This study aimed to investigate non-alcoholic fatty liver disease (NAFLD) occurrence and factors associated with the disease in phenylketonuria (PKU) patients undergoing exclusive dietary treatment.
MethodThis cross-sectional study included 101 adolescents 10 to < 20 years of age with PKU, who were undergoing exclusive dietary treatment and monitored since early diagnosis at a single reference service. Anthropometric and biochemical assessments were performed and food intake was documented, and an ultrasound diagnosis of NAFLD was established. Data were evaluated using the Student's t-test for continuous variables, the chi-square for categorical variables, and logistic regression using the Wald chi-squared test; differences with p < 0.05 were considered to be statistically significant.
ResultsNAFLD was detected in 26 (25.7%) teenagers. There was no difference in prevalence between the sexes or nutritional status. The final logistic regression model revealed low sensitivity (26.1%) and high specificity (94.7%). The specificity suggested a lower likelihood of NAFLD in older adolescents, in the presence of normal or high levels of alkaline phosphatase, lower carbohydrate intake, and adequate protein and lipid intake.
ConclusionsThe prevalence of NAFLD in adolescents with PKU was higher than that found in healthy Brazilian adolescents and similar to that found in obese Brazilian children, suggesting a higher risk for NAFLD in patients with PKU treated exclusively by dietary modification.
Non-alcoholic fatty liver disease (NAFLD) may be defined as liver abnormalities caused by the accumulation of >5% of lipids in the cytoplasm of hepatocytes in patients with no history of excessive alcohol consumption, viral hepatitis, or autoimmune disease. It is an important cause of morbidity and mortality associated with liver disease.1 The prevalence of NAFLD has increased concomitantly with the increase in obesity and represents an important current public health problem.1–5 NAFLD is prevalent in all age groups, with a higher prevalence in adults. Schwimmer et al.6 found steatosis (>5% of liver fat) in 13% of liver tissue samples from necropsies of children and adolescents between two and 19 years of age; it was predominant among obese children (38%). Steatosis was found in 5.3% of the evaluated samples in a similar study from Poland,7 and it was also frequently observed in overweight children (55.6%).
NAFLD may occur with increased liver enzyme and inflammatory cytokine levels, which indicate hepatic tissue damage, progression to steatosis, steatohepatitis, fibrosis, and cirrhosis, and consequently, increased mortality among young adults. Due to its evolution―which is often asymptomatic in the early stages―the detection of early liver changes is very important.1–4 NAFLD may evolve into remission in some cases.3
Liver biopsy is considered the gold standard method to diagnose NAFLD.4,8 However, magnetic resonance imaging with spectroscopy is less invasive and can also be used alternatively.4,8 More recently, hepatic ultrasound has become an effective and widely used technique for diagnosis because it reduces the costs and risks of the procedure.4 In this method, the hepatorenal index (HRI) is used and calculated by dividing the average brightness found in the image corresponding to the liver by the average brightness of the image corresponding to the right kidney. This is a simple, non-invasive, reliable, and objective technique for diagnosing NAFLD, yielding results similar to those obtained by gold-standard methods and additionally confirmed in studies.4,8
Risk factors for NAFLD include age, sex, and race. Increased abdominal fat, body mass index (BMI), insulin resistance, and family history are all associated with the development of NAFLD.2 As per previous literature, the other risk factors for NAFLD include obesity and other metabolic diseases such as type 2 diabetes mellitus, hyperlipidemia, and metabolic syndrome.1 Although the consumption of certain foods and nutrients, such as excessive intake of glucose, fructose, saturated and trans fat, has also been reported, few studies have confirmed an association between NAFLD and specific dietary patterns.5
Phenylketonuria (PKU) (OMIM # 261600) is an inborn autosomal recessive error in metabolism due to the lack or insufficient activity of the hepatic enzyme phenylalanine hydroxylase. The phenylalanine hydroxylase enzyme is involved in the conversion of phenylalanine (Phe) into tyrosine. Untreated, PKU evolves into various clinical manifestations, with intellectual disability being the most severe.9
The diet plays a key role in the treatment of PKU, and the patients are recommended to consume a peculiar diet. Dietary treatment includes natural protein restriction, Phe-free-L-amino acid supplements, low protein foods, and complete restriction of animal protein sources, such as meat, eggs, milk, dairy products, and beans. For protein complementation, a metabolic formula is offered to contain all amino acids except Phe, along with vitamins and minerals. The diet aims to maintain adequate levels of serum Phe.9 Non-adherence to dietary treatment may cause intellectual disability and neurological alterations. Dieting with a restriction of most natural proteins, however, may pose a potential risk of excessive consumption of carbohydrates and fats to meet energy needs, which may lead to obesity and other comorbidities.10
The authors hypothesized that patients with PKU undergoing exclusive dietary treatment are at a higher risk of developing NAFLD. However, to our knowledge, no studies have been reported on this topic so far. Therefore, the aim of the present study was to evaluate the occurrence of NAFLD in adolescents with PKU, who were undergoing exclusive dietary treatment, and the factors associated with it.
Material and methodsThis was a cross-sectional study involving adolescents between 10 and 20 years of age. The patients who were diagnosed with PKU at an early stage and undergoing exclusive dietary treatment were enrolled in this study. The patients were regularly monitored by a multidisciplinary team at a public hospital and a reference service that performs the diagnosis, treatment, and follow-up of all children screened for the disease in the state.
A total of 128 adolescents with an early diagnosis of PKU (starting treatment before 21 days) in the age group of interest were identified throughout the state. Of these, three were excluded: one due to drug use; one due to pregnancy; and one for treatment abandonment. Seven patients refused to perform the necessary procedures. Seventeen patients could not be contacted because they had moved away from the addresses they had initially provided. Patients with late diagnoses of the disease were excluded. Ultimately, the study was performed with 101 adolescents between 10 and 20 years of age. Data were collected between January 2017 and December 2018.
Weight and height were measured. Nutritional status was assessed according to BMI, which was calculated using the formula BMI = weight (kg)/height2 (m2). Patients >18 years of age were classified according to World Health Organization (WHO) criteria,11 and patients <18 years of age were classified according to the standards established by the WHO curves.12 The patients were classified based on Z-scores as overweight (Z-scores >1 and ≤2), obese (Z-score>2), eutrophic (Z-scores ≥-2 and ≤1), and with low BMI (Z-score< - 2).
Food intake was assessed using the quantitative food frequency questionnaire (FFQ), validated by Colucci.13 FFQ calculations were performed using Diet Pro software version 5i, with total calories, carbohydrates, proteins, and lipids evaluated according to the Brazilian Table of Food Composition (i.e., TACO).14 Phe intake was evaluated using the North American Table (United States Department of Agriculture).15 The consumption of macronutrients was classified as adequate, inadequate, or excessive according to values established by the dietary reference intake (DRI).16 Protein intake, plus 50%, and Phe intake were evaluated according to the recommendations of Acosta and Yannicelli.17 Biochemical evaluations included total cholesterol (TC) and fractions (low-density lipoprotein cholesterol [LDL-c] and high-density lipoprotein cholesterol [HDL-c]), triglycerides, ultrasensitive C-reactive protein, alanine aminotransferase, aspartate aminotransferase (AST), gamma-glutamyltransferase, alkaline phosphatase (AP), fasting glucose, and basal insulin. The Homeostasis Model Assessment of Insulin Resistance (i.e., HOMA-IR) was calculated according to the mathematical model described by Matthews et al.18
To evaluate disease control, the mean values of 4,447 serum Phe evaluations obtained from the patients during adolescence (10 to < 20 years of age) were considered. Levels were considered to be inadequate if the values were outside the reference values for the age group studied: ≥ 120 and ≤ 700 μmol/L.19
The abdominal ultrasound was performed in a specialized clinic by a single experienced examiner, who did not have access to the biochemical test results of the patients, after a 6-h fast, with adolescents positioned supine and arms elevated. The Supersonic Aixplorer version 8.5.02 (Aix-en-Provence, France) CONVEXA SC6-1 probe with an average frequency of 3.5 MHz was used. Images were evaluated based on the ratio of the echogenicity of the liver to that of the right kidney. Average brightness was calculated using numerical values assigned to grayscale pixels. The average brightness of the liver was divided by the average brightness of the kidney to generate the HRI. The degree of hepatic steatosis was determined according to the HRI assessment and the cut-off value proposed by Martin-Rodriguez et al.4 According to HRI classification, two groups, one with NAFLD and the other without, were formed.
To verify whether the prevalence of overweight and obesity found in the present study population was similar to that in the general population, the authors compared the nutritional status of 46 patients aged 13–17 years with that of healthy students of the same age from the PeNSE (2015).20 This survey studied the frequency of overweight and obesity in all Brazilian states using a sample of 102,301 students aged 13–15 years and 10,926 students aged 16–17 years; the maximum sampling error was 3% with a 95% confidence interval. The prevalence rate of overweight and obesity in the survey was 23.7%.
The data are expressed as numbers (percentages) and the mean ± standard deviations (SD). The Student's t-test was used to compare continuous variables, while the chi-squared test was used for the comparison of categorical variables in the univariate analysis. The multivariate logistic regression analysis was performed using variables with a p-value < 0.40 using the Wald chi-squared test. To compare the nutritional status of patients aged 13–17 years with that of healthy students of the same age in the PeNSE (2015),20 the authors used the chi-square test for adherence. Differences with p < 0.05 were considered statistically significant.
This study was approved by the Research Ethics Committee, and an informed consent form was obtained from the legal guardians.
ResultsThe present study included 101 adolescents, corresponding to 79% of adolescents with PKU in Minas Gerais. There were 55 males (54.5%) and 46 females (45.5%), with a mean (± SD) age of 14.8 ± 3.2 years. Sixty-nine adolescents (68.3%) were eutrophic, 16 (15.8%) overweight, 12 (11.9%) obese, and four (4%) with low BMI.
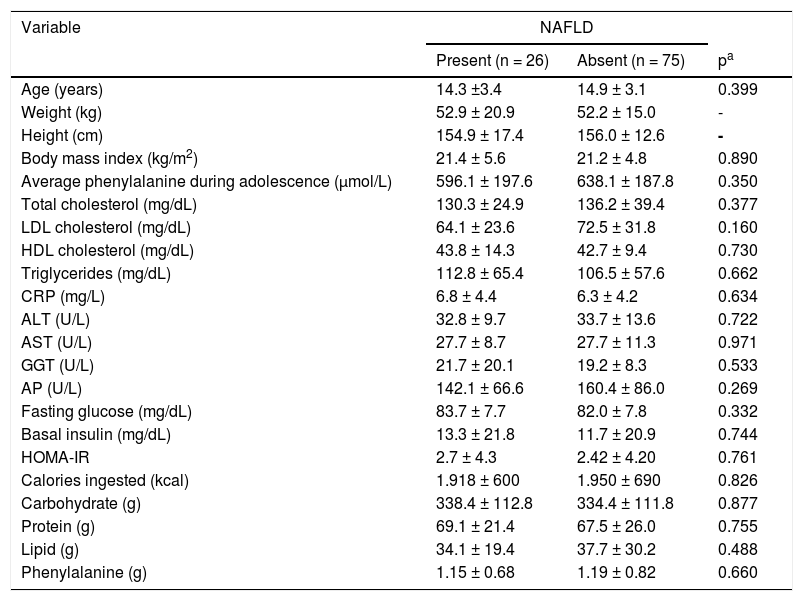
The presence of NAFLD was detected in 26 (25.7%) adolescents: 14 (53.8%) males and 12 (46.2%) females (p = 0.942). Table 1 shows a comparison and analysis of the association between NAFLD and the variables that were examined.
Comparative analysis and association between the presence of NAFLD and anthropometric variables, biochemical tests, and food intake.
| Variable | NAFLD | ||
|---|---|---|---|
| Present (n = 26) | Absent (n = 75) | pa | |
| Age (years) | 14.3 ±3.4 | 14.9 ± 3.1 | 0.399 |
| Weight (kg) | 52.9 ± 20.9 | 52.2 ± 15.0 | - |
| Height (cm) | 154.9 ± 17.4 | 156.0 ± 12.6 | - |
| Body mass index (kg/m2) | 21.4 ± 5.6 | 21.2 ± 4.8 | 0.890 |
| Average phenylalanine during adolescence (μmol/L) | 596.1 ± 197.6 | 638.1 ± 187.8 | 0.350 |
| Total cholesterol (mg/dL) | 130.3 ± 24.9 | 136.2 ± 39.4 | 0.377 |
| LDL cholesterol (mg/dL) | 64.1 ± 23.6 | 72.5 ± 31.8 | 0.160 |
| HDL cholesterol (mg/dL) | 43.8 ± 14.3 | 42.7 ± 9.4 | 0.730 |
| Triglycerides (mg/dL) | 112.8 ± 65.4 | 106.5 ± 57.6 | 0.662 |
| CRP (mg/L) | 6.8 ± 4.4 | 6.3 ± 4.2 | 0.634 |
| ALT (U/L) | 32.8 ± 9.7 | 33.7 ± 13.6 | 0.722 |
| AST (U/L) | 27.7 ± 8.7 | 27.7 ± 11.3 | 0.971 |
| GGT (U/L) | 21.7 ± 20.1 | 19.2 ± 8.3 | 0.533 |
| AP (U/L) | 142.1 ± 66.6 | 160.4 ± 86.0 | 0.269 |
| Fasting glucose (mg/dL) | 83.7 ± 7.7 | 82.0 ± 7.8 | 0.332 |
| Basal insulin (mg/dL) | 13.3 ± 21.8 | 11.7 ± 20.9 | 0.744 |
| HOMA-IR | 2.7 ± 4.3 | 2.42 ± 4.20 | 0.761 |
| Calories ingested (kcal) | 1.918 ± 600 | 1.950 ± 690 | 0.826 |
| Carbohydrate (g) | 338.4 ± 112.8 | 334.4 ± 111.8 | 0.877 |
| Protein (g) | 69.1 ± 21.4 | 67.5 ± 26.0 | 0.755 |
| Lipid (g) | 34.1 ± 19.4 | 37.7 ± 30.2 | 0.488 |
| Phenylalanine (g) | 1.15 ± 0.68 | 1.19 ± 0.82 | 0.660 |
NAFLD, non-alcoholic fatty liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; AP, alkaline phosphatase; GGT, gamma-glutamyltransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, Homeostasis Model Assessment– Insulin Resistance.
Data are presented as mean ± standard deviation unless otherwise indicated.
For the considered variables, the univariate analysis showed that there were no significant differences between adolescents with and without NAFLD.
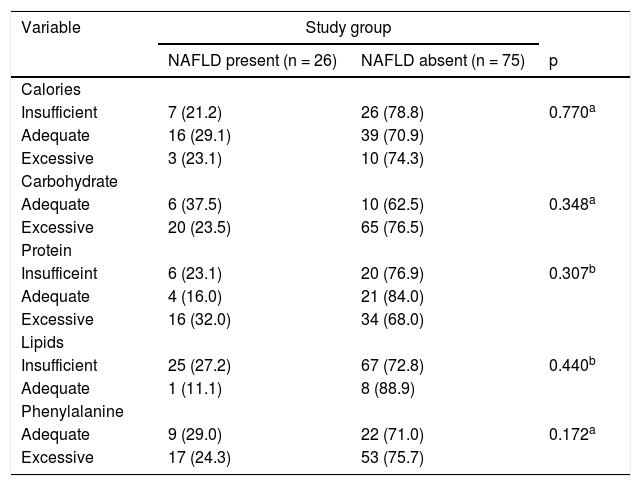
Analyses related to adequacies in the intake of macronutrients and Phe are shown in Table 2.
Comparative and association analyses between adequacy of food intake and occurrence of NAFLD.
| Variable | Study group | ||
|---|---|---|---|
| NAFLD present (n = 26) | NAFLD absent (n = 75) | p | |
| Calories | |||
| Insufficient | 7 (21.2) | 26 (78.8) | 0.770a |
| Adequate | 16 (29.1) | 39 (70.9) | |
| Excessive | 3 (23.1) | 10 (74.3) | |
| Carbohydrate | |||
| Adequate | 6 (37.5) | 10 (62.5) | 0.348a |
| Excessive | 20 (23.5) | 65 (76.5) | |
| Protein | |||
| Insufficeint | 6 (23.1) | 20 (76.9) | 0.307b |
| Adequate | 4 (16.0) | 21 (84.0) | |
| Excessive | 16 (32.0) | 34 (68.0) | |
| Lipids | |||
| Insufficient | 25 (27.2) | 67 (72.8) | 0.440b |
| Adequate | 1 (11.1) | 8 (88.9) | |
| Phenylalanine | |||
| Adequate | 9 (29.0) | 22 (71.0) | 0.172a |
| Excessive | 17 (24.3) | 53 (75.7) | |
NAFLD, non-alcoholic fatty liver disease.
Data are presented as n (%), unless otherwise indicated.
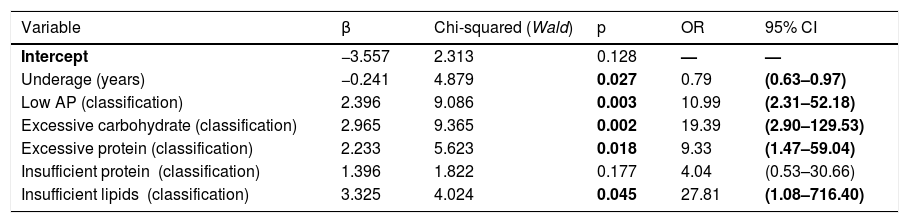
Among the considered variables, age, mean serum Phe level, AP, TC, LDL-c, fasting glycemia, and carbohydrate, protein, and lipid intake met the cut-off value for the multivariate analysis (p < 0.4). Although serum Phe values reached a p-value sufficient to enter into the logistic regression model, it did not reach statistical significance in the initial model and, as such, was removed from the final model (Table 3).
Final model derived from logistic regression analysis.
AP, alkaline phosphatase; CI, confidence interval; OR, odds ratio.
Bolded values indicate statistically significant differences.
Database: 98 children (23 cases WITH steatosis, 75 cases WITHOUT steatosis). Three outlier cases were removed from the study. Sensitivity 26.1/%; Specificity: 94.7%.
Subtitle:
AP (classification): 1 = Low AP; 0 = Normal AP.
Carbohydrate (classification): 1 = Adequate; 0 = Excessive.
Protein (classification):
Excessive protein = 1 and Insufficient protein = 0 Excessive.
Excessive protein = 0 and Insufficient protein = 1 Insufficient.
Excessive protein = 0 and Insufficient protein = 0 Adequate.
Lipids (classification): 1 = Insufficient lipids and 0 = Adequate.
1 = NAFLD present; 0 = NAFLD absent.
The final logistic regression model yielded a sensitivity of 26.1% and a specificity of 94.7%. More specifically, the model demonstrated a good ability to predict the absence of NAFLD, although its ability to predict the presence of NAFLD was low.
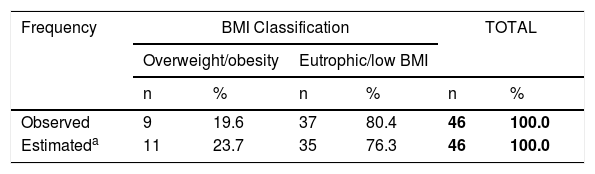
Table 4 shows the comparison of the prevalence of obesity and overweight in patients with PKU aged 13–17 years and in healthy students of the same age from the PeNSE.20 The equality hypothesis was not rejected (p = 0.488).
The observed and estimated results of the Brazilian National School Health Survey (PeNSE, 2015)20 with regard to the prevalence of overweight and obesity.
| Frequency | BMI Classification | TOTAL | ||||
|---|---|---|---|---|---|---|
| Overweight/obesity | Eutrophic/low BMI | |||||
| n | % | n | % | n | % | |
| Observed | 9 | 19.6 | 37 | 80.4 | 46 | 100.0 |
| Estimateda | 11 | 23.7 | 35 | 76.3 | 46 | 100.0 |
DATABASE: 46 patients.
Chi-square test for adherence: p = 0.488.
Percentages were calculated in relation to the total of the cases and percentages.
Values extracted from reference article “The Brazilian National School Health Survey (PeNSE, 2015)”,20 in which the numbers were calculated based on the prevalence of overweight/obesity of 23.7%.
BMI, body mass index.
Even though PKU is a rare disease, it is the most common inborn error in metabolism of the amino acids. It is the disease that newborns are screened for the most frequently. Numerous countries screen for PKU, thus, thousands of patients are monitored globally. It is important to evaluate not only the intellectual evolution of the patients but also the adverse effects of the treatments.
Although there are different types of treatments, diet plays a key role in all of them, which includes natural protein restriction. Restricting the consumption of most natural proteins can pose a potential risk of overconsumption of carbohydrates and fats to meet energy needs. This may lead to obesity and other comorbidities. Patients undergoing exclusive dietary treatment may be more susceptible to these risks. Hence, it was suspected that these patients were at a higher risk of developing NAFLD. Unfortunately, studies about NAFLD in patients with PKU or hepatic effects of the diet in these patients were not found in the literature. Thus, this is the first study to address this topic.
The metabolic formula with free amino acids used by adolescents with PKU has faster absorption and leads to higher plasma concentrations, however, there is less effective nitrogen retention and greater hepatic oxidation of these amino acids when compared to natural protein intake. The data in the literature on the digestibility and bioavailability of L-amino acids as well as the association of these amino acids’ consumption with the development of NAFLD, were found to be insufficient.9
As hypothesized, it was found that 26/101 (25.7%) adolescents in this study following an exclusively PKU diet developed NAFLD, which suggests the possibility of this comorbidity in the follow-up of these patients.
The prevalence of NAFLD found in this study (25.7%) was significantly higher than expected in adolescents. It is much higher than that found in Brazilian adolescents with adequate weight (3.4%)21 and in Australian adolescents (15.2%).22 The overall prevalence of NAFLD has been estimated to be 25.4%, with the highest in the Middle East and South America and the lowest in Africa.1 However, this prevalence was also estimated in adults, and the prevalence is more common among adults than in adolescents.
Some characteristics of this sample should be emphasized. The study was performed in the only care center for patients with PKU in Minas Gerais, the second most populous state in Brazil, with more than 21 million inhabitants. This means that the studied population had greater socio-environmental and cultural similarity and reduced research bias compared to multicenter studies. All patients evaluated were, and continue to be, followed up on a regular basis in a unique referral center since birth, and they were treated exclusively through diet. For this reason, any confounding factors that could result from a treatment using the diet associated with other available tools for the treatment of PKU were removed; the sample comprises 79% of all known patients diagnosed with PKU in the State of Minas Gerais, until that.
The study was conducted exclusively among adolescents. Since the disease can progress with age to hepatic steatosis, which is more common in adults,23 knowing its prevalence among adolescents with PKU will help prevent steatosis and liver cirrhosis, possibly reducing the number of patients who are candidates for liver transplantation.
There are currently more recent recommendations9 than those of Acosta and Yannicelli,17 but those patients started treatment between 10 and 20 years ago when the recommendations of Acosta and Yannicelli17 were the most used in the world.
Diet has been considered one of the risk factors for NAFLD. However, most studies have focused on individualized foods or nutrients, which are consumed in different combinations. A few studies have addressed the role of dietary patterns in NAFLD.5,22 The dietary pattern of patients with PKU is unique, and no studies have addressed the relationship between the PKU diet and NAFLD. The results of the study may indicate some kind of relationship between PKU adolescents who were exclusively treated with diet and NAFLD.
Obesity is considered to be one of the most important risk factors for NAFLD, and several studies have studied only obese patients or have observed a higher prevalence of the disease in overweight and obese patients.2,5,21,24,25 Since there was no control group of healthy adolescents, it was only possible to compare the prevalence of obesity in adolescents aged 13–17 years with PKU with the prevalence of obesity in students of the same age participating in a large cohort carried out in all regions of Brazil.20 The hypothesis of equality was not ruled out by the chi-square test of adherence.
The association between obesity and NAFLD was not found in the present study, and no significant difference in the prevalence of NAFLD was observed between obese and non-obese adolescents when considering weight (p = 0.86), BMI (p = 0.89), and abdominal circumference (p = 0.72). This suggests that other factors, such as PKU itself or the diet consumed by patients, may play some role in its development.
Concerning other associated factors with NAFLD, the role of sex is controversial.3 In this study, no difference was found in the distribution of NAFLD between the sexes, similar to the results in previous studies.21,25
The final logistic regression model was not satisfactory. The sensitivity was only 26.1%; however, its specificity (94.7%) was good, indicating that the model is better for identifying patients who are less likely to develop the disease than those more likely to develop it. This could mean that the variables used in the study were insufficient for the test.
The specificity of the final model suggests that NAFLD is less likely to occur in older PKU adolescents who have normal or high levels of AP, lower carbohydrate intake, and adequate protein and lipid intake.
In agreement with the indication of the specificity of the logistic regression model, Suzuki et al.26 found a higher propensity for NAFLD and hepatic fibrosis in prepubescent adolescents, compared to pubertal or post-pubertal adolescents. Similarly, adult studies suggest an increased risk of developing NAFLD in older individuals.2,23
Additionally, compatible with what was suggested by the specificity of the logistic regression model, Couce et al.27 reported a higher carbohydrate intake in patients with PKU than in controls without the disease. Interestingly, some authors have demonstrated a positive relationship between excessive carbohydrate intake and excessive consumption of saturated or trans fats with the occurrence of NAFLD.28,29 The authors did not find an association between protein consumption and the occurrence of NAFLD in the literature.
The protective role of normal or elevated levels of AP in the development of NAFLD suggested by the specificity of the final model could not be explained. In contrast, Almeida and Borges et al.8 reported higher levels of AP and other liver enzymes in patients with NAFLD. Lira et al.22 found no significant differences in AST and AP levels among obese adolescents with NAFLD and controls.
It is noteworthy that the prevalence of NAFLD in adolescents with PKU treated exclusively through diet was much higher than that reported in healthy Brazilian and Australian adolescents. This prevalence may depend on the diet or the PKU itself. Further studies are needed to verify whether this is a consistent finding to guide the treatment of adolescents with PKU.
Study conducted at the Universidade Federal de Minas Gerais (UFMG), Faculdade de Medicina, Belo Horizonte, MG, Brazil.