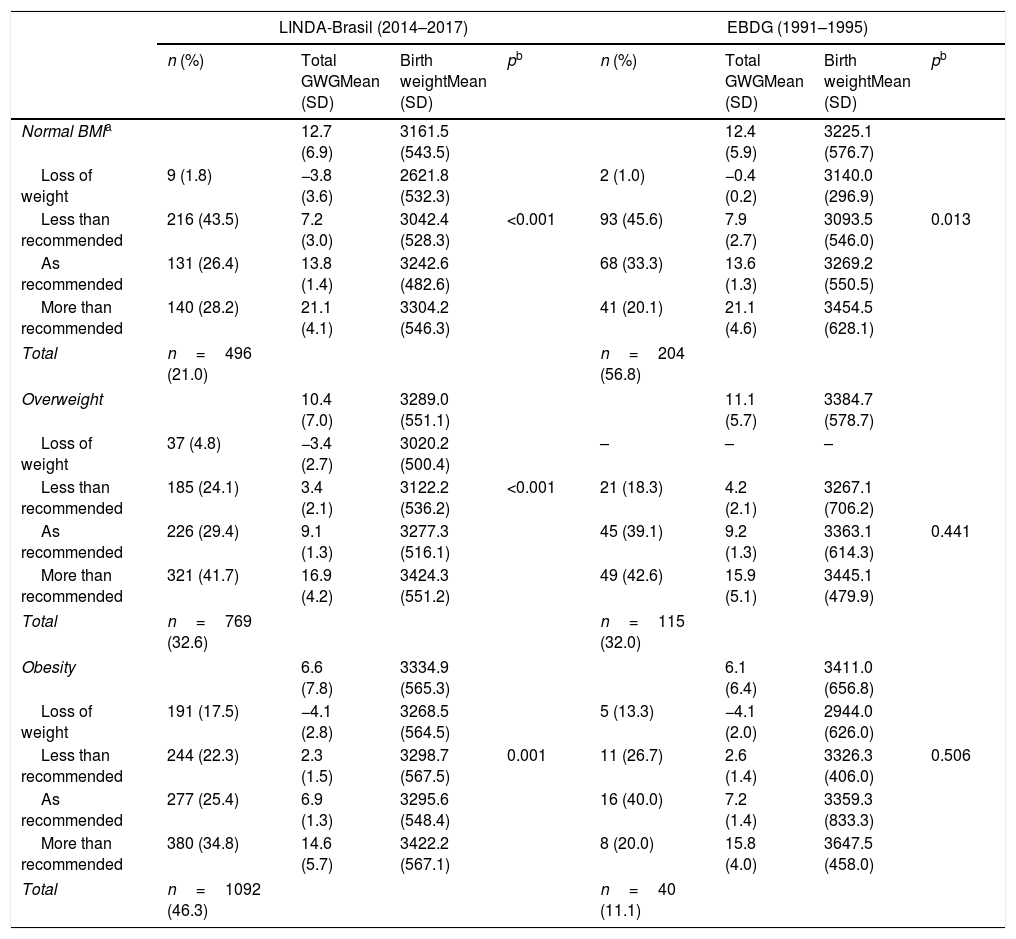
Table 2 presents outcomes of birth weight in relation to maternal total GWG according to pregestational BMI categories. In normal BMI women, gaining weight less than recommended (less than 11.5kg) was frequent, ∼45% in both cohorts. In the overweight group, gaining more than recommended was frequent, ∼42%, while in obese women, it almost doubled in the contemporary cohort (34.8% vs. 20.0%). Birth weight was, in general, higher as GWG increased, across all BMI categories. In normal BMI women, those who lost weight in the LINDA-Brasil study (1.8%) delivered newborns with the lowest birth weight among all women. In both cohorts, normal BMI women who lost, gained less than recommended, or even gained adequate weight, delivered neonates with a significantly lower birth weight, compared to those gaining weight more than recommended. In the LINDA-Brasil cohort, the same was observed in pregnancies of overweight or obese women, whereas in the EBDG cohort, these differences were not significant.
Birth weight according to adequacy of the total gestational weight gain in two cohorts of gestational diabetes women: LINDA-Brasil and EBDG.
| LINDA-Brasil (2014–2017) | EBDG (1991–1995) | |||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Total GWGMean (SD) | Birth weightMean (SD) | pb | n (%) | Total GWGMean (SD) | Birth weightMean (SD) | pb | |
| Normal BMIa | 12.7 (6.9) | 3161.5 (543.5) | 12.4 (5.9) | 3225.1 (576.7) | ||||
| Loss of weight | 9 (1.8) | −3.8 (3.6) | 2621.8 (532.3) | 2 (1.0) | −0.4 (0.2) | 3140.0 (296.9) | ||
| Less than recommended | 216 (43.5) | 7.2 (3.0) | 3042.4 (528.3) | <0.001 | 93 (45.6) | 7.9 (2.7) | 3093.5 (546.0) | 0.013 |
| As recommended | 131 (26.4) | 13.8 (1.4) | 3242.6 (482.6) | 68 (33.3) | 13.6 (1.3) | 3269.2 (550.5) | ||
| More than recommended | 140 (28.2) | 21.1 (4.1) | 3304.2 (546.3) | 41 (20.1) | 21.1 (4.6) | 3454.5 (628.1) | ||
| Total | n=496 (21.0) | n=204 (56.8) | ||||||
| Overweight | 10.4 (7.0) | 3289.0 (551.1) | 11.1 (5.7) | 3384.7 (578.7) | ||||
| Loss of weight | 37 (4.8) | −3.4 (2.7) | 3020.2 (500.4) | – | – | – | ||
| Less than recommended | 185 (24.1) | 3.4 (2.1) | 3122.2 (536.2) | <0.001 | 21 (18.3) | 4.2 (2.1) | 3267.1 (706.2) | |
| As recommended | 226 (29.4) | 9.1 (1.3) | 3277.3 (516.1) | 45 (39.1) | 9.2 (1.3) | 3363.1 (614.3) | 0.441 | |
| More than recommended | 321 (41.7) | 16.9 (4.2) | 3424.3 (551.2) | 49 (42.6) | 15.9 (5.1) | 3445.1 (479.9) | ||
| Total | n=769 (32.6) | n=115 (32.0) | ||||||
| Obesity | 6.6 (7.8) | 3334.9 (565.3) | 6.1 (6.4) | 3411.0 (656.8) | ||||
| Loss of weight | 191 (17.5) | −4.1 (2.8) | 3268.5 (564.5) | 5 (13.3) | −4.1 (2.0) | 2944.0 (626.0) | ||
| Less than recommended | 244 (22.3) | 2.3 (1.5) | 3298.7 (567.5) | 0.001 | 11 (26.7) | 2.6 (1.4) | 3326.3 (406.0) | 0.506 |
| As recommended | 277 (25.4) | 6.9 (1.3) | 3295.6 (548.4) | 16 (40.0) | 7.2 (1.4) | 3359.3 (833.3) | ||
| More than recommended | 380 (34.8) | 14.6 (5.7) | 3422.2 (567.1) | 8 (20.0) | 15.8 (4.0) | 3647.5 (458.0) | ||
| Total | n=1092 (46.3) | n=40 (11.1) | ||||||
LINDA-Brasil, Lifestyle Intervention for Diabetes Prevention After Pregnancy-Brasil; EBDG, Estudo Brasileiro de Diabetes Gestacional; BMI, body mass index, GWG, gestational weight gain.
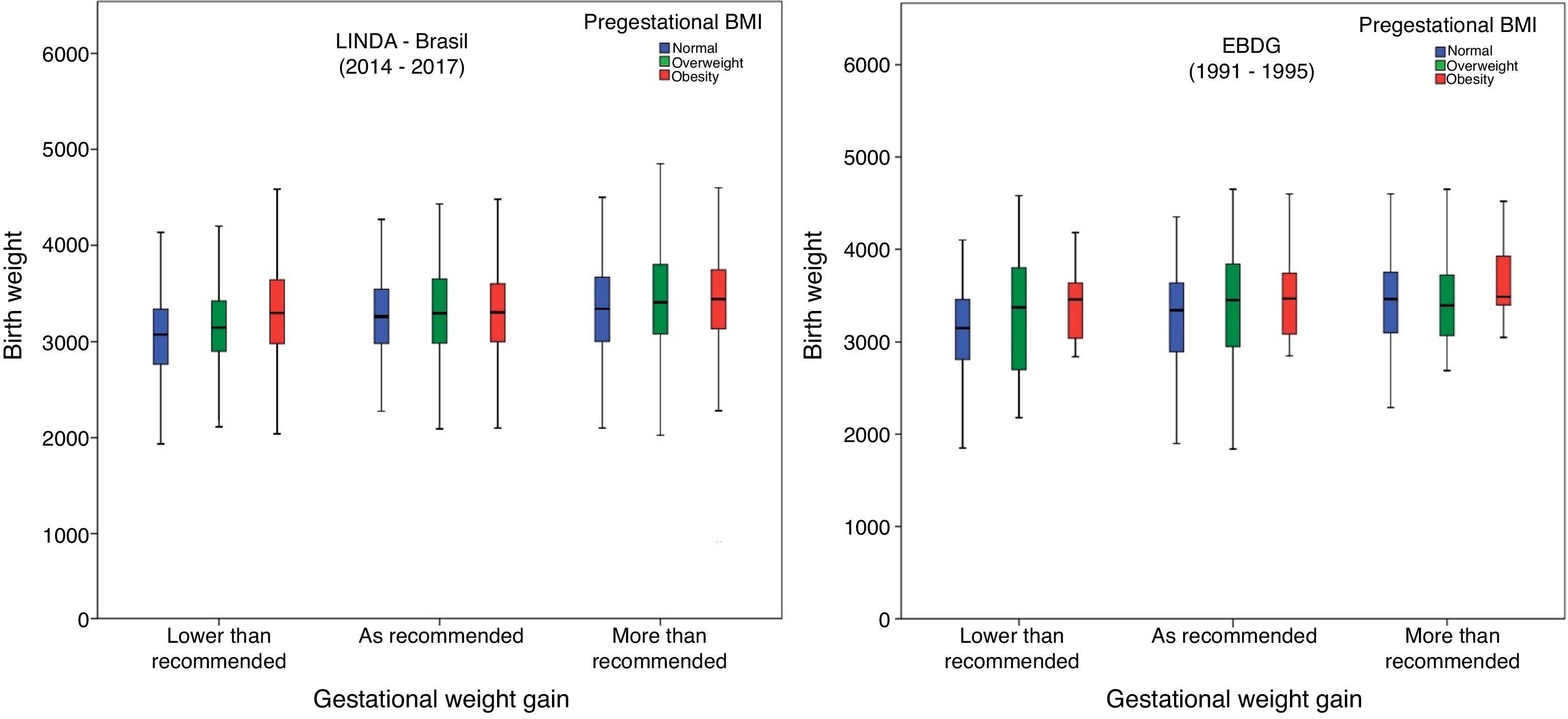
In Fig. 1, birth weight according to GWG and prepregnancy BMI is illustrated. In both cohorts, in mothers who had insufficient GWG, newborns had a lower median birth weight when their mothers had normal BMI, compared to those with overweight or obesity. In the LINDA-Brasil study, a higher birth weight median was observed with increased pregestational BMI in the excessive GWG category. Conversely, in the EBDG study, those with pregestational overweight had lower median birth weight in the excessive GWG category.
Birth weight according to weight gain by the Institute of Medicine categories in two cohorts of gestational diabetes: LINDA-Brasil (2014–2017) and EBDG (1991–1995).
The authors analyzed underweight and normal pregnancy BMI together, as there were only 18 (0.8%) participants in LINDA-Brasil with underweight and nine (2.5%) in EBDG.
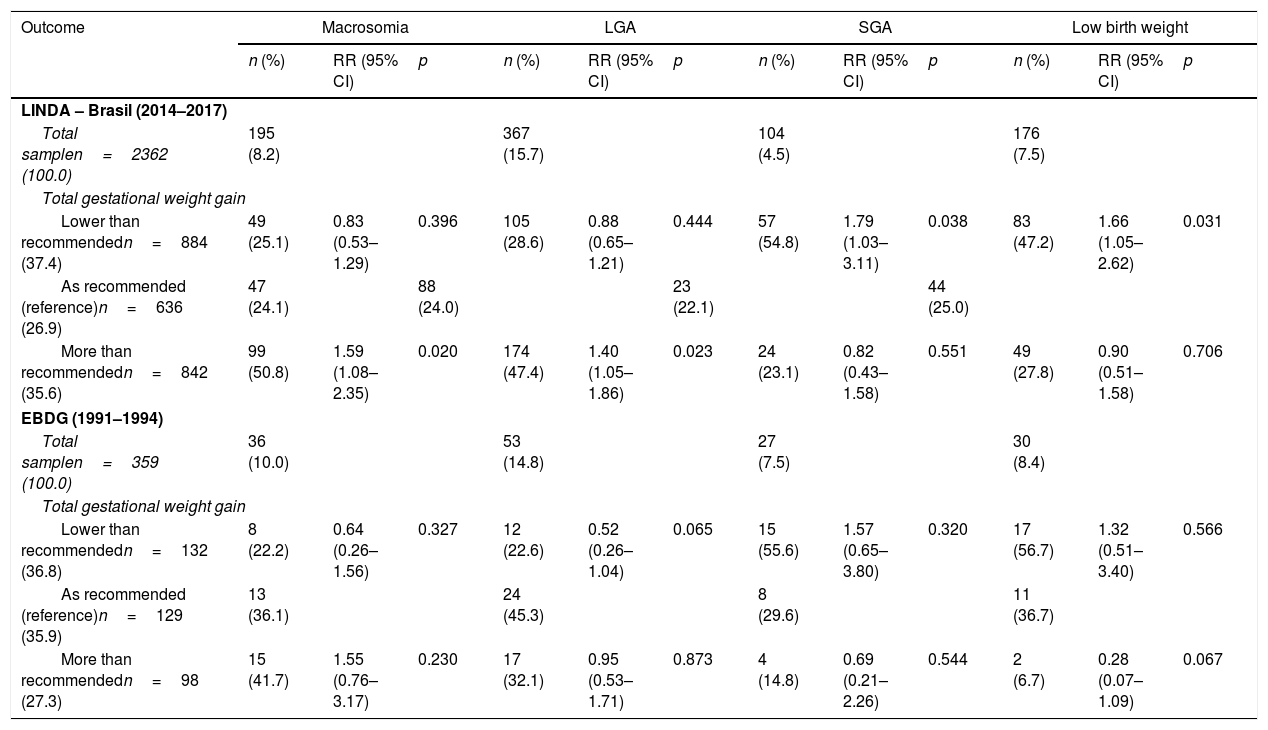
In Table 3, number and percent of cases of macrosomia, low birth weight, LGA and SGA in each cohort, and the relative risk of each outcome adjusted for the variables significant in the univariable analysis (Supplementary Table) are presented. In LINDA-Brasil, GWG more than recommended raised the risk of macrosomia in 63% and of LGA infants in 38%, whereas lower weight gain increased the risk of newborns with low birth weight in 59% and of SGA in 76%. In EBDG, these associations were similar, but not statistically significant.
Risks of birth weight deviations according to gestational weight gain in two Brazilian cohorts of women with gestational diabetes: LINDA-Brasil and EBDG.
| Outcome | Macrosomia | LGA | SGA | Low birth weight | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | RR (95% CI) | p | n (%) | RR (95% CI) | p | n (%) | RR (95% CI) | p | n (%) | RR (95% CI) | p | |
| LINDA – Brasil (2014–2017) | ||||||||||||
| Total samplen=2362 (100.0) | 195 (8.2) | 367 (15.7) | 104 (4.5) | 176 (7.5) | ||||||||
| Total gestational weight gain | ||||||||||||
| Lower than recommendedn=884 (37.4) | 49 (25.1) | 0.83 (0.53–1.29) | 0.396 | 105 (28.6) | 0.88 (0.65–1.21) | 0.444 | 57 (54.8) | 1.79 (1.03–3.11) | 0.038 | 83 (47.2) | 1.66 (1.05–2.62) | 0.031 |
| As recommended (reference)n=636 (26.9) | 47 (24.1) | 88 (24.0) | 23 (22.1) | 44 (25.0) | ||||||||
| More than recommendedn=842 (35.6) | 99 (50.8) | 1.59 (1.08–2.35) | 0.020 | 174 (47.4) | 1.40 (1.05–1.86) | 0.023 | 24 (23.1) | 0.82 (0.43–1.58) | 0.551 | 49 (27.8) | 0.90 (0.51–1.58) | 0.706 |
| EBDG (1991–1994) | ||||||||||||
| Total samplen=359 (100.0) | 36 (10.0) | 53 (14.8) | 27 (7.5) | 30 (8.4) | ||||||||
| Total gestational weight gain | ||||||||||||
| Lower than recommendedn=132 (36.8) | 8 (22.2) | 0.64 (0.26–1.56) | 0.327 | 12 (22.6) | 0.52 (0.26–1.04) | 0.065 | 15 (55.6) | 1.57 (0.65–3.80) | 0.320 | 17 (56.7) | 1.32 (0.51–3.40) | 0.566 |
| As recommended (reference)n=129 (35.9) | 13 (36.1) | 24 (45.3) | 8 (29.6) | 11 (36.7) | ||||||||
| More than recommendedn=98 (27.3) | 15 (41.7) | 1.55 (0.76–3.17) | 0.230 | 17 (32.1) | 0.95 (0.53–1.71) | 0.873 | 4 (14.8) | 0.69 (0.21–2.26) | 0.544 | 2 (6.7) | 0.28 (0.07–1.09) | 0.067 |
LINDA-Brasil, Lifestyle Intervention for Diabetes Prevention After Pregnancy-Brazil; EBDG, Estudo Brasileiro de Diabetes Gestacional; LGA, large for gestational age; SGA, small for gestational age; RR, relative risk; 95% CI, 95% confidence interval; Poisson regression with robust variance; adjustments as below.
LINDA-Brasil:
Macrosomia adjusted for age, schooling, gravidity, pregestational body mass index, fasting and 2-h glycemia, total gestational weight gain, and gestational age at delivery;
LGA adjusted for age, schooling, gravidity, pregestational body mass index, fasting and 2-h glycemia, total gestational weight gain, and gestational age at delivery;
SGA adjusted for age, schooling, smoking, gravidity, pregestational body mass index, fasting and 2-h glycemia, total gestational weight gain, and gestational age at delivery in weeks and pregestational body mass index;
Low birth weight adjusted for age, schooling, race, smoking, gravidity, pregestational body mass index, fasting and 2-h glycemia, total gestational weight gain, and gestational age at delivery.
EBDG:
Macrosomia adjusted for age, schooling, pregestational body mass index, fasting and 2-h glycemia, total gestational weight gain, and gestational age at delivery;
LGA adjusted for age, schooling, pregestational body mass index, fasting and 2-h glycemia, total gestational weight gain;
SGA adjusted for age, schooling, smoking, pregestational body mass index, fasting and 2-h glycemia, total gestational weight gain;
Low birth weight adjusted for age, schooling, race, smoking, pregestational body mass index, fasting and 2-h glycemia, total gestational weight gain, and gestational age at delivery.
The present study discloses a marked increase in pregestational BMI in women with gestational diabetes, leading to a distinct BMI profile: higher rates of adequate BMI in the 1990s and higher rates of obesity in the 2010s, with current obesity rates almost four times higher. Despite this, the results show that women in the contemporary cohort (LINDA-Brasil, 2014–2017) had lower weight gain during pregnancy. Women with less than recommended GWG were at increased risk of delivering low birth weight and SGA newborns, while those with excessive gain delivered macrosomic and LGA newborns.
A relevant change in pregestational nutritional profile of women of childbearing age, leading to increased rates of overweight and obesity at the beginning of pregnancy, has been documented in Brazil across time.3,4 This is also prominently reflected in women with GDM: in the earlier cohort, EBDG (1991–1995), only 11.1% of the women started pregnancy in obesity category, whereas this number quadrupled (to 41.3%) in the current cohort (LINDA-Brasil). Overweight was found in around 32% of women in both cohorts. These rates largely reflect the nutritional transition that occurred in Brazil, with even higher rates of pregestational obesity in GDM women. This is consistent with the well-known association of GDM with higher pregestational BMI, mainly with obesity.8
Taking into account the 2009 IOM recommendations for adequacy of GWG, the authors observed high frequencies of inadequate GWG, either excessive or insufficient, in both cohorts. In LINDA-Brasil, GWG more than recommended was verified in 34.8% of obese women, compared to only 20.0% in EBDG. Kase et al.18 reported that, among pregnant women with GDM, 71% were obese, and excessive weight gain was frequent (40%), even higher than the frequencies found in LINDA-Brasil (35.6%). Conversely, in obese Polish GDM women, 21% gained excessive weight,19 a frequency similar to the one found in EBDG (1991–1995), 27.3%. Excessive GWG was also common in Irish GDM women: 35% in overweight and 48% in obese women.20 Although few studies reported weight gain across BMI categories, excessive GWG was common in overweight and obese women (∼44% in each stratum).9
Although a large proportion of the LINDA-Brasil cohort began pregnancy with obesity, total GWG was lower than in the 1990s cohort (EBDG). The results also showed a high frequency of GWG below that recommended (loss or less than recommended) among women with normal pre-gestational BMI: 45.3% in LINDA-Brasil and 46.6% in EBDG, figures similar to those previously reported, ∼40%.9 In an Australian cohort, it was reported that more than 45% of women with normal or low pre-gestational BMI did not reach the target weight recommended by IOM in 2009.21 Less than recommended GWG was close to 20% in overweight GDM women, and close to 28% in those with obesity.9
It is well known that not only prepregnancy BMI but also GWG have impact on birth weight. In a meta-analyses including individual data on 265,270 births born to women without hyperglycemia, obesity posed a risk factor of 2.15 of delivering an LGA newborn, with a population-attributable risk fraction (PARF) of 6.6%, while excessive weight gain posed a risk of 2.11, with a PARF of 31.6%.22 Weight gain and prepregnancy BMI affect birth weight in such a way that GWG is relevant in lower BMI categories and less important in women with higher BMI.23 Low GWG is generally associated with both low birth weight and SGA, even in obese women,24 whilst excessive GWG is linked to increased rates of macrosomia and LGA. In GDM, this modifiable factor has been frequently evaluated but it is still not well established how much weight women must gain to prevent birth weight deviations. For example, it was only possible to disclose that weight loss impacted birth weight in women with normal BMI in the contemporary cohort, in which mean birth weight was close to what is considered low birth weight. In other studies, women with GDM who gained below the 2009 IOM recommendations had a greater chance of having SGA and low birth weight infants.25 Weight loss or insufficient gain in overweight or obese pregnant women is associated with an increased risk of SGA and low birth weight infants26; the present data add to these findings in GDM women.
Concerning weight gain more than recommended and its consequences for the fetus, high frequencies of macrosomia and LGA were associated with excessive GWG in Irish women.20 The results are similar herein, although in general, women in the contemporary cohort gained less weight than those in the past. It is of note that high rates of weight gain above current recommendations also occurred in overweight women (in both cohorts), emphasizing the need to slow GWG in this group. Although birth weight was not importantly affected, these mothers have increased risk of future obesity and diabetes.27
Besides wider access to treatment nowadays, other factors, such as continuous and early glucose monitoring, seemed to impact GWG: the rate of women with excessive gain was lower in the monitored group and women who started early monitoring gained less weight.28 It can be speculated that better management of GDM associated with routine follow-up at high-risk prenatal care clinics in the 2017 cohort of LINDA-Brasil occurred more commonly than in the earlier cohort, and despite the higher rates of pregestational obesity being more common now, adequate or lower weight gains, and, perhaps, more strict glycemic targets, may have contributed to the results herein. These improvements in the delivery of pregnancy care were seen almost nationwide, since 2002.29 Free distribution of medications and better care of diabetes may also have contributed, since self-monitoring of capillary glycemia is considered an important tool for the adequate control of GDM.28 There were also improvements in other social and health indicators, such as higher schooling and a decrease in smoking rates.29 The findings of the present study reflect these improvements: increased mean educational level, from 2.5±1.5 in the 1990s cohort to 4.1±1.4 in the 2010s, while smoking decreased from 16.7% to 7.3%.
The strengths of this study merit mention: as far as we know, EBDG is the largest Brazilian study on GDM and LINDA-Brasil is the largest cohort including postpartum follow-up in Brazilian women with previous GDM. The comparison permitted evaluation of the nutritional transition from the 1990s to the 2010s, disclosing an important increase in maternal obesity rates, favoring the delivery of heavier newborns.
Limitations also have to be pointed out. Prepregnancy weight was reported by women in both cohorts, but high agreement between reported and measured weight is described in pregnant women.30 Height was reported in LINDA-Brasil; the weight of the newborn was collected from the mother's information in the immediate postpartum in LINDA-Brasil, but women were requested to have the newborn's card in hands at the time of the interview. GDM definition varied overtime. It is possible that current definition includes milder cases diagnosed solely on having altered fasting plasma glucose or one altered cutpoint on the OGTT. In the EBDG cohort, cases of GDM occurred in less than 10% of the sample, precluding subgroup analyses.
The present study illustrates a large increase in maternal obesity from the 1990s to the 2010s, and contributes to a better understanding of GWG and birth weight imbrications in women with GDM. The alarming rate of maternal obesity found in the 2014–2017 cohort and the high frequencies of inadequate weight gain found in the two cohorts, with their effects on birth weight, impose the need to prepare women even before pregnancy, through prepregnancy counseling to avoid overweight/obesity at conception, as well as careful follow-up of their pregnancies regarding weight gain.
FundingThe authors would like to thank the National Council for Scientific and Technological Development (CNPq) and Lilly for the financial assistance and support to carry out the original studies.
Conflicts of interestThe authors declare no conflicts of interest.
Vânia Naomi Hirakata, for helping with the statistical analysis.