Amplitude-integrated electroencephalography (aEEG) is a simplified bedside neurophysiology tool that has been implemented in the neonatal intensive care unit and studied in an extensive range of clinical applications in the past decade. This critical review aimed to evaluate a variety of clinical applications of aEEG monitoring in diagnosis, clinical management, and prognosis assessment in critically ill neonates.
SourcesThe databases of Pubmed, SciELO, Lilacs, and Cochrane, books, and other online resources were consulted, as well as sources of professional experiences.
Summary of findingsThe clinical use of aEEG to access real-time brain function, background activity, and utility in seizures detection has been described. A critical review was realized considering the authors’ professional experience. Newborns with hypoxic-ischemic encephalopathy and seizures screening represent the most common studied population. However, several studies have shown interesting applications on preterm infants, newborns with congenital heart disease, and other clinical situations of high risk of injury to the developing brain.
ConclusionThe aEEG has shown to be a useful non-invasive bedside monitor that aids in evaluating brain function, background activity, and cyclicity. aEEG findings have also demonstrated good prognostic value in a group of critically ill neonates. The aEEG seizure diagnosis capability has limitations, which have been already well established. The use of neonatal brain monitoring such as aEEG was shown to give valuable information in several high-risk clinical situations.
Despite recent advances in perinatal care, the incidence of impaired outcomes in neonates at high risk for brain injury is still significant and represents a challenge in neonatal care. Precise evaluation and early diagnosis of brain injury play an important role in preventing neurological impairment. In the last several years, brain monitoring devices have been steadily gaining ground in neonatal intensive care units (NICU).1
Brain damage and the majority of electroencephalographic seizures occur in high-risk groups such as infants with hypoxic-ischemic encephalopathy, extreme prematurity, cardiac surgery, stroke, extracorporeal membrane oxygenation (ECMO), or meningitis. Still, they can also occur in other neurologic and systemic conditions.2–10
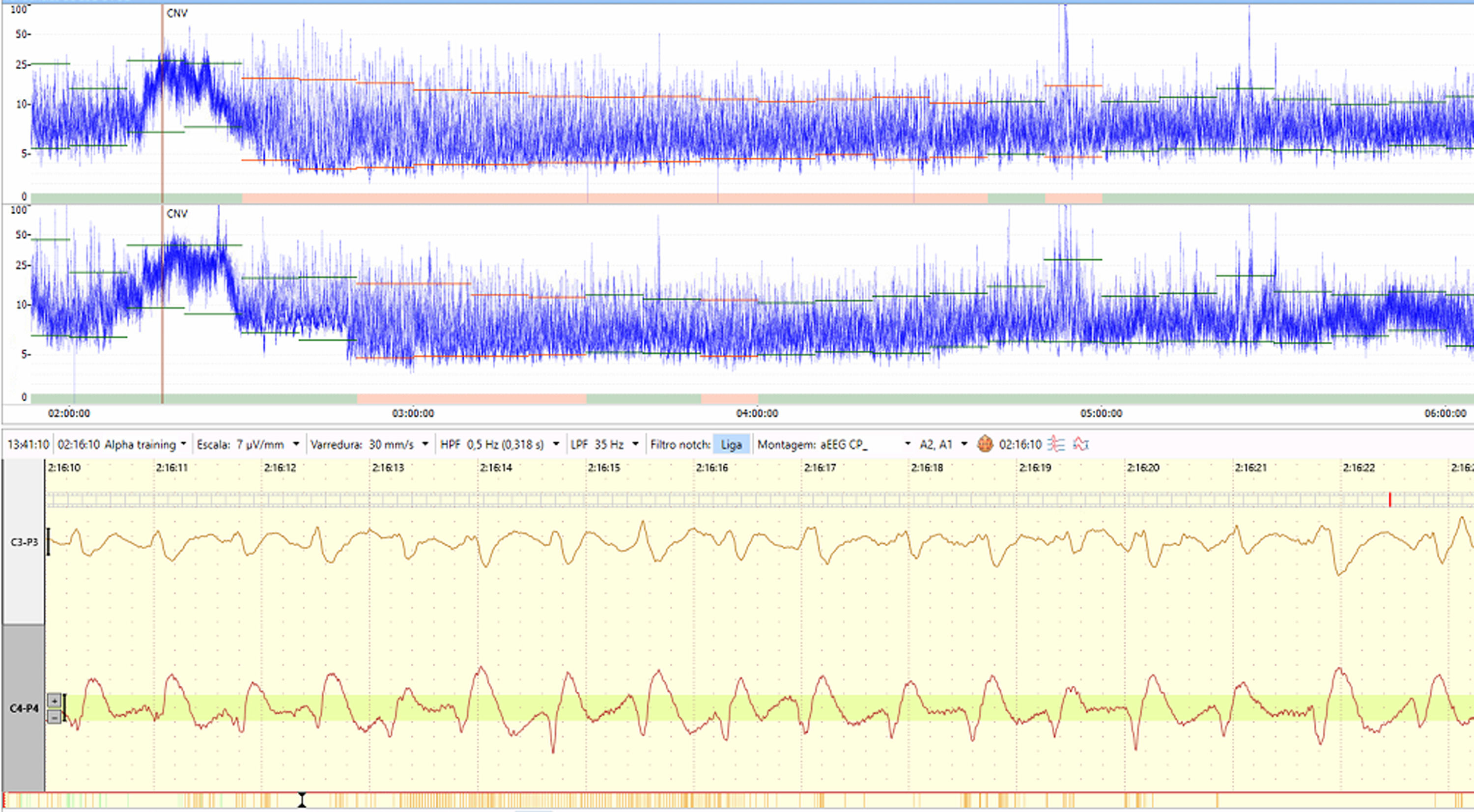
Amplitude-integrated electroencephalography (aEEG) represents a non-invasive, bedside, and simplified method of continuous brain monitoring, mainly accessed by the neonatologist, which has been increasingly used in the NICU in order to access brain function. In this method, a minimum of three electrodes are placed on the scalp (more electrodes may be used to record a multichannel aEEG). Similar to EEG, differences in electrical potentials are recorded, and changes in electrical activity are displayed over time. Then, the electrical activity is time-compressed, rectified, smoothed, and displayed semilogarithmically in a 6cm/h chart after being filtered for frequency. Newer equipment can associate aEEG with raw EEG traces and video imaging, allowing better identification of seizures and artifacts in the NICU.11–14
Previous findings have shown that aEEG, as well as the conventional EEG, is a valuable tool to access background activity, cyclicity, which can be represented by periodical changes in the pattern of aEEG neonatal recording, often referred to as sleep-wake cycling (SWC), and to detect seizures in critically ill neonates as a continuous bedside monitoring.11–16
This review aimed to discuss a variety of clinical applications of aEEG for monitoring critically ill neonates.
RevisionThe databases of Pubmed, SciELO, Lilacs and Cochrane, books, and other online resources were consulted, as well as sources of professional experiences, in order to carry out this critical review about the use of aEEG in newborns at high-risk for brain injury. The central-temporal areas are the source of most newborn seizures, and the features of EEG ictal activity differ between preterm and full-term babies.16,17
Although traditional EEG is the gold standard for seizure diagnosis, and short or low voltage seizures may be missed in aEEG depending on the examiner, the aEEG has the advantage of being more straightforward and quicker to apply. Also, more extended periods can be monitored, and thus, there are more chances of not missing seizures as in a punctual cEEG for a few hours. It should also be remembered that aEEG can be applied almost immediately on demand, and aEEG has become an interesting option to be used as a bedside tool for monitoring neonates. Several studies and reviews have accessed the accuracy of aEEG for seizure detection. Using two-channel aEEG associated with raw EEG interpretation has improved accuracy.16,17–24 Seizure activity is characterized in aEEG by a sudden change in background activity as an abrupt rise in minimum and maximum aEEG amplitudes correlated with an evolving, stereotypical, and rhythmic wave pattern, repeating form such as spikes or sharp waves, often with high amplitude in raw EEG, with a total duration of at least ten seconds25 (Figure 1). The central-temporal areas are the source of most newborn seizures, and the features of EEG ictal activity differ between preterm and full-term babies.16,17
Shellhaas et al.16 evaluated 125 aEEG single-channel C3-C4 readings from patients with gestational ages ranging from 36 to 44 weeks. In this study, 78% of all neonatal seizures appeared in the single-channel aEEG. Compared to traditional electroencephalography, however, aEEG seizures were shorter and had a lower amplitude.16 Shah et al.17 monitored 21 term newborns referred to a tertiary hospital with clinical seizures. Thirty-one of forty-one non-status epilepticus episodes were recognized properly (sensitivity, 76%; specificity, 78%; positive predictive value, 78%; negative predictive value, 78%). When amplitude-integrated electroencephalography was accessed (one or two-channel) without the use of raw EEG readings, a lower sensitivity (27–56%) was found.17
Frenkel et al.20 evaluated 38 high-risk babies for seizures and compared the sensitivity and specificity of seizures detection across users with varying levels of aEEG competence. When students assessed the raw EEG trace for seizure detection, the sensitivity and specificity were 84% and 66%, respectively, and 71% and 97% for neonatologists. In the hands of expert users, the technique offers excellent sensitivity and specificity, according to the author.20
A systematic review by Rakshasbhuvankar et al.23 included ten studies (patient sample of 433) and found a median sensitivity of 76% and specificity of 85% for seizure detection when aEEG was evaluated together with raw EEG tracings. When aEEG data was evaluated by experienced aEEG readers, the accuracy was higher.16,23,24
The diagnosis and proper treatment of seizures are extremely important in the neonatal period since most episodes occur without associated clinical manifestations. Previous studies demonstrate that epileptic seizures are directly associated with brain damage. At the same time, if the authors use anticonvulsants inappropriately and inadvertently, the authors will also cause brain damage from drug-triggered apoptosis. With continuous bedside monitoring, the authors use fewer anticonvulsants and more assertively.
Hypoxic ischemic encephalopathy (HIE)In term newborns, HIE is a significant cause of early death and persistent severe impairment.26 The degree of the brain injury and neurological outcome should be assessed to guide management, therapy selection, prognosis evaluation, and parent counseling.
HIE is the most prevalent cause of neonatal seizures.27 Since most neonatal seizures have no clinical signs, continuous brain monitoring has an essential role in this population. Beside seizure detection, aEEG has been used for prognosis assessment and as entry criteria for cooling in some clinical trials.
Prognosis assessmentBefore the cooling era, abnormal aEEG background patterns during the first 3–6 postnatal hours were significantly predictive of unfavorable outcomes.28–30 Hellström-Westas et al.30 described that early aEEG background activity correctly predicted neurological outcomes in 91.5% of infants in a cohort of 47 neonates with HIE.30
In 2007, Spitzmiller et al.31 carried out a meta-analysis to assess the evidence for utilizing aEEG as a quantitative predictor of neurodevelopmental outcome in full-term infants with HIE. They found that aEEG had an overall sensitivity of 91% (95% and confidence interval: 87–95) and a negative probability ratio of 0.09 (95% confidence interval: .06-.15) for properly predicting a bad outcome, which shows how aEEG can help predict long-term neurodevelopmental outcomes for high-risk babies.31
The prognostic value of aEEG at 6 h of life had changed when cooling was implemented. The authors have described time to recovery to normal background pattern as a very important predictor of outcome after 48 h of life, and an abnormal aEEG tracing is linked to subsequent abnormal neurodevelopmental outcomes.32,33 Thoresen et al.32 studied a cohort of 74 infants with HIE. They discovered that an abnormal aEEG pattern at the age of 3 to 6 h had a positive predictive value of 84% for normothermia and 59% for hypothermia. The recovery time to normal background pattern was the best predictor of poor outcome (96.2% in hypothermia, 90.9% in normothermia).32
Some investigations studied the effect of therapeutic hypothermia (TH) may have on aEEG accuracy in predicting neurodevelopmental outcomes. Chandrasekaran et al.34 also conducted a meta-analysis to examine the prognostic accuracy for predicting long-term neurodevelopmental outcomes in term newborn infants undergoing TH for moderate or severe EHI. The authors systematically evaluated the predictive value of an abnormal trace acquired at 6, 24, 48, and 72 h of age. They concluded that the aEEG background at six h of age has a good sensitivity of 96%, but poor specificity 39%, and at 48 h or later, a persistently severe abnormal aEEG background predicts a poor prognosis (positive predictive value 85% and diagnostic odds ratio 67 at 48 h). According to this study, the predictive ability of aEEG background at six hours of age during TH induction is sub-optimal. Because consistently abnormal traces at 48 h or more were linked with poor long-term outcomes, aEEG examination at 48 h of age may be beneficial as an additional bedside inquiry for clinical decision making.34
A systematic review by Del Río et al.35 included 17 studies and found that infants not treated with hypothermia with abnormal aEEG background at six hours of life had a positive predictive value of 88.20% (95%CI 79.80 to 93%) in the prediction of death or moderate/severe disability. In this cohort, the highest dependability of an abnormal aEEG in predicting prognosis was found at 36 h of life, with a positive post-test probability of 97.90% (95% and confidence interval: 88.40 to 99.40%). Maximum predictive reliability was reached in babies treated with TH at 72 h of life, with a post-test probability of 95.70% (95%CI 84.40 to 98.50%).35
Entry criteria for therapeutic hypothermiaIt is well established that TH significantly improves survival and reduces disability, including cerebral palsy and neurocognitive outcomes, in full-term infants with moderate to severe HIE.36
Due to the good prognostic value of early aEEG in infants with HIE evaluated before the cooling era,28–30 some of the main clinical trials that evaluated the efficacy of TH added an abnormal aEEG background as needed entry criteria for initiation of cooling.37,38 However, it is still unclear whether abnormal aEEG background should be used to identify infants for TH. The accurate identification of infants suffering from encephalopathy is crucial. Clinical studies of TH in newborns without indications of moderate or severe encephalopathy have yet to be conducted. It is also essential not to exclude patients who could benefit from neuroprotective strategies.
A recent study evaluated 47 infants who underwent TH and aimed to examine whether using aEEG severity pattern should be used as entry criteria for TH. Results suggested that an abnormal aEEG would improve the selection of infants with HIE.39 The authors think it is reasonable to use aEEG in the first 6 h of life as another tool for evaluating newborns possibly eligible for treatment with TH. However, not necessarily a pathological background activity should be mandatory as entry criteria, and a normal background activity isolated should not exclude newborns from treatment with hypothermia. Future and ongoing studies need to better evaluate the use of aEEG in the first six hours of life as an entry criterion for therapeutic hypothermia for more solid conclusions.
Brain monitoring in asphyxiated newborns has been shown to be important in clinical practice at the bedside due to: assessment of brain function over time, providing support in the indication or not of TH, diagnosing neonatal seizures commonly present in this condition, and also predicting long-term neurological prognosis.
Preterm infantsThe first studies of using aEEG in premature infants have been described in the 80’s by Verma et al.40 and in the 90’s by Hellström-Westas et al.41 Although the use of aEEG in term newborns is well acknowledged, research into its use in preterm infants is still ongoing.
In terms of background activity, in extremely preterm babies, the EEG background is discontinuous, with high-voltage bursts and low-voltage interburst periods. Discontinuity decreases with increasing gestational age.42 A concordance between sleep states and continuity/discontinuity is not established in the smallest preemies, even though the cyclicity represents a very early phenomenon in cerebral function even before 27 weeks.15,41,43,44 More frequent 'bursts' are conceivable, and the raw EEG tracing may reveal periods of relatively low voltage activity interspersed with brief but rare bursts of high activity.45
Seizures are a tricky issue in preterm infants, as they are the ones who are either missed (short and of the same voltage of the background activity) or misdiagnosed due to many rhythmic events in the NICU mimicking seizures.46 Though many authors evaluated seizures in preterm infants, not many included EEG,47 and only four used aEEG (two are mentioned in the manuscript: Shah et al.48 and Vesoulis et al.49).
The incidence of described electrographic seizures in the preterm infant varies widely from 4% to 48%.41,48,50,51 Intraventricular hemorrhage (IVH), white matter injury (WMI), mortality in the neonatal period, and moderate to severe cognitive impairment on follow-up have all been linked to electrographic seizures in preterm infants.49
Vesoulis et al.49 evaluated a cohort of 95 very preterm infants for 72 h after birth. They found an incidence of 48% of seizures, which were associated with poor early and long-term outcomes.49 Preterm infants with seizures have shown in studies to be more likely to die, which means that seizures may be an indicator of pathologies that could lead to death, even though seizures alone do not represent a predictor of death.52
In the first postnatal week, continuous background activity and cyclicity have been linked to a positive neurodevelopmental result.53 Abnormal background activity and absence of cyclicity in the first days/weeks of life were associated with severe IVH, WMI, neonatal death,12,54 and neurological impairment at two years of age.53,55
Soubasi et al.54 monitored 115 infants from 25 to 32 weeks of gestational age within 72 h of life. The presence of pathological trace (defined as burst suppression, continuous low voltage or flat) or discontinuous low voltage displayed a sensitivity of 88.9% severe brain damage in cranial ultrasonography results or death.54
Klebermass et al.55 study evaluated 143 preterm infants with gestational age below 30 weeks during the first two weeks of life. aEEG was classified into a graded score according to background activity, the appearance of cyclicity, and seizure activity and correlated with the good neurological outcome at three years of age. Specificity and sensitivity were 95% and 83%, respectively in aEEG findings in the second week of life and were superior when compared with cranial ultrasound findings.55
The use of aEEG in seizures and brain function evaluation in preterm newborns has shown to be beneficial. Electrographic seizures in the first week of life are linked to poor outcomes, according to research.48 A study conducted by Wikström et al.53 evaluated 49 preterm infants between 22 and 30 weeks of gestational age to characterize aEEG and EEG for prediction of long-term outcome. They reported that aEEG/EEG could predict long-term outcomes with 75–80% accuracy at 24 h of life, even within those with no early indication of brain damage.53
The use of early aEEG examination in preterm infants may help in providing helpful counseling information. Some clinical neonatologists consider hazardous the need to manipulate the newborn's head in the first 72 h of life to place the aEEG electrodes in very preterm infants regarding prevention of periventricular hemorrhage. The authors consider that this possible risk can be minimized with gentle care and is outweighed by the benefits highlighted above.
The use of brain monitoring in preterm infants has evolved as new scientific research has been published, but some questions still need to be elucidated, such as: is it safe to install the aEEG in the period of minimal handling? Does it alter the incidence of peri- and intraventricular hemorrhage? Will the care be better with brain monitoring, avoiding neurological damage? These are some questions that the literature must answer in the future.
Congenital heart diseaseBrain injury is a common and sometimes fatal complication of severe congenital heart disease (CHD) that needs surgery during the neonatal period.56 The perioperative period represents a moment of risk when the developing brain is vulnerable to acute cerebral perfusion and oxygenation changes.
Clinical and electrographic seizures in the perioperative period have been associated with neurological impairment in cohorts of infants with CHD. Several authors used aEEG for evaluating the incidence of seizures, early recovery to normal background activity, and presence of SWC after cardiac surgery with good neurological outcome in this population.57,58
Gunn et al.57 studied a large group of 150 newborn children who had heart surgery and were monitored with aEEG during the perioperative phase (before and during surgery, and for 72 h postoperatively). Perioperative electrical seizures were found in 30%, of whom 1/4 had any clinical correlation. Failure to return to a continuous background at aEEG by 48 h postoperatively was linked to impairment in all outcome categories, and abnormal aEEG at seven days postoperatively was strongly linked to death.57
During the management of infants with CHD, in addition to survival, the aim is to minimize the risk of neurological sequelae; therefore, the authors recommend brain monitoring before, during, and after cardiac surgery. Using brain monitoring in these 3 phases, the authors can have an early diagnosis of seizures and electroencephalographic changes associated with brain injury, enabling assertive therapy and possibly impacting long-term neurodevelopment.
Other applicationsThe neonatal intensive care unit usually counts with many infants at high risk of brain injury. Beside neonates with HIE, extreme prematurity, and congenital heart diseases, other conditions pose true moments of high risk for brain injury and incidence of seizures. Metabolic disorders, inborn errors of metabolism (IEM), stroke, cerebral malformations, congenital infections, sepsis, meningitis, hemodynamic and ventilator instability, ECMO, and recovery after cardiac arrest are examples of these conditions.
Since aEEG is a useful non-invasive tool to access real-time brain function and seizure detection in the neonatal population, there are many possible situations where aEEG could be applied, and several studies have addressed this issue.59–61
Olischar et al.59 evaluated a cohort of 30 infants with IEM and found abnormal background activity in 70% and presence of seizures in 60% of monitored patients. Infants with metabolic energy problems, hyperammonemia, and organic/amino acidopathies frequently had aEEG depression and seizure activity. The aEEG of individuals with peroxisomal diseases, on the other hand, did not reveal any significant background abnormalities.59
Toso et al.61 evaluated 21 infants with different causes of neonatal encephalopathy, neurologic disturbance, or severe respiratory distress syndrome and found the incidence of 38% of seizures.61
Helderman et al.60 examined a large group of 108 preterm children born before 28 weeks of gestation and used an electroencephalogram (EEG) every month from 28 to 36 weeks of postmenstrual age. During the first bout of sepsis in the babies, further aEEG recordings were made. At the moment of recording, burst suppression was identified in 22% of aEEG recordings from newborns without sepsis and 57% of babies with sepsis, showing that sepsis is associated with acute electroencephalographic changes.60
Several medications such as sedation and antiepileptic drugs affect brain function and can influence aEEG background activity. This influence usually is related to depression on background activity and depends on the type and dose of the used drug. Norman et al.62 performed a randomized controlled trial using aEEG/EEG as a brain monitoring assessment to compare medications for rapid sequence intubation (including thiopental and remifentanil) with morphine. Their findings demonstrate that morphine treatment causes longer cerebral depression, more discontinuous aEEG/EEG background activity, and a lack of SWC that lasts for 24 h in extremely preterm babies. Morphine dosages often used as analgesics exhibited a neurodepressive impact that lasted at least six hours.62 In recent research, Peled et al.63 found that six hours after morphine beginning, background aEEG activity score worsened in 40% of extremely preterm babies, and cycling, which was originally evident in 70% of the children, could only be identified in 10% after ten hours.63
Yuan et al. have published a study on the potential predictive neurological prognostic value of early continuous aEEG in term and near-term infants with severe hyperbilirubinemia. They discovered that early aEEG had a sensitivity of 35.7% and a specificity of 92.0% in predicting poor neurodevelopmental outcomes in newborns with severe hyperbilirubinemia. On the other hand, the sensitivity was significantly lower than the auditory brainstem response.64
Another common procedure that usually affects aEEG trace is surfactant administration, which often can lead to a noticeable but transient depression on brain activity lasting around ten minutes.65
From our point of view, every newborn with a disorder leading to the possibility of brain damage, with a risk of impacting the quality of life, will benefit from being monitored with aEEG, providing real-time information on brain function and seizure diagnosis, giving opportunity for immediate treatment with less chance of permanent neurological sequelae. It is survival with quality of life, impacting the child, family and society.
LimitationsThe aEEG can be used as a screening tool for seizures, although brief, focused, and low-amplitude seizures may be missed.29 Moreover, in some specific clinical scenarios, seizure activity can arise from cerebral areas not covered by the aEEG electrodes, leading to false negatives. Using two-channel aEEG and correlating with raw EEG interpretation (with readers' expertise) is mandatory to increase sensibility and specificity.20,23 It is important to point out that full array continuous EEG remains the gold standard for seizures detection. Current technology and remote access possibilities may allow experienced specialists to provide long-term and distant assistance.
ConclusionThe aEEG has shown to be a useful non-invasive bedside monitor that aids in evaluating brain function, background activity, and cyclicity. In a group of critically sick neonates, aEEG data have also demonstrated high predictive value, particularly in babies with HIE, where aEEG might potentially assist select infants eligible for neuroprotective treatments such as therapeutic hypothermia. The seizure detection capability of the use of aEEG and its limitations have been accessed in many studies.
With the evolution of brain-focused neonatal care, including early diagnoses of brain injury and neuroprotective strategies, the use of bedside brain monitoring such as aEEG may be suitable for neonates at high risk in a large number of clinical situations.